Abstract
A monoclonal antibody against Toxoplasma gondii of Tg556 clone (Tg556) blotted a 29 kDa protein, which was localized in the dense granules of tachyzoites and secreted into the parasitophorous vacuolar membrane (PVM) after infection to host cells. A cDNA fragment encoding the protein was obtained by screening a T. gondii cDNA expression library with Tg556, and the full-length was completed by 5'-RACE of 2,086 bp containing an open reading frame (ORF) of 669 bp. The ORF encoded a polypeptide of 222 amino acids homologous to the revised GRA3 but not to the first reported one. The polypeptide has 3 hydrophobic moieties of an N-terminal stop transfer sequence and 2 transmembrane domains (TMD) in posterior half of the sequence, a cytoplasmic localization motif after the second TMD and an endoplasmic reticulum (ER) retrival motif in the C-terminal end, which suggests GRA3 as a type III transmembrane protein. With the ORF of GRA3, yeast two-hybrid assay was performed in HeLa cDNA expression library, which resulted in the interaction of GRA3 with calcium modulating ligand (CAMLG), a type II transmembrane protein of ER. The specific binding of GRA3 and CAMLG was confirmed by glutathione S-transferase (GST) pull-down and immunoprecipitation assays. The localities of fluorescence transfectionally expressed from GRA3 and CAMLG plasmids were overlapped completely in HeLa cell cytoplasm. In immunofluorescence assay, GRA3 and CAMLG were shown to be co-localized in the PVM of host cells. Structural binding of PVM-inserted GRA3 to CAMLG of ER suggested the receptor-ligand of ER recruitment to PVM during the parasitism of T. gondii.
-
Key words: Toxoplasma gondii, GRA3, cDNA sequence, yeast two-hybrid, CAMLG, PVM-ER interaction
INTRODUCTION
The protozoan parasite
Toxoplasma gondii infects humans and other warm-blooded animals, causing clinical symptoms of toxoplasmosis in immunocompromised patients and sometimes even in immunocompetent individuals [
1].
T. gondii is an obligate intracellular parasite that can penetrate actively into any type of nucleated cells. After entry of
T. gondii parasitophorous vacuole (PV) is formed within host cells bordered by a membrane (PVM), in which the parasites multiply and grow. In the PV and PVM many proteins are secreted from secretory organelles of
T. gondii such as micronemes, rhoptries and dense granules, which interact with cytosolic components and subcellular organelles of the host cells as evidenced morphologically by the attachment of host mitochondria and endoplasmic reticulum (ER) to the PVM [
2].
PVM has been formed as early as
T. gondii penetrates into host cells with an active process distinctly different from phagocytosis [
3], which offers primarily the parasite to reside in a compartment that excludes delivery of protein and lipid components from the host cell endocytic and exocytic pathways [
4]. As the structural interface between parasite and host cell cytoplasm, PVM functions in metabolite uptake, nutrient transport, signaling interference and protein trafficking as reviewed previously [
5]. Until now, the origin of PVM in host cells infected with
T. gondii and other apicomplexan parasites has been unclear but explained by 2 controversial hypotheses of the bilayer insertion model and the induced envagination model. In the former, PVM is formed of the lipids secreted from apical organelles of the parasite and inserted into host cell membrane [
6,
7], and in the latter, invading parasite induces the host cell membrane to envaginate to form the PVM [
8]. PVM has been expanding as the parasite inside grows and duplicates and decorated by secreted transmembrane proteins from rhoptries and dense granules of
T. gondii, which attracts host cell mitochondria and ER outside the PVM. For the expanding PVM, lipids of attached organelles are scavenged such as lipoic acid via the PVM-mitochondria association [
9], phospholipids and sterols through the PVM-ER association [
10], cholesterol from the LDL-pathways and lipid bodies of host lipid storage bodies [
11,
12]. In the PVM-mitochondria association, ROP2 of
T. gondii secreted from rhoptries and inserted into the PVM mediates the association through the N-terminal domain of the protein exposed to the cytosol as a mitochondrial targeting signal [
13]. The PVM-ER association has been always mentioned with the PVM-mitochondria association, but there is no interacting mechanism between PVM and ER until now.
