Abstract
Free-living Naegleria fowleri leads to a fatal infection known as primary amebic meningoencephalitis in humans. Previously, the target cell death could be induced by phagocytic activity of N. fowleri as a contact-dependent mechanism. However, in this study we investigated the target cell death under a non-contact system using a tissue-culture insert. The human microglial cells, U87MG cells, co-cultured with N. fowleri trophozoites for 30 min in a non-contact system showed morphological changes such as the cell membrane destruction and a reduction in the number. By fluorescence-activated cell sorter (FACS) analysis, U87MG cells co-cultured with N. fowleri trophozoites in a non-contact system showed a significant increasse of apoptotic cells (16%) in comparison with that of the control or N. fowleri lysate. When U87MG cells were co-cultured with N. fowleri trophozoites in a non-contact system for 30 min, 2 hr, and 4 hr, the cytotoxicity of amebae against target cells was 40.5, 44.2, and 45.6%, respectively. By contrast, the cytotoxicity of non-pathogenic N. gruberi trophozoites was 10.2, 12.4, and 13.2%, respectively. These results suggest that the molecules released from N. fowleri in a contact-independent manner as well as phagocytosis in a contact-dependent manner may induce the host cell death.
-
Key words: Naegleria fowleri, cell death, cytotoxicity, microglial cells
INTRODUCTION
Naegleria fowleri is a free-living amebae found in widespread areas such as moist soil, sewage water, and sediment, and exists as a virulent pathogen causing fatal primary amebic meningoencephalitis (PAME) in experimental animals and humans [
1,
2]. Trophozoites are invasive, able to enter the nervous system through the olfactory nerve, and digest neuronal tissues by unusually effective cytolysis and phagocytosis as observed in culture or in sections of infected brain tissues [
3,
4]. PAME is a fulminant infection that typically leads to death with 1 to 2 wk from the onset of symptoms [
5,
6].
The adhesion of amebae to the host cells is clearly a critical first step in the pathogenesis of infection. According to the same-free living amebae,
Acanthamebae, subsequent to adhesion, the parasite produces a potent cytopathic effect leading to target host cell death [
7,
8]. In addition, Han et al. [
9] reported that an integrin-like protein and protein kinase C in
N. fowleri adhesion to fibronectin and amebic cytotoxicity was involved. Although the cytopathogenic effects of amebae against host cells require adhesion of amebae to host cells and that adhesion is the crucial step for the pathogenicity of amebae, it has been proposed that secretory-excretory protein released from amebae show the cytotoxic effect on target cells [
7,
10]. We suggested that pathogenicity would be a complex process which involves both the contact-dependent and contact-independent pathways in order to kill host cells.
Until now, the factors that determine the pathogenicity of
N. fowleri have not been fully established. In case of a contact-dependent mechanism, we reported that a gene (called
nfa1) was cloned from a cDNA library of
N. fowleri by immunoscreening methods with infected and immune sera [
11]. The Nfa1 protein was located with pseudopodia and involved in the formation of food cup structure (amoebastome), which plays an important role in phagocytic activity as a contact-dependent mechanism in pathogenic
N. fowleri [
12]. In another study, the treatment of an anti-Nfa1 polyclonal antibody showed the decreasing cytotoxic effect of
N. fowleri trophozoites against target cells in a dose-dependent manner [
13,
14]. In addition, the heat shock protein 70 (HSP70) cloned from
N. fowleri showed the localization on pseudopodia, and should be an important survival strategy in high temperature tolerance, ameba proliferation, and pathogenicity of
N. fowleri [
15]. Based on these observations, Nfa1 protein and HSP70 might be related with a contact-dependent mechanism for pathogenicity of
N. fowleri.
In the present study, to investigate a contact-independent mechanism for pathogenicity of N. fowleri, human microglial cells were co-cultured indirectly with N. fowleri trophozoites under a non-contact system, and the morphological change and cell death were observed by a light microscope and FACS analysis, In addition, the in vitro cytotoxicity of N. fowleri against human microglial cells was measured.
MATERIALS AND METHODS
Culture of N. fowleri, N. gruberi, and U87MG cells
N. fowleri trophozoites (ATCC No. 30215) were cultured under axenic conditions in Nelson's medium at 37℃ [
16]. Before using
N. fowleri trophozoites, their pathogenicity and cytotoxicity were tested in mice or target cells, respectively [
13].
