Abstract
Cryptosporidium parvum is a well-known waterborne and opportunistic intracellular protozoan parasite that causes diarrheal illness. In this study, we quantitatively investigated reduction of the infectivity of C. parvum after gamma irradiation and repair of the infectivity during incubation time after irradiation. C. parvum oocysts were subjected to gamma irradiation at various doses (1, 5, 10, and 25 kGy), and the in vitro infectivity was measured by real-time PCR every day up to 7 days after irradiation. The in vitro infectivity of C. parvum on human ileocecal adenocarcinoma cells (HCT-8) was effectively reduced (> 2 log10) by irradiation at 10 kGy or more. However, in the experiment to find out repair of the infectivity, recovery was not noted until day 7 post-incubation.
-
Key words: Cryptosporidium parvum, gamma irradiation, infectivity
INTRODUCTION
Cryptosporidium parvum is an obligate intracellular protozoan that infects a wide range of vertebrates, including humans and animals [
1]. Most infections are acquired from water or food contaminated with infectious oocysts [
2,
3].
Cryptosporidium has been a threat to the water industry and public health since 1980 when AIDS became a public health issue, and its low infection dose and high resistance to various disinfectants facilitate its high infection rate [
4-
6].
Gamma irradiation has been used to prevent some food-borne infections derived from parasites and to produce vaccines for parasitic infections [
7-
10]. We have previously shown that gamma irradiation at 50 kGy is necessary for a complete elimination of
C. parvum oocyst excretion in vivo [
11], which is the highest reported resistance to irradiation among parasites. In the present study, we investigated the reduction of the infectivity of
C. parvum by gamma irradiation and the repair of the infectivity using absolute quantitative real-time PCR (qPCR). As it has been already known that in vitro infectivity of
C. parvum using human ileocecal anenocarcinoma cell line (HCT-8) could be a useful substitute for animal infectivity study, and CP2 gene is a useful viability marker for quantitation of
C. parvum [
12,
13], we evaluated infectivity change and repair of
C. parvum after gamma irradiation quantitatively using an in vitro system. Several biological characteristics of non-irradiated
C. parvum were also evaluated quantitatively, including changes in viability and in vitro infectivity during 12 months of storage, and the time point when the number of parasites was maximal in a cell culture system.
MATERIALS AND METHODS
C. parvum oocyst preparation and gamma irradiation
The oocysts of
C. parvum (KKU isolate) maintained in specific pathogen-free C57BL female mice were purified according to the method of previous papers [
14,
15]. The purified oocysts were surface sterilized by placing them in 10% sodium hypochlorite solution for 10 min, and they were stored for less than 2 wk at 4℃ before the experiment. As we found that
C. parvum sporozoites maintained their infectivity up to 13 days in this study, we irradiated sporozoites directly and used them for the viability and in vitro infectivity studies. For excystation, oocysts were incubated in acidified Hanks' balanced salt solution (pH 2.75) for 10 min at 37℃ in a shaking incubator, followed by incubation in 4 mM sodium taurocholate (Sigma, St. Louis, Missouri, USA) in phosphate-buffered saline (PBS, pH 7.4) at 37℃ for 15 min [
16]. A 1.5-ml microcentrifuge tube containing 8 × 10
7 of
C. parvum sporozoites with 1 ml of Dulbecco's modified Eagle's medium (DMEM) (Sigma) was immersed in a 50-ml tube filled with distilled water, both to obtain the backscattered ray and to reduce the temperature increase due to energy absorption from the high-dose radiation. Irradiation was performed at room temperature (22℃ for 2 hr with a
60Co IR221 High Performance Tote Irradiator (MDS Nordion, Ottawa, Canada). The irradiation doses were 1, 5, 10, and 25 kGy, with dose rates ranging from 5 × 10
2 to 1.25 × 10
4 Gy/hr. The control group was kept at room temperature during irradiation. The temperatures of the room and irradiated sample tubes were measured before and immediately after irradiation.