A monoclonal antibody Tg556 clone (Tg556) detected a dense granular 29 kDa protein [
14]. During the screening of a
T. gondii cDNA expression library, Tg556 had been found to bind to GRA3 of
T. gondii of which the sequence was homologous to that revised by a previous author [
15] but not to the first reported one [
16]. In the yeast two-hybrid assay with GRA3 as a bait to the prey of HeLa cDNA expression library, GRA3 interacted with calcium modulating ligand (CAMLG), an ER-anchoring protein [
17]. We describe here the specific binding between GRA3 and CAMLG biochemically, the localization of both proteins in transfectionally expressed cells and the immunofluorescence of both proteins in
T. gondii infected cells, which enable us to suggest the role of these proteins as linking molecules of the PVM-ER interactions.
MATERIALS AND METHODS
Parasite and host cells
The RH strain of
T. gondii was maintained by peritoneal passage in BALB/c mice under a specific pathogen-free (SPF) condition. Prior to use, the tachyzoites were purified by centrifugation over 40% Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) in phosphate-buffered saline (PBS) solution [
18]. HeLa (ATCC CCL-2) cells were cultured in MEM supplemented with 10% FBS and used as host cells.
These procedures were performed according to previously described methods [
19]. A
T. gondii λZAPII cDNA expression library was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH and screened in
E. coli XL1-Blue MRF'(Stratagene, La Jolla, California, USA) using Tg556 by detection with the ECL Detection System (Amersham Pharmacia Biotech). Positive pBluescript SK phagemids were isolated by co-infection of the λZAPII phage and ExAssist helper phage (Stratagene) to excise and propagate in the
E. coli SOLR host strain, and the DNA was purified using the Wizard Plus SV Miniprep kit (Promega, Madison, Wisconsin, USA).
The total
T. gondii tachyzoite RNA was extracted using Tri reagent (Sigma Chem Co., St. Louis, Missouri, USA), and the 5' untranslated region was amplified using the 5'-RACE procedure [
20]. First strand cDNA was synthesized from 1 µg of total RNA using the Superscript Preamplification System (Life Technologies, Gaithersburg, Maryland, USA) to add deoxyCTPs to the 3' end of the non-coding cDNA using terminal deoxynucleotide transferase. PCR amplification of C-tailed cDNA was performed with an anchor primer (5'-CTA ATA CGA CTC ACT ATA GGG CAA GCA GTG GTA TCA ACG CAG AGT-3'; Bionia Co., Daejeon, Korea) and a gene-specific primer (5'-CGC CTG ATT GTC CAC CTT ATC CGA-3'). The first-round product was further amplified with the abridged universal anchor primer (5'-AAG CAG TGG TAT CAA CGC AGA GT-3') and an internal gene-specific primer (5'-GCG GTA GCT TCT TGG ACA CCC GAA-3'). The second-round PCR product was cloned into the pGEM-T EASY vector (Promega) to sequence the inserted DNA using primers of T7 and T3 vector promoter sequences.
Sequences of cDNA clones were used to search for homologous sequences in the Toxoplasma dbEST (Database of Expressed Sequence Tags) using the BLASTn algorithm with default settings. The protein sequences were compared to the GenBank database using BLASTp. PROSITE and SignalP were used to search for signal sequence, motifs and post-translational modification sites. Hydrophobicity of the amino acid sequence was checked by ProtScale and DAS in ExPASy.
Glutathione S-transferase (GST) pull-down assay and immunoprecipitation
cDNA of human CAMLG (GeneBank No. NM001745) was amplified with primers of CAMLG up (5'-CGG ATC CAT GGA GTC GAT GGC C-3') and CAMLG low (5'-CGA ATT CAC TAA TCA GCT GTT TCC TTA GC-3'), and inserted into pGEX4T-1 (Amersham Pharmacia Biotech) with BamHI/EcoRI restriction.
