Abstract
The nfa1 gene was cloned from a cDNA library of pathogenic Naegleria fowleri by immunoscreening; it consisted of 360 bp and produced a 13.1 kDa recombinant protein (rNfa1) that showed the pseudopodia-specific localization by immunocytochemistry in the previous study. Based on the idea that the pseudopodia-specific Nfa1 protein mentioned above seems to be involved in the pathogenicity of N. fowleri, we observed the effect of an anti-Nfa1 antibody on the proliferation of N. fowleri trophozoites and the cytotoxicity of N. fowleri trophozoites on the target cells. The proliferation of N. fowleri trophozoites was inhibited after being treated with an anti-Nfa1 polyclonal antibody in a dose-dependent manner for 48 hrs. By a light microscope, CHO cells co-cultured with N. fowleri trophozoites (group I) for 48 hrs showed severe morphological destruction. On the contrary, CHO cells co-cultured with N. fowleri trophozoites and anti-Nfa1 polyclonal antibody (1:100 dilution) (group II) showed less destruction. In the LDH release assay results, group I showed 50.6% cytotoxicity, and group II showed 39.3%. Consequently, addition of an anti-Nfa1 polyclonal antibody produced a decreasing effect of in vitro cytotoxicity of N. fowleri in a dosedependent manner.
-
Key words: Naegleria fowleri, cytotoxicity, proliferation, nfa1 gene
INTRODUCTION
Naegleria fowleri, a free-living amoeba widespread in moist soil, water and sediment, exists as a virulent pathogen causing fatal primary amoebic meningoencephalitis (PAME) in experimental animals and humans (
Culbertson, 1971;
Marciano-Cabral, 1988;
Ma et al., 1990;
Kollars and Wilhelm, 1996). To obtain antigenic molecules to be used as diagnostic agents in pathogenic
N. fowleri infection, we previously cloned an antigenic gene (called
nfa1) from a cDNA library of
N. fowleri by immunoscreening, using infected and immune mouse sera (
Shin et al., 2001). We reported that the
nfa1 gene had the coding nucleotide sequence of 360 bp, producing a recombinant protein (rNfa1) of 13.1 kDa (
Shin et al., 2001). An anti-Nfa1 polyclonal antibody obtained from mice immunized with a rNfa1 protein was used in immunocytochemistry, showing the Nfa1 protein as an indication of the pseudopodiaspecific immunolocalization on a trophozoite of
N. fowleri (
Cho et al., 2003).
We produced an anti-Nfa1 polyclonal antibody based on the hypothesis that Nfa1 protein involved in pseudopodia activity may be concerned with the pathogenicity of
N. fowleri. And then we obtained a preliminary data of the anti-Nfa1 antibody causing a decreasing effect on the cytotoxicity of
N. fowleri trophozoites against CHO (Chinese hamster ovary) cells, much like treating an anti-Nfa1 antibody on a co-cultivating system (
Cho et al., 2003).
In this research, for a more detailed analysis, we observed whether an anti-Nfa1 polyclonal antibody effects the proliferation of N. fowleri trophozoites in cultivation and on the in vitro cytotoxicity of pathogenic N. fowleri in a time- or a dose-dependent manner.
MATERIALS AND METHODS
Cultivation of N. fowleri and CHO cells
N. fowleri trophozoites (Cater NF69 strain, ATCC No.30215) were axenically cultured at 37℃ in Nelson' media (
Willaert, 1971). CHO cells were cultured with EMEM (Earle's Minimum Essential Medium) containing 10% fetal bovine serum (complete EMEM) at 37℃ in a 5% CO
2 incubator (
Im and Shin, 2003).
Expression of the nfa1 gene and production of a rNfa1 protein
To obtain a rNfa1 fusion protein, the expression and purification of the
nfa1 gene product were performed accordingly by the method mentioned in the previous paper (
Shin et al, 2001). The purified DNA (5 µg/µl) obtained from PCR-T7/NT TOPO expression vector (Invitrogen, Grohingen, Netherlands) containing the
nfa1 gene was subsequently transformed into the BL21(DE3)pLysS
E. coli strain by the heat-shock method. Cells were cultured at 37℃ in the LAC (Luria-Bertani media containing 100 µg/ml of ampicillin and 34 µg/ml of chloramphenichol) plates for selection. A transformed-colony was selected and cultured at 37℃ in the LAC broth until the absorbance reached 0.5-0.8 at 600 nm; then 1 mM IPTG was added to the media. After 4 hrs of incubation, the cells were harvested by centrifugation (6,000 × g for 15 min). Cell extracts were compared with those of non-transformed BL21(DE3)pLysS by SDS-PAGE, and the presence of the expressed gene product was confirmed by Western blotting using both the immune and the infected sera, and anti-His and Xpress antibodies (Invitrogen).
For the production of anti-Nfa1 polyclonal serum, the rNfa1 protein (50 µg/mouse) was mixed with an equal volume of Freund's complete adjuvant (Sigma), and the mixture was injected intraperitoneally into an 8-week-old female BALB/c mice (purchased from the Korea Institute of Science and Technology, Daejeon, Korea). The mouse was boosted biweekly for another 4 weeks with the rNfa1 protein (25 µg/mouse) containing an equal volume of incomplete Freund's adjuvant (Sigma). After the third boosting, the rNfa1 protein (5 µg/mouse) was injected intravenously without the adjuvant. Four days later, anti-Nfa1 polyclonal serum was collected from the mouse blood by centrifuging at 2,500 × g for 30 min at 4℃. ELISA was performed with a purified Nfa1 protein (5 µg/ml) and with a rabbit anti-mouse whole immunoglobulin (1:10,000 dilution) conjugated with alkaline phosphate (Sigma). Western blotting for the recombinant Nfa1 protein was performed according to the method in a previous paper (
Cho et al., 2003).
