INTRODUCTION
Schistosomiasis is an important disease problem in several Asian countries. Schistosomiasis japonica is prevalent in China and the Philippines, where millions of people are affected. Schistosomiasis mekongi is prevalent in the Mekong River basin (MRB) from the Khong district in southern Laos to Kratie province in northern Cambodia. The total population at risk for schistosomiasis mekongi is estimated to be 60,000 in Laos and 80,000 in Cambodia [
1]. The first human case of
Schistosoma mekongi infection was reported as
Schistosoma japonicum infection in 1957 [
2]. Afterwards,
S. mekongi was identified as a new species of
Schistosoma [
3].
S. japonicum and
S. mekongi have a complicated mode of transmission. As part of the life cycle occurs in the environment outside of the host, it is difficult to measure the transmission rate on the basis of field observations [
4]. Therefore, a mathematical model for
Schistosoma transmission could be useful for estimating its prevalence, and model simulations can be instrumental in managing various control strategies. There have been many studies involving the mathematical modeling of transmission for
S. japonicum since 1965 [
4-
12], while there have been only 2 studies on mathematical modeling of the transmission of
S. mekongi [
13,
14]. This review focuses on the epidemiological and environmental factors in
S. japonicum and
S. mekongi infections and the recent advances in mathematical models of
Schistosoma transmission.
S. japonicum and
S. mekongi carry out their transmission cycle in definitive and intermediate hosts. Humans are the major definitive hosts, and many domestic and wild mammals were found to be reservoirs [
15-
19], whereas the intermediate hosts are only snails. Cercariae, which are released from infected snails, penetrate into the definitive host via the skin and develop into mature adults in the host; female adults produce eggs throughout their life-span. A mathematical model that quantitatively describes
Schistosoma transmission needs to include the following components [
4,
14].
Dynamics of the human population and behavior of humans related to water contact
Dynamics of the intermediate host population
Fluctuation of water level in the Mekong River (only for S. mekongi)
Contribution of animal reservoirs
Effect of control measures
DYNAMICS OF THE INTERMEDIATE HOSTS: SNAILS FOR S. JAPONICUM
Oncomelania spp. snails were recognized as intermediate hosts of
S. japonicum;
O. quadrasi and
O. hupensis were identified in the Philippines [
20,
21] and in China [
16], respectively. As snail density varies according to time and circumstances, it is difficult to make accurate estimates of snail density. In Bohol, the Philippines, a water area fluctuates seasonally according to rainfalls [
4]. In regard to the longevity of snails, the mortality rate among infected snails is higher than that among uninfected snails [
20,
22].
DYNAMICS OF THE INTERMEDIATE HOSTS: SNAILS FOR S. MEKONGI AND FLUCTUATION OF THE MEKONG RIVER FLOW
Neotricula aperta is recognized as the intermediate host of
S. mekongi, which is composed of 3 races (α, β, and γ). Races α and γ range in the MRB from Khong to Kratie, whereas race β lives in the Mun River, a tributary of the Mekong River [
23]. Race γ shows the highest susceptibility to miracidia among the 3 races [
24].
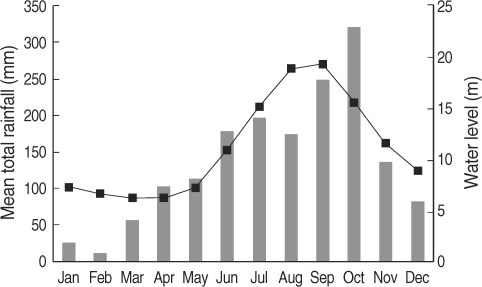
In Cambodia, the rainy season begins in March, and heavy rainfall lasts from June to October; the rainfall drops dramatically in November, and thereafter the dry season lasts from December to February. The heavy rainfall and the arrival of the dry season results in rising and dropping water levels in the Mekong River (World Weather Information Service; Mekong River Commission) (
Fig. 1). The biology of
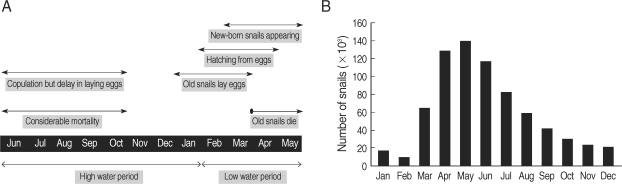
N. aperta is still largely unknown because of the impracticality of field observations during the high water period of the Mekong River. The "Post-Spate Survival" hypothesis that
N. aperta survive and copulate during the high water period of the Mekong River, but that laying eggs could be delayed until next January, and that thereafter the eggs hatch from February has been used to represent the population dynamics of
N. aperta [
14,
25] (
Fig. 2).