GST pull-down assay was performed according to the method described previously [
21]. Two µg of GST or GST-fused CAMLG were mixed with 40 µl of 50% suspension of glutathione-Sepharose 4B beads for 2 hr in a binding buffer (25 mM HEPES-NaOH, pH 7.5, 12.5 mM MgCl
2, 10% glycerol, 5 mM DTT, 0.1% NP-40, 150 mM KCl and 20 µM ZnCl
2). Herein Histagged GRA3 lysate was added and followed by incubation for another 2 hr. The pellets were washed extensively and boiled. The bound proteins were resolved in 12% SDS-PAGE and blotted by Tg556.
Immunoprecipitation was followed the method described previously [
22]. RH tachyzoites of
T. gondii were lysed with RIPA buffer containing protease/phosphatase inhibitors. RH lysates were then sonicated, insoluble materials were removed and the supernatant was transferred to new tubes. Immunoprecipitation was performed by rocking for 4 hr at 4℃ the precleared extracts with His-tagged CAMLG and Tg556, and then by precipitating the immunocomplexes with protein A agarose. The immunoprecipitates were washed with 1 ml RIPA buffer and with 1 ml TBS. The beads were then suspended in SDS sample buffer containing 0.125 M DTT and boiled for 5 min. The samples were analyzed by 10% SDS-PAGE followed by immunoblotting using anti-His antibody.
GRA3 cDNA was amplified with 5'-GCG GCA AGC TTG CCT GAA AAT CAT CA-3' and 5'-AAT GG ATC CGT TTG TTT CTT GGA GGC-3') to insert into pDSRed2-N1 (BD Biosciences Clontech, Mountain View, California, USA) by EcoRI/KpnI digestion for GRA3-Red fusion protein. CAMLG cDNA was amplified with 5'-AAA GCT TGA TGG AGT CGA TGG CC-3' and 5'-CGG ATC CCT TCG TGG TAC TTC AGA-3' to insert into pEGFP-N1 by HindIII/BamHI digestion for CAMLG-GFP fusion protein.
Transient transfection of both pDSred2-GRA3 and pEGFP-CAMLG plasmids into HeLa cells was achieved using the calcium phosphate co-precipitation method [
23]. Plasmid DNA (1-2 µg) was diluted in 42 µl of H
2O, mixed with 7 µl of 2 M CaCl
2 and added by drops to 50 µl of 2 x HeBS (280 mM NaCl, 1.5 mM Na
2HPO
4 and 50 mM HEPES, pH 7.05). After incubation for 20 min at room temperature, the mixture was added to the cells. The cells were incubated further for 24 hr and fixed with cold absolute methanol for 5 min. The fluorescence was observed under a fluorescence microscopy (Axiophot, Carl Zeiss, Oberkochen, Germany).
HeLa cells cultured on 18 mm coverslips in 24-well plates were infected with tachyzoites. Cells were fixed either with cold absolute methanol for 5 min or with 3% formaldehyde for 10 min and then permeabilized by 0.05% Triton X-100 for 5 min, separately. Cells were incubated with mouse Tg556 and rabbit polyclonal anti-CAMLG antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) diluted in 1 : 200 of 3% BSA/PBS for 1 hr, and then incubated with TRITC-conjugated goat anti-mouse IgG antibody and FITC-conjugated goat anti-rabbit IgG antibody (Sigma Chemical Co.) diluted in 1 : 500. Fluorescence was observed under a fluorescence microscopy.
RESULTS
Molecular characterization of GRA3 recognized by Tg556
Tg556 was used to screen a
T. gondii λZAPII cDNA expression library, which resulted in the isolation of a cDNA clone from the pBluescript SK phagemid. The nucleotide sequence of the clone contained a poly (A+) tail. The complete 5' untranslated sequence, determined by 5'-RACE, yielded a cDNA which spanned a total of 2,086 bp and included a predicted open reading frame (ORF) of 669 bp. A search of the
Toxoplasma database of expressed sequence tags identified a contig assembly containing 125 expressed sequence tags with a high BLAST score. A full cDNA sequence included a 945 bp cDNA registered on GenBank (accession number AF414079) as a 29 kDa GRA3 of
T. gondii, of which the ORFs were perfectly matched to encode a polypeptide of 222 amino acids using the first in-frame ATG as the starting site downstream of the consensus sequence of protozoal/toxoplasmal translation initiation (
Fig. 1).