N. fowleri lysate was prepared according to the method of a previous paper [
12]. Non-pathogenic
N. gruberi (ATCC No. 30960) was cultured at 27℃ in modified PYNFH medium [
17]. U87MG cells were grown in a monolayer in 75-cm
2 flasks containing 12 ml of Dulbecco's modified Eagle's medium (DMEM) (Sigma, St. Louis, Missouri, USA) with 10% fetal bovine serum in a fully-humidified incubator containing 5% CO
2 at 37℃.
Preparation of a non-contact culture system and light microscopic observations
The U87MG cells were seeded on 6-well tissue culture plate (Nunc, Roskilde, Denmark). For non-contact culture system, a tissue-culture insert (Nunc) with a membrane pore size of 0.2 µm was placed in a 6-well tissue culture plate containing 2 ml of DMEM without fetal bovine serum. Then, N. fowleri trophozoites were put onto a tissue-culture insert and co-cultured with U87MG cells for 30 min, 2 hr, and 4 hr in 5% CO2 incubator at 37℃. For a control group, 3 × 105/well of U87MG cells or N. fowleri trophozoites were seeded in a 6-well tissue culture plate. For other control experiments, N. fowleri trophozoites were cultured with Nelson's medium, and U87MG cells were cultured with DMEM. After 30 min, 2 hr, and 4 hr, the conditioned media were collected and used for LDH release assay, and U87MG cells for FACS analysis. The cultures were assessed under an inverted microscope (Nikon, Tokyo, Japan) and photographed for documentation during culture.
Flow cytometric assay
Flow cytometry experiments were performed to identify early apoptotic cells and to discriminate late apoptotic/necrotic and living cells. Apoptotic cells were detected by flow cytometry using a FACS Vantage (Becton Dickinson, Franklin Lakes, New Jersey, USA), after staining with fluorescein isothiocyanate-conjugated annexin V and propidium iodide (PI) using the Annexin V-FITC kit™ (Trevigen Inc., Gaithersbug, Maryland, USA). The U87MG cells and N. fowleri trophozoites were incubated at density of 3 × 105 in a 6-well tissue culture plate for 30 min, 2 hr, and 4 hr. The U87MG cells were harvested by washing with PBS followed by treatment with trypsin-EDTA solution (Sigma). The cell pellet was resuspended in the binding buffer at a concentration of 1 × 105 cells/ml. After adding annexin V fluorescein isothiocyanate and PI and incubating for 15 min at room temperature in the dark, the cells were run on a FACS Vantage. In this study, we used tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or N. fowleri lyaste for the control. TRAIL is known as the death ligand, which induces preferential apoptosis of transformed tumor cells, and N. fowleri lysate shows the cytotoxic effect on target cells. For each experiment, 1 × 104 cells were analyzed using the CellQuest software (Beckton Dickinson). In FACS analysis, the lower right quadrant represents early apoptotic cells positive for annexin V and negative for PI, and the upper right quadrant represents apoptotic or necrotic cells positive for both annexin V and PI. The lower left quadrant represents healthy cells negative for annexin V and PI staining as a control.
LDH release assay
To evaluate the cytotoxicity of
N. fowleri on target cells under a non-contact culture system, lactate dehydrogenase (LDH) release assay was performed according to the previous study [
13]. Briefly, 3 × 10
5 of
N. fowleri trophozoites as effector cells and 3 × 10
5 of U87MG cells as target cells were co-cultured in a non-contact condition which was previously described above. The supernatant containing LDH released from target cells was collected at 30 min, 2 hr, and 4 hr. As control groups, U87MG cells were directly treated with
N. fowleri lysate or
N. gruberi lysate (1 mg/ml) for 4 hr. For LDH release assay, 50 µl of reacted supernatant in each well was transferred onto 96-well assay plates (Nunc). After 50 µl of the reconstituted assay buffer in CytoTox96® Non-radioactive Cytotoxicity Assay Kit (Promega, Madison, Wisconsin, USA) was added, the plate was incubated for 30 min at room temperature, and then 50 µl of stop solution was added. The reactions were read at 490 nm with ELISA reader. Values were means ± standard errors of 3 experiments in triplicate. The formula of in vitro cytotoxicity was as follows: Cytotoxicity (%)=Sample release-Spontaneous release/Maximum release-Spontaneous release×100
Statistical differences between groups or samples were determined with Student's 2-sample t-test. The difference was considered significant when P was < 0.05.