Human ileocecal adenocarcinoma cells (HCT-8; Korean Cell Line Bank, Seoul, Korea) were incubated in DMEM (Sigma) with 10% heat-inactivated fetal bovine serum (Gibco BRL, Gaithersburg, Maryland, USA) supplemented with L-glutamine (Sigma), 15 mM HEPES (
N-2-hydroxyethylpiperazine-
N'-2-ethanesulfonic acid, pH 7.5), 14 mM sodium bicarbonate, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B. For routine cell passages, the HCT-8 cells were incubated at 37℃ in a humidified incubator containing 6% CO
2 and 94% air. Before inoculation of excysted oocysts (sporozoites), HCT-8 cells at a confluence of approximately 70% in a 35-mm dish were carefully rinsed with 0.1 M PBS (pH 7.2). Sporozoites (8 × 10
6) diluted with prewarmed cryptosporidia infection medium [
17] were inoculated gently onto the HCT-8 cells. After 2 hr of incubation, the infected HCT-8 cells were gently washed 3 times with 0.1 M PBS and then reincubated for another 22 hr in a humidified incubator containing 6% CO
2 and 94% air. The infection was stopped at each time point in the experiment evaluating time point of maximal infection. For the study of infectivity after gamma irradiation, infection was stopped at 24 hr after inoculation of sporozoites. After removing the culture medium, the cell monolayer was washed with 0.1 M PBS and used for RNA purification.
C. parvum viability was assayed by treating with 4 cycles of freezing (liquid nitrogen for 2 min) and thawing (95℃ for 2 min) to extract total RNA, and the infectivity was assayed by lysing
C. parvum-infected HCT-8 cells in Trizol® reagent (Invitrogen, Carlsbad, California, USA). Total RNA was extracted in accordance with the manufacturer's instructions as described by Lee et al. [
13]. For reverse transcription (RT), the reaction mixtures included 0.5-1.0 µg of total RNA, 10 pmol of oligo (dT)
20 primer (Bioneer, Daejon, Korea), 1 × Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV) RT reaction buffer, 0.25 mM dNTP mixture (TaKaRa, Otsu, Shiga, Japan), 1 U of recombinant RNasin ribonuclease inhibitor (Promega, Madison, Wisconsin, USA), and 10 U of M-MLV RT enzyme (Enzynomics, Daejeon, Korea). RT reactions were carried out for 1 hr at 42℃ and were stopped by incubation at 70℃ for 10 min.
Absolute qPCR
Plasmid pGEMT-CP2 was prepared as previously described [
13]. Absolute qPCR reactions were performed in a 20-µl volume using cDNA as a template. Reaction mixtures included 0.1 × LightCycler® FastStart HybProbe master mix (Roche, Mannheim, Germany), each
C. parvum primer set at 0.5 µM (CP2-F, 3'-AAAACAAAAACTACTCAGACTCAAG-5', CP2-R, 3'-GGGTTGTAAACTTCATTGACTTT-5'; Bioneer), and each probe set at 0.1 µM (CP2FL, TCAATATCAGAGGTTACTTCAACTTCGC-FL; CP2LC, 705-TGGGACTGGTAAAACTATGACGTCTTC-P; TIB MOLBIO, Germany). qPCR was carried out with a LightCycler® real-time PCR system (Roche). Each qPCR mixture was subjected to an initial denaturation at 95℃ for 10 min, followed by 55 cycles of denaturation at 95℃ for 5 sec, annealing at 55℃ for 15 sec, and extension at 72℃ for 8 sec, with a final cooling step at 40℃ for 30 sec. The results were analyzed by LightCycler® software (version 4.05, Roche). DNase/RNase-free water was included as a negative control.
RESULTS
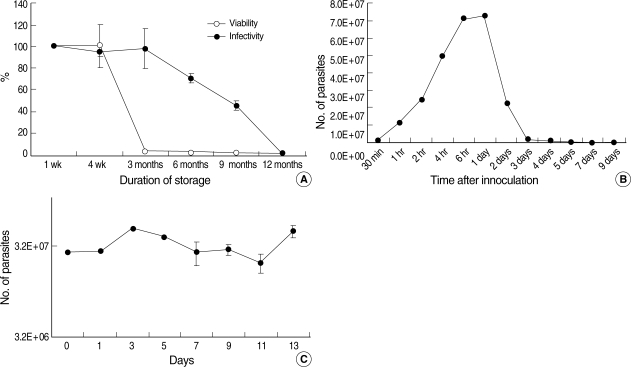
Viability and infectivity of non-irradiated C. parvum
The viability and infectivity changes in non-irradiated
C. parvum oocysts according to storage duration were measured by qPCR using a CP2 gene-specific primer and probe with cDNA converted from total RNA extracted from oocysts and HCT-8 cells infected with
C. parvum. The viability of
C. parvum oocysts at 4 wk was not changed, but it was reduced rapidly thereafter, and was less than 3% after 3 months compared to 1-wk-old oocysts (
Fig. 1A). The infectivity of
C. parvum oocysts remained at up to 98% after 3 months of storage compared to 1-wk-old fresh oocysts (
Fig. 1A), but it was reduced to 71% and 45% after 6 and 9 months, respectively, and to only 0.7% after 12 months (
Fig. 1A). The number of parasites infected in the HCT-8 cell line was maximal (7.2 × 10
7) at 6 to 24 hr after inoculating cells (
Fig. 1B), which represented increases of 9- and 3-folds in the inoculation dose (8 × 10
6 sporozoites) and the number of infected parasites at 2 hr after inoculation (2.4 × 10
7), respectively. However, the number of infected parasites decreased rapidly, with no parasites being detected at 7 days after infection (
Fig. 1B).