There was no hydrophobic region just after the first ATG which would constitute a potential signal sequence, but after 20 amino acids downstream of the ATG 23 hydrophobic amino acids appeared as a potential stop transfer sequence (STS). A PROSITE search yielded several obvious sequence motifs or post-translational modification sites such as cytoplasmic localization motif (CM) of Yxxφ and ER retrival motif (ERM) of KKxx. In the middle part of the sequence, 2 transmembrane domains (TMD) of approximately 17 amino acids were searched within the GRA3 sequence.
Interaction between GRA3 and CAMLG
CAMLG was a prey protein to GRA3 in the yeast two-hybrid technique in the previous report [
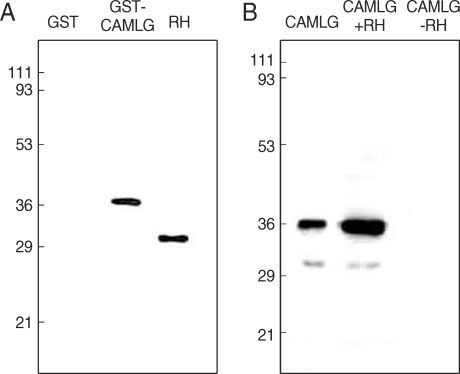
17]. Specific interaction between CAMLG and GRA3 was confirmed by GST pull-down assay and immunoprecipitation assay (
Fig. 2). In the GST pull-down assay (
Fig. 2A), GRA3 was bound to glutathione-Sepharose beads to be blotted by Tg556 under the presence of GST-CAMLG fusion protein, while not bound to the beads in the GST only fraction without CAMLG. The size of GRA3 expressed and purified from bacteria was bigger than that blotted in RH tachyzoites. By the immunoprecipitation assay (
Fig. 2B), Histagged CAMLG was precipitated with Tg556 to Protein A beads under the presence of RH lysate which contained GRA3, but not precipitated in the fraction without RH lysate.
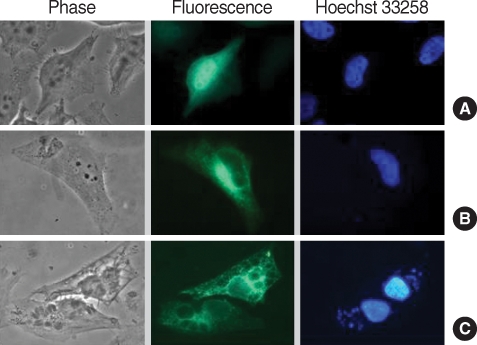
Transfectionally expressed fluorescent CAMLG was localized in the cytoplasm near the nucleus in HeLa cells (
Fig. 3B). After
T. gondii infection, fluorescence of CAMLG was dispersed around the PVM (
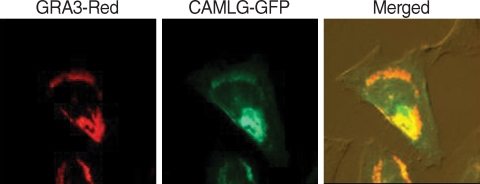
Fig. 3C). GRA3 and CAMLG were co-localized in the perinuclear cytoplasm after co-transfection (
Fig. 4). Red fluorescence from GRA3-Red overlapped perfectly with green fluorescence of CAMLG-GFP as revealed by merged image.