N. fowleri trophozoites (1 × 104 cells) were put in triplet culture tubes containing 1 ml of Nelson's medium. Then an anti-Nfa1 polyclonal antibody (1:200, 1:100 and 1:50 dilution) obtained from mice was added in each tube. Cultivating in a 37℃ incubator, the number of trophozoites in each tube was counted with an haematocytometer up to 48 hrs post-incubation.
Microscopic findings and LDH release assay for the cytotoxicity of N. fowleri
N. fowleri trophozoites containing CHO cells cultured in complete EMEM were put in each well of a 96-well plate (group I). For the experimental group (group II), an anti-Nfa1 polyclonal antibody (1:200, 1:100 and 1:50 dilution) obtained from mice was added in the triplet wells refer to the 96-well plate. After incubating at 37℃ for 24 hrs and 48 hrs, respectively, the effect of the anti-Nfa1 polyclonal antibody was observed with a light microscope and LDH (lactate dehydrogenase) release assay. The cytotoxicity from the LDH release assay was observed with the CytoTox 96™ Non-Radioactive Cytotoxicity Assay Kit (Promega, WI, USA). About 1 × 104 CHO cells were prepared with 100 µl of EMEM in a round-bottomed 96-well plate. The amoeba trophozoites (1 × 105) and/or an anti-Nfa1 polyclonal antibody (1:200, 1:100 and 1:50 dilution) were added to each well, and five sets of triplet wells for controls including spontaneous releasing from medium and naive cells were prepared. The casted plate was incubated in a humidified chamber at 37℃ and in a 5% CO2 incubator for 45 min consecutively, and then centrifuged at 250 × g for 4 min. The supernatant (50 µl) from each well was transferred to a fresh flat-bottomed 96-well plate. Equal volumes of the reconstituted Substrate Mix were added to each well. The plate was incubated at room temperature for 30 min. Fifty µl of Stop Solution was added, and after 1 hr, the absorbance was recorded at 490 nm.
Cytotoxicity (%) =Absorbance of (experimental group - control group)Absorbance of control group×100
RESULTS
Characterization of an anti-Nfa1 polyclonal antibody
To obtain a purified recombinant Nfa1 protein, a fusion protein of 17 kDa from the BL21
E. coli strain containing the
nfa1 gene was purified using a nickel resin column and enzyme digestion. Finally the purified protein eluted from the gels had a molecular weight of 13.1 kDa, which corresponded well to the size in the previous research (
Cho et al., 2003) (data not shown). An anti-Nfa1 polyclonal antibody was collected from mice immunized with a recombinant Nfa1 protein, and it's specific reactivity against the Nfa1 protein was observed with ELISA and Western blotting. Values of anti-Nfa1 polyclonal antibodies by ELISA ranged from 0.212 ± 0.021 (1:10,000 dilution, M ± SD, N=5) to 1.213 ± 0.024 (1:200 dilution), which showed a difference from the 0.112 ± 0.005 (1:200 dilution) of the normal sera and also the 0.109 ± 0.005 of the PBS control.
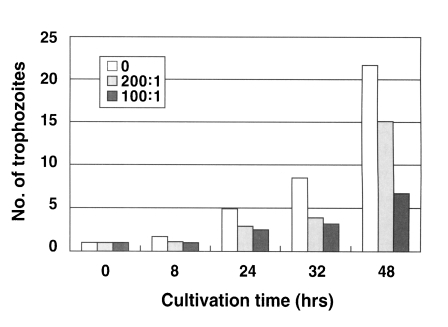
N. fowleri trophozoites (1 × 10
4 cells) cultured from the beginning with an anti-Nfa1 polyclonal antibody (1:200 dilution) proliferated to 1.1 × 10
4 cells in 8 hrs, 2.9 × 10
4 cells in 24 hrs, 3.9 × 10
4 cells in 32 hrs and 15.1 × 10
4 cells in 48 hr post-incubation (
Fig. 1). When an anti-Nfa1 antibody (1:100 dilution) was treated in cultivating system,
N. fowleri trophozoites (1 × 10
4 cells) proliferated to 1.0 × 10
4 cells in 8 hrs, 2.5 × 10
4 cells in 24 hrs, 3.2 × 10
4 cells in 32 hrs and 6.7 × 10
4 cells in 48 hrs post-incubation (
Fig. 1). In the control group, without the anti-Nfa1 antibody, the amoeba proliferated to 1.7 × 10
4 cells in 8 hrs, 4.9 × 10
4 cells in 24 hrs, 8.5 × 10
4 cells in 32 hrs and 21.7 × 10
4 cells in 48 hrs post-incubation (
Fig. 1). Consequently, the addition of an anti-Nfa1 polyclonal antibody showed an inhibition on the proliferation of
N. fowleri in a dosedependent manner (p < 0.05).
Effects of the anti-Nfa1 antibody on the cytotoxicity of N. fowleri