The infectivity of
C. parvum sporozoites was maintained for 13 days (the last day we studied) after excystation when they were preserved at 4℃ in cell culture medium (DMEM) without FBS (
Fig. 1C). Based on the above results of biological characteristics of non-irradiated
C. parvum oocysts, we irradiated sporozoites directly and observed the in vitro infectivity at 24 hr post-inoculation of parasites into host cells.
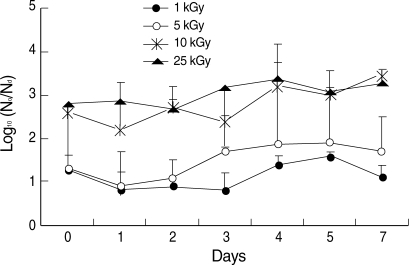
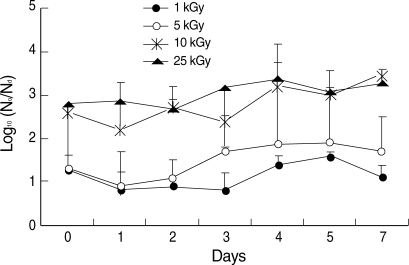
The temperatures of the irradiated sample tubes were not different before and immediately after irradiation such as in a range of 21.7 to 22.0℃ The effective reduction of in vitro infectivity (> 2 log
10) was achieved by more than 10 kGy irradiation (
Fig. 2). During 7 days after irradiation, there was no recovery of infectivity in irradiated group with more than 10 kGy (
Fig. 2). In the irradiated group with less than 5 kGy, in vitro infectivity was not reduced effectively and effective reduction of infectivity (> 2 log
10) was not achieved during 7 days after irradiation (
Fig. 2).
DISCUSSION
In the present study, we found that
C. parvum oocyst viability was the same after 4 wk of storage as in 1-wk-old oocysts. It was also noted that the viability decreased rapidly after 4 wk and remained less than 3% in oocysts after 3 months. On the other hand, the infectivity of
C. parvum was the same as in fresh oocysts for up to 3 months, but then decreased to 0.7% at 12 months. Both viability and infectivity of
C. parvum were almost lost when oocysts were 12-month-old. These data showed that there is no correlation between viability and infectivity of
C. parvum oocysts aged from 1 to 12 months when they are measured by the CP2 gene. A previous study found that several genes used as viability targets also decreased before infectivity was lost [
18]. Ribosomal RNA gene was detected at 40-50% for 3-7 months and at 20% after 8 months by fluorescence in situ hybridization, and the RT-PCR signals of the amyloglucosidase CPAG decreased relative to that in 1-month-old oocysts by 50% at 3 months and were not detected after 4 months of storage, although
C. parvum oocysts suspended in deionized water at 0-20℃ exhibited infectivity for mice and cell cultures after 6-7 months of storage [
18,
19]. Therefore, it remains necessary to develop a viability marker that has a strong correlation with the infectivity of
C. parvum oocysts.
It has been pointed out that once liberated from the oocyst, the sporozoite has limited energy resources and must find a host cell quickly, and that morphological changes in isolated sporozoites from an elongated banana shape to a rounded pear shape results in a loss of active mobility that helps invasion to host cells [
20]. In the present study, we stored sporozoites in serum-free cell culture medium at 4℃ infected host cells every day, and checked the infectivity at 24 hr after inoculation. Unexpectedly, the infectivity of sporozoites was maintained for 13 days (until the last day of study). Based on this result, we applied gamma irradiation to sporozoites directly to rule out an experimental bias from an excystation procedure after gamma irradiation.