Interaction between GRA3 and CAMLG was further confirmed in HeLa cells infected with
T. gondii especially focused on the localization of both proteins in the PVM (
Fig. 5). In the methanol-fixed HeLa cells in which the PVM was preserved (
Fig. 5A), red fluorescence of GRA3 localized in the PVM in addition to the growing
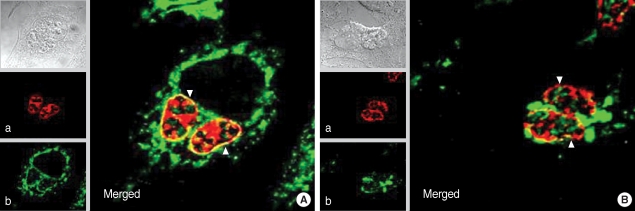
T. gondii inside the PVM by Tg556 and TRITC-conjugated anti-mouse IgG antibody (a) and green fluorescence of CAMLG was dispersed in the cytoplasm of the host cells and PVM by anti-CAMLG antibody and FITC-conjugated anti-rabbit IgG antibody (b). Accordingly in the merged image, yellow fluorescence appeared only in the PVM which reflected the co-localization of red GRA3 and green CAMLG (Merged). In the paraformaldehyde-fixed and then permeabilized with Triton X-100 in which the PVM and other cytoplasmic membraneous structures were defeated (
Fig. 5B), red GRA3 fluorescence was detected within the growing
T. gondii (a) and green CAMLG fluorescence was not dispersed over the cytoplasm but aggregated in the cytoplasm near PVM (b). Therefore, the yellow fluorescence was diappeared near the PVM with smudges near the aggregate of CAMLG in the merged image (Merged).
DISCUSSION
Tg556 recognized a 29 kDa dense granular protein of T. gondii tachyzoite that was secreted into the PVM of infected host cells. By the screening of an expression library of T. gondii and 5'-RACE, a full length cDNA of 2,086 bp was obtained and searched to include the homologous cDNA of GRA3 recently revised by others. ORF of 669 bp was used in yeast two-hybrid assay to define the interacting proteins of host cells when GRA3 was secreted to insert into PVM during the growth of T. gondii within host cells, which resulted in the interaction with CAMLG, an ER-anchoring protein. Specific interaction between GRA3 and CAMLG was further confirmed by GST pull-down assay, immunoprecipitation assay and co-transfection. Morphologically GRA3 and CAMLG co-localized in the PVM formed in T. gondii infected cells by the immunofluorescence microscopy, which suggested the interacting molecules of PVM-ER interaction such as ROP2 as a key molecule in PVM-mitochondia interaction.
In the full length cDNA sequence, the first ATG was followed by GTAACA which is similar to the
T. gondii consensus translation initiation sequence of gNcAAA in addition to a G at position +4 [
24]. A second ATG 39 bp downstream of the first one was followed by TTCACA and a T at position +4. Two ways of translation were possible on the same in-frame of the sequence as noted by previous authors [
15,
16]. The first ATG was selected as the starting of translation by applying the consensus rule strictly in this study, which provided an ORF of 669 bp. In the deduced amino acid sequence, GRA3 had 3 hydrophobic moieties of N-terminal STS instead of signal sequence (SS) and 2 TMD in the middle of the posterior half of the peptide, CM downstream of the second TMD and ERM in the C-terminal end (
Fig. 1). SignalP predicted a potential SS of approximately 43 amino acids of the N-terminal, but it was comparable to the generally accepted average lenth of SS of 22 for eukaryotes and 24 to 32 for prokaryotes [
25]. Actually, 20 non-hydrophobic amino acids preceded hydrophobic 23 amino acids in the predicted poteintial SS of 43 amino acid sequence, only the latter hydrophobic moiety seemed to be deserved as the STS rather than total sequence as SS [
26,
27]. This explanation could be applied to GRA6 of which the N-terminal sequence was similar to that of GRA3 [
28,
29]. Hydrophobicity analysis predicted 2 putative TMDs in the middle of GRA3, which is different from the previous report [
15]. GRA7 and GRA10 also have 2 TMDs in their polypeptide, in which the cell attachment sequence of RGD motif is present in the middle of TMDs [
19,
30], whereas no significant motifs in GRA3. Downstream of the second TMD, a CM of Yxxφ appeared to sort the remaining C-terminal sequence to the cytoplasmic face of membrane [
31,
32]. In the C-terminal end, an ERM of KKxx [
15,
33] appeared to function of GRA3 in PVM-organelle association. With the consideration of STS, 2 TMDs and CM, it is suggested that GRA3 belongs to a type III transmembrane protein rather than type I.