DEFINITIVE HOSTS: ANIMAL RESERVOIRS
When animal reservoirs play a part in the reproduction of
Schistosoma, it is necessary to give careful consideration to them because of the difficulty in eliminating
Schistosoma by means of chemotherapy for humans only [
26]. In Lyte, the Philippines, many domestic animals such as dogs, pigs, and cows were found to be reservoirs for
S. japonicum [
15], while in Bohol, the Philippines, only rats were infected by
S. japonicum with a low prevalence rate from the results of field surveys [
21]. In China, more than 40 species of domestic and wild mammalians have been identified as reservoirs for
S. japonicum [
16]. In the MRB, dogs and pigs have been recognized as reservoirs for
S. mekongi [
18,
19]. In Laos, the prevalence in dogs was estimated as 11% [
18] and 29.2% [
17].
MATHEMATICAL MODELS OF SCHISTOSOMA TRANSMISSION
Macdonald [
5] first proposed a mathematical model for
Schistosoma transmission, and thereafter a number of mathematical models for
Schistosoma transmission have been published, mainly from the theoretical point of view [
5-
8]. Anderson and May [
22] studied the prevalence of snail infection based on empirical evidence. A stochastic model for schistosomiasis developed from a model for onchocerciasis aimed to evaluate control strategies [
9]. A series of studies on modeling of
S. japonicum transmission and control in China have been performed [
10-
12]. Ishikawa et al. [
4] developed a model of
S. japonicum transmission that took account of seasonal variations in snail density, animal reservoirs, rats, and high and low cercarial shedding stages in snails to predict the effects of control measures against
S. japonicum in Bohol, the Philippines (
Fig. 3). A couple of studies proposed a mathematical model for the transmission of
S. mekongi in Cambodia with age-structure, which was aimed at estimating the coverage rate and range of ages in targeted mass treatment (TT) to interrupt
Schistosoma transmission [
13,
14]. The fluctuation of water level in the Mekong River, dynamics of the intermediate snail host population, and the contribution of an animal reservoir, dogs, were incorporated into this model [
14].
CONTROL MEASURES AND THEIR EVALUATION THROUGH SIMULATIONS
A collaborative project of the Schistosomiasis Control Service of the Philippine Department of Health and the Sasakawa Memorial Health Foundation of Japan has been continuing since 1981 in Bohol [
21]. The major approach to the control of
S. japonicum consists of 2 methods: the detection of infected individuals and chemotherapeutic treatment, snail control by environmental changes such as land reclamation and cement lining of ditches, and using molluscicides. The comparative simulations showed that the prevalence in inhabitants and the density of infected snails could be restored swiftly after the completion of 4 courses of yearly selected mass treatments without snail control, that the prevalence in inhabitants could be reduced gradually by snail control measures alone, and that the prevalence in inhabitants and the density of infected snails would be eliminated by human control together with snail control [
4] (
Fig. 4).
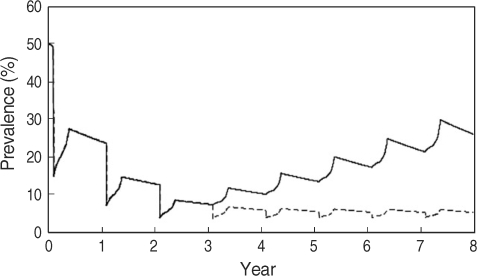
In the MRB, snail control measures such as using molluscicides are ineffective because of the Mekong River flow rate. In Cambodia, a universal treatment campaign (UT) was initiated by the Cambodian government, WHO, and Medecine Sans Frontieres in 1995. The Sasakawa Memorial Health Foundation has rendered support for control of schistosomiasis mekongi since 1997. In Laos, although the average prevalence of schistosomiasis mekongi decreased to less than 1% after mass treatment with praziquantel during a 10-year control program [
27], a resurgence of schistosomiasis in the Khong district was confirmed by epidemiological surveys by WHO in 2003 [
28]. Comparative simulations for the situation of an interruption in mass treatments after 3 courses of yearly UT showed that the prevalence in inhabitants increased swiftly, while the effect of yearly TT for children of 5-19 years old, who show higher prevalence and intensity of infection, after 3 courses of yearly UT would keep the prevalence in inhabitants low throughout an 8-year simulation [
14] (
Fig. 5).
PROSPECTS
Recent advances in mathematical modeling of the transmission of S. japonicum and S. mekongi are summarized here. There has been steady progress in the mathematical modeling of Schistosoma transmission taking into consideration of the ecology of snails and the behavior of inhabitants. The model simulations suggested that, among various possible control measures, a selective mass treatment program coordinated with snail control would be effective for the elimination of S. japonicum. The model simulations also predicted that the suppression of schistosomiasis mekongi could be possible in Cambodia by maintaining control strategies for humans such as biyearly UT or yearly TT with high coverage.
ACKNOWLEDGEMENTS
We appreciate the helpful comments of Profs. H. Matsuda, Y. Chigusa, and M. Kirinoki of Dokkyo Medical University. We are indebted to Dr. T. Matsumoto of the National Institute for Rural Engineering for providing Mekong River water level data.