In this study, we found that the number of parasites was maximal (9-fold increase of inoculation dose) after 6 to 24 hr of in vitro infection, with the number of parasites decreasing thereafter. The increased number of parasites suggests that only asexual development cycle occurred because 8 merozoites are developing from 1 sporozoite. After 24 hr, no more asexual or sexual propagation was evident in our in vitro culture system.
Toxoplasma gondii and
Eimeria necatrix, both coccidian protozoans, were reportedly to be controlled by irradiation at 0.6-2.0 kGy [
10,
21,
22]. To the best of our knowledge,
C. parvum is the most tolerant of the tested parasites to gamma irradiation. Kato et al. [
23] reported that the excystation rate was the same in
C. parvum oocysts receiving 2 kGy irradiation and non-irradiated oocysts, but they also showed that 20 and 50 kGy irradiations reduced excystation rates of 50% and 0%, respectively. We have previously shown viabilities of 89-94% and 46-59% in
C. parvum oocysts irradiated at 0-10 and 25-50 kGy, respectively, using nucleic acid staining [
11]. Previous studies have found different effects of gamma irradiation on the viability and infectivity of
C. parvum. Irradiation at 0.25 kGy was found to be necessary to kill oocysts in a neonatal mouse infectivity model [
24], and 0.4 kGy was reported to be sufficient to kill oocysts in a neonatal calf model [
25]. We have previously reported successful infection in a weaning mouse model with up to 25 kGy although infection duration was transient in the early period [
11]. The discrepancy of the results between previous studies might be due to not only the
C. parvum strain difference between studies but also the use of different experimental methods to measure viability and infectivity, such as semiquantitative PCR [
24] and fecal examination [
11], and using different host animals such as neonatal calves [
25] and mice [
11,
24].
The present study showed that there was a 5-fold difference in irradiation dose for the complete loss of infectivity of
C. parvum sporozoites between mouse (50 kGy) [
11] and HCT-8 cell line models (10 kGy). It is quite understandable that there must be a big difference in the infection environment between in vivo and in vitro. Therefore it could be more difficult in an in vitro culture system to succeed in infection by sporozoites with the same degree of damage than an in vivo system. The infectivity was not changed significantly in the present study during 7 days after irradiation in all of the experimental groups, which suggested that there was no post-irradiation infectivity repair over time. Previously, no phenotypic evidence of either light or dark repair of UV-induced DNA damage of
C. parvum was found by Shin et al. [
26] as well. The present study showed that for the purpose of disinfection of
C. parvum, 10 kGy of irradiation could be an effective dose. The molecular mechanism of the high radioresistance of
C. parvum should be investigated in more details in the future.
ACKNOWLEDGEMENTS
This work was supported by the Second Phase of the BK (Brain Korea) 21 Project in 2008 and the program of the Basic Atomic Energy Research Institute (BAERI), which forms part of the Nuclear R & D Programs funded by the Ministry of Science and Technology (MOST) of Korea in 2008. This study complied with the current laws of the Republic of Korea, where the experiments were performed.
References
Fig. 1(A) Temporal viability and infectivity changes in C. parvum oocysts by qPCR with CP2 gene-specific primers. C. parvum oocyst viability was measured from extracted total RNA of oocysts, and the infectivity was measured at 24 hr after the inoculation of oocysts from extracted total RNA of C. parvum infected HCT-8 cells. (B) Tentative number of C. parvum sporozoites in HCT-8 cells by absolute qPCR using CP2 gene-specific primers. The number of infected C. parvum on HCT-8 cells was maximal at 1 day after inoculation. Absolute qPCR was performed using CP2 gene primers with extracted total RNA. (C) Maintenance of infectivity of C. parvum sporozoites on HCT-8 cells measured by absolute qPCR using CP2 gene-specific primers. The number of infected C. parvum sporozoites did not change markedly over 13 days in comparison with freshly excysted sporozoites.
Fig. 2Infectivity changes of gamma-irradiated C. parvum sporozoites in HCT-8 cells measured by qPCR with a LightCycler® system. Infectivity is shown as log10 (N0/Nd), where N0 and Nd are the numbers of live parasites in the non-irradiated and irradiated groups, respectively. Data are mean ± SD values.