Among 10 GRA proteins, GRA3, GRA5 and GRA10 are preferentially detected as PVM-associated proteins. Interactions of PVM-associated GRA proteins with host cell proteins and organelles will provide a way to elucidate the function of the GRA protein in the parasitism of
T. gondii. Host cell proteins are profiled that bind to GRA3 by yeast two-hybrid method. CAMLG of host cells interacted with GRA3 in the yeast two-hybrid technique, of which the specific binding was proved by GST pulldown assay and immunoprecipitation assay (
Fig. 2). CAMLG is an integral membrane protein of ER, contains 296 amino acids with N-terminal region projecting into the cytoplasm and with 3 predicted TMDs in C-terminal region as a typical type II transmembrane protein, which appears to be a new participant in the calcium signal transduction pathway [
34,
35]. Fluorescent CAMLG was localized in the perinucler region of cytoplasm which was thought to be on the ER and then dispersed to surround the PVM when the cells were infected with
T. gondii (
Fig. 3), which verified the recruitment of ER to the PVM. GRA3 and CAMLG were co-localized in the cytoplasm after co-transfection (
Fig. 4), which confimed the interaction of both proteins. Interaction of GRA3 and CAMLG on the PVM was further confirmed by the immunofluorescence assay in
T. gondii infected cells (
Fig. 5). The conventional methods to preserve or permeabilize PVM were applied to clarify the interaction of GRA3 and CAMLG on the PVM. Yellow fluorescence, resulted from mixing of red and green fluorescences, appeared in the PVM only when the PVM was preserved. Structural binding between GRA3 of PVM and CAMLG of ER suggests that the linking role in the PVM-ER association during the parasitism of
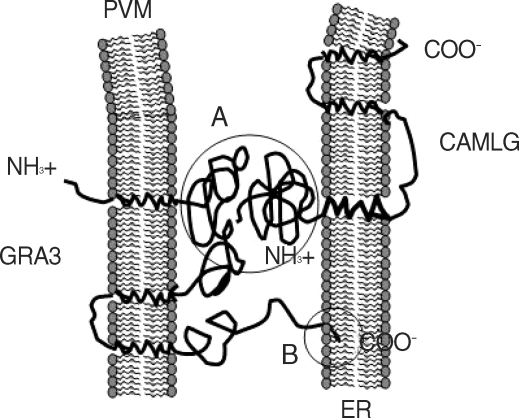
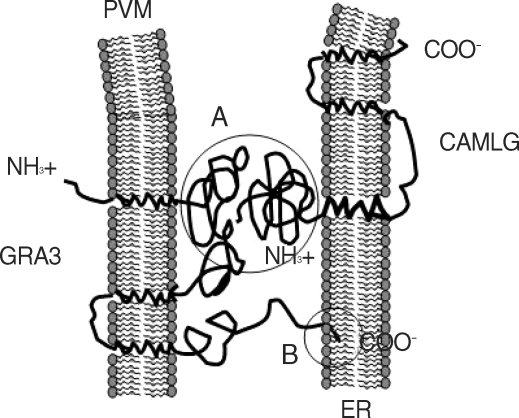
T. gondii may occur through the interaction of the N-terminal moiety to the cytoplasmic face of the PVM as type III transmembrane protein of GRA3 and the N-terminal moiety of the cytoplasmic face of the ER-anchoring CAMLG in addition to the direct insertion of ER retrival motif of GRA3 (
Fig. 6). This protein-protein interacting mode is different from the previous suggestions that PVM-ER association occurs via direct insertion of ER ritrieval motif in C-terminal end of PVM-inserted GRA3 into the approaching ER [
15], and that the PVM-mitochndira association occurs through the direct targeting of N-terminal domain of PVM-inserted ROP2 to the nearby mitochondria [
13].
Both PVM-organelle associations have been related to the anti-apoptosis of the infected cells functionally through the different processes to provide longer time for the growing T. gondii. Further analysis of the GRA3-CAMLG interation related with modulation of intracellular [Ca++] will ultimately lead to a better understanding the role of PVM-ER association in addition to PVM-mitochondria association in the survival of T. gondii within host cells.
ACKNOWLEDGEMENTS
This study was supported by the Academic Research Service Program of the Korean Center for Disease Control and Prevention, 2007 (No. 4847-302-260-00).
References
Fig. 1Characteristics of deduced amino acid sequence and Clustal W sequence alignment of GRA3 proteins. Tg556: deduced amino acid sequence obtained from cDNA by mAb Tg556 screening in this study; GRA3r: revised GRA3 sequence from cDNA of AF414079; and GRA3: first reported sequence from cDNA of U13771. Highly conserved residues are presented as bold and non-homologous GRA3 C-terminal after 149 aa as italic.

Fig. 2Interaction of GRA3 with CAMLG by GST pull-down assay and immunorecipitation assay. Nitrocellulose sheet was blotted, (A) with monoclonal anti-GRA3 antibody (Tg556) in the GST pull-down assay and (B) with anti-His antibody after immunoprecipitation by Tg556 in the immunoprecipitation assay. Numerals on the left side indicated the molecular weights in kDa.
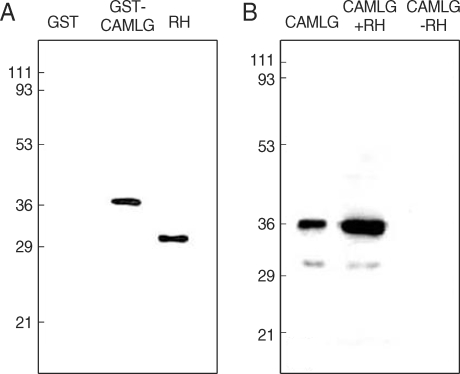
Fig. 3Localization of GFP-CAMLG fusion protein expressed in HeLa cells infected with T. gondii. (A) control of pEGFP-N1 plasmid only; (B) expression of pEGFP-CAMLG plasmid near nucleus; and (C) expression of pEGFP-CAMLG plasmid near nucleus spreads to the PVM after T. gondii infection. In each set green color indicated the fluorescence of GFP chimera and blue color the fluorescence of Hoechst 33258 from nucleus of the cell or T. gondii.
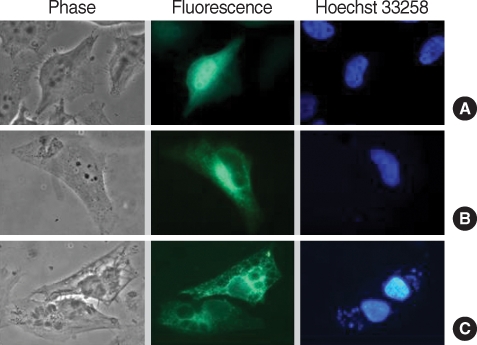
Fig. 4Localization of transfectionally expressed GRA3 and CAMLG in HeLa cells. Fluorescence of GRA3-RED and CAMLG-GFP were overlapped perfectly in the Merged image.

Fig. 5Immunofluorescence localization of GRA3 and CAMLG in HeLa cells infected with T. gondii. (A) cells fixed with methanol to preserve the PVM 16 hr after infection and (B) fixed with paraformaldehyde then permeabilized with Triton X-100 to defeat the PVM. a: fluorescence of GRA3 by Tg556 and TRITC-conjugated anti-mouse IgG antibody, b: fluorescence of CAMLG by FITC-conjugated anti-rabbit IgG antibody, and Merged: merged image of a and b, arrow head indicates the PVM.
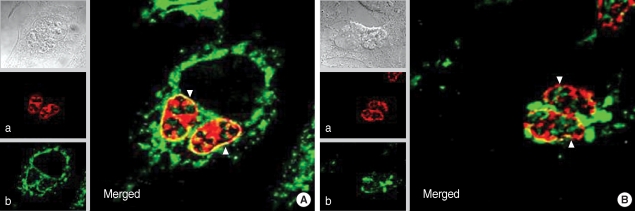
Fig. 6Schematic orientation of GRA3 in PVM and CAMLG in ER and proposed interaction of them to PVM-ER association. (A) interaction between N-terminal parts of GRA3 and CAMLG, both are faced to the cytoplasm, and (B) interaction of ER retrival motif of C-terminal end of GRA3 and ER membrane.