RESULTS
Cytopathic changes of U87MG cells
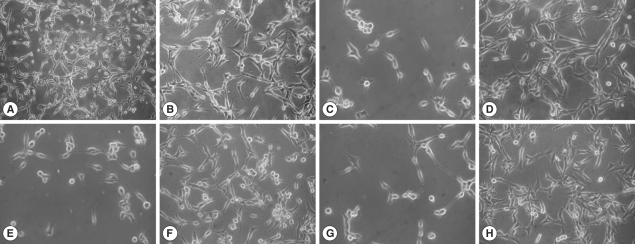
The U87MG cells co-cultured with
N. fowleri trophozoites at 30 min, 2 hr, and 4 hr in a non-contact system showed serious morphologic changes such as the cell membrane destruction and a reduction in cell number during the culture periods (
Fig. 1). However, U87MG cells treated with 1 mg/ml of
N. fowleri lysate revealed only a little morphologic change similar to an untreated control group (
Fig. 1).
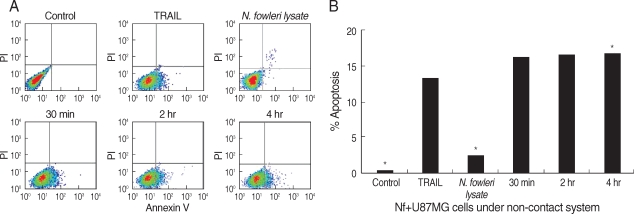
To detect the externalization of phosphatidylserine in early phase of apoptosis, we performed annexin V-FITC binding assay.
N. fowleri trophozoites induced the apoptosis of U87MG cells in a non-contact culture system, as which showed the distinguishment between viable, apoptotic, or necrotic cells by staining of annexin V in combination with PI (
Fig. 2). In FACS dot plots, apoptotic cells shifted the lower right quadrants, annexin V-positive and PI-negative staining populations. The U87MG cells co-cultured with
N. fowleri trophozoites in a non-contact system significantly increased the percentage of apoptotic cells in comparison with the control or
N. fowleri lysate-treated group (
P < 0.01). When U87MG cells were co-cultured with
N. fowleri for 30 min, 2 hr, and 4 hr in a non-contact system, the populations of annexin V-positive and PI-negative cells were 16.1, 16.6, and 16.7%, respectively (
Fig. 2). The U87MG cells treated with
N. fowleri lysate for 4 hr showed the decreasing populations of the necrotic and apoptotic cells (2.5 and 2.7%, respectively). Otherwise, when U87MG cells were treated with 100 ng/ml of TRAIL (a positive control) for 4 hr, the population of annexin V-positive and PI-negative cells was 13.3% (
Fig. 2).
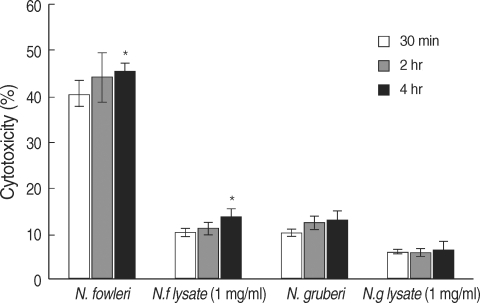
To further confirm the in vitro cytotoxicity of
N. fowleri trophozoites, we performed LDH release assay. When U87MG cells were co-cultured with
N. fowleri trophozoites for 30 min, 2 hr, and 4 hr in a non-contact system,
N. fowleri trophozoites showed 40.5, 44.2, and 45.6% cytotoxicity in a time-dependent manner, respectively (
Fig. 3). When the U87MG cells were treated with 1 mg/ml of
N. fowleri lysate, the cytotoxicity of amebae on target cells ranged from 10.2 to 13.6% (
Fig. 3). In other words,
N. fowleri trophozoites co-cultured with target U87MG cells for 4 hr in a non-contact system showed maximum 45.6% cytotoxicity, whereas
N. fowleri lysate showed maximum 13.6% cytotoxicity (
P < 0.01). Under the same conditions, non-pathogenic
N. gruberi trophozoites showed 10.2, 12.4, and 13.2% of cytotoxicity, whereas the cytotoxicity of
N. gruberi lysate ranged from 6.1 to 6.4% (
Fig. 3).