References
Fig. 1Monthly average rainfall levels (bars) for 5 years during 1997-2001 in Phnom Penh (World Weather Information Service) and monthly average water levels of the Mekong River (line) for 14 years during 1989-2002 in Kratie province, Cambodia (Mekong River Commission) [
14].
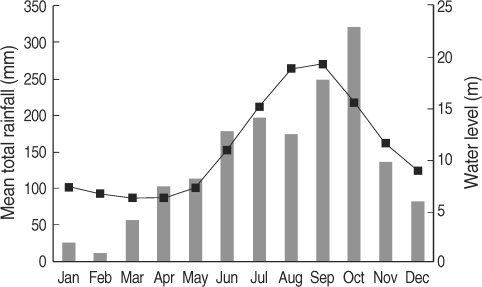
Fig. 2Population dynamics of
Neotricula aperta on the basis of the Post-Spate Survival hypothesis. (A) The life cycle of
N. aperta, (B) The monthly variation of the total snail population [
14].
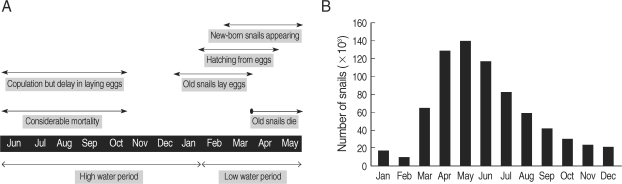
Fig. 3The basic scheme of the transmission model for
S. japonicum showing the transfers among epidemiological classes [
4].
Fig. 4Variations in the infection of
S. japonicum in Bohol (Sto. Thomas) for the human-control case with selective mass treatment at 1-year interval with a coverage rate of 50% (solid line), the snail-control case with the use of molluscicides at half-year intervals under the assumption that its effective rate would be 50% (dotted line), and both the human and snail-control case (dashed line), respectively. (A) Variations in prevalence (%) in the human population, (B) Variations in infected snail densities per 1
a (100 m
2) [
4].
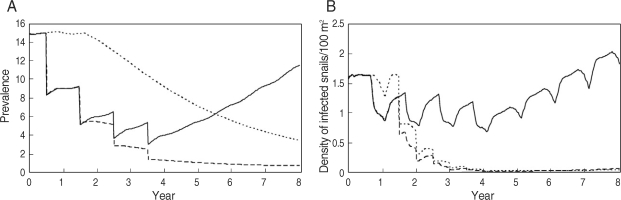
Fig. 5Variations in the prevalence in humans with 2 control measures. 1: Yearly universal treatment (UT) with a 70% coverage rate for the initial 3 years (solid line), 2: After 3 years of annual UT targeted mass treatment (TT) with a 85% coverage rate (dashed line) [
14].

Citations
Citations to this article as recorded by

- Current advances in serological and molecular diagnosis of Schistosoma mekongi infection
Adrian Miki C. Macalanda, Atcharaphan Wanlop, Kevin Austin L. Ona, Eloiza May S. Galon, Virak Khieu, Somphou Sayasone, Aya Yajima, Jose Ma. M. Angeles, Shin-ichiro Kawazu
Tropical Medicine and Health.2024;[Epub] CrossRef - Trematodoses in Cattle and Buffalo Around Schistosomiasis Endemic Areas in Central Sulawesi Province of Indonesia
Novericko Ginger Budiono, Fadjar Satrija, Yusuf Ridwan, Defriska Nur, . Hasmawati
Jurnal Ilmu Pertanian Indonesia.2018; 23(2): 112. CrossRef - Serological approaches for the diagnosis of schistosomiasis – A review
Rebecca Hinz, Norbert G. Schwarz, Andreas Hahn, Hagen Frickmann
Molecular and Cellular Probes.2017; 31: 2. CrossRef - Schistosoma japonicum in Samar, the Philippines: infection in dogs and rats as a possible risk factor for human infection
H. CARABIN, S. T. McGARVEY, I. SAHLU, M. R. TARAFDER, L. JOSEPH, B. B. DE ANDRADE, E. BALOLONG, R. OLVEDA
Epidemiology and Infection.2015; 143(8): 1767. CrossRef - Schistosomiasis japonica: Modelling as a tool to explore transmission patterns
Jun-Fang Xu, Shan Lv, Qing-Yun Wang, Men-Bao Qian, Qin Liu, Robert Bergquist, Xiao-Nong Zhou
Acta Tropica.2015; 141: 213. CrossRef - Exploring the Contribution of Host Susceptibility to Epidemiological Patterns of Schistosoma japonicum Infection Using an Individual-Based Model
Robert C. Spear, Shuo Wang
The American Journal of Tropical Medicine and Hygiene.2015; 92(6): 1245. CrossRef - Development of a real-time PCR assay with fluorophore-labelled hybridization probes for detection of Schistosoma mekongi in infected snails and rat feces
O. SANPOOL, P. M. INTAPAN, T. THANCHOMNANG, P. SRI-AROON, V. LULITANOND, L. SADAOW, W. MALEEWONG
Parasitology.2012; 139(10): 1266. CrossRef