DISCUSSION
It is known that the adhesion to target cells plays an important role in cytopathogenicity of
N. fowleri. In case of
Entamebae histolytica, it was reported that the contact with host cells is needed to cause apoptosis [
18]. But, recently, it was reported that released or secreted molecules as well as adhesion to host cells induce the cell death of target cells [
19].
N. fowleri trophozoites release cytolytic molecules, phospholipolytic enzymes and acid phosphatase which are required for the invasiveness in vivo as well as the cytopathogenicity in vitro [
20]. In another free-living amebae,
Acanthamoeba spp., cytopathogenic effects on host cells require the adhesion to host cells in order to phagocyte the target cells [
21]. In addition, there are many molecules related to killing of target cells, such as proteolytic enzymes, including serine proteases, metalloproteases, elastases and cysteine proteases [
22,
23].
In the present study, we demonstrated that some molecules released from
N. fowleri trophozoites induce apoptosis of human microglial cells (U87MG cells). This apoptosis occurred without the contact of amebae to target cells. This result suggests that
N. fowleri trophozoites produce certain virulent molecules that can induce the early apoptotic pathway in U87MG cells. The in vitro cytotoxicity of
N. fowleri trophozoites against target cells was observed previously and most of them were related to a contact-dependent mechanism including food cup formation [
12]. Until now, the contact-independent mechanism of cell death due to amebae was not noted clearly. These results suggest that
N. fowleri trophozoites have the potential virulent factors to cause cytotoxic effects without adhering to host cells. Our study show for the first time that
N. fowleri could cause apoptosis of human microglial cells in a contact-independent mechanism.
FACS analysis in the present study revealed that the target cells co-cultured with N. fowleri trophozoites in a non-contact system showed significantly increasing percentage of apoptotic cells in comparison with the target cells treated with N. fowleri lysate. Thus, we observed that molecules released from N. fowleri stimulated with some excretory-secretory products of target cells could induce apoptosis, and the apoptosis was increased more than that of N. fowleri lysate. Particularly, the apoptosis was induced within early time, 30 min. In addition, in case of non-pathogenic N. gruberi, the target cells in a non-contact culture system showed lower level of cytotoxicity or apoptotic cell death in comparison with that of N. fowleri. Therefore, it is suggested that molecules released or secreted from amebae may be related with the amebic pathogenicity.
In summary, our findings demonstrate for the first time that N. fowleri trophozoites in a non-contact culture system induce the apoptosis of target cells. We suggest that pathogenicity of N. fowleri would be complex processes which involve contact-independent pathway as well as contact-dependent pathway to kill the target cells. Further studies are needed to identify the virulent factors released from N. fowleri, and to investigate the role of virulent factors in N. fowleri infection.
ACKNOWLEDGEMENTS
This study was supported by a grant from National Institute of Health, Korean Center for Disease Control and Prevention (2008-E0091-00).
References
Fig. 1Light microscopic images. These images show the cytopathic effects of N. fowleri trophozoites on U87MG cells in a contact or a non-contact culture system. N. fowleri lysate treated directly at concentration of 1 mg/ml for 4 hr. The control was cultured with U87MG cells only at the same condition. (A, B) control (×100, ×200), (C, E, and G) U87MG cells co-cultured with N. fowleri trophozoite in a non-contact system for 30 min, 2 hr, and 4 hr, respectively (×200), (D, F, H) The U87MG cells treated with N. fowleri lysate for 30 min, 2 hr, and 4 hr, respectively (×200).

Fig. 2FACS analysis for apoptosis of U87MG cells. (A) The U87MG cells were co-cultured with N. fowleri trophozoites for 30 min, 2 hr, and 4 hr. The U87MG cells (1 mg/ml) were directly treated with N. fowleri lysate or TRAIL (100 ng/ml) for 4 hr. Untreated U87MG cells and TRAIL was used as a control and positive control for apoptosis, respectively. (B) Quantitative analysis calculated from FACS data. The values were significantly different from the value for the negative control for all samples.

Fig. 3In vitro cytotoxicity of N. fowleri against U87MG cells in a non-contact system by LDH release assay. The N. fowleri trophozoites showed higher cytotoxicity than those of N. gruberi trophozoites, N. fowleri lysate and N. gruberi lysate used as the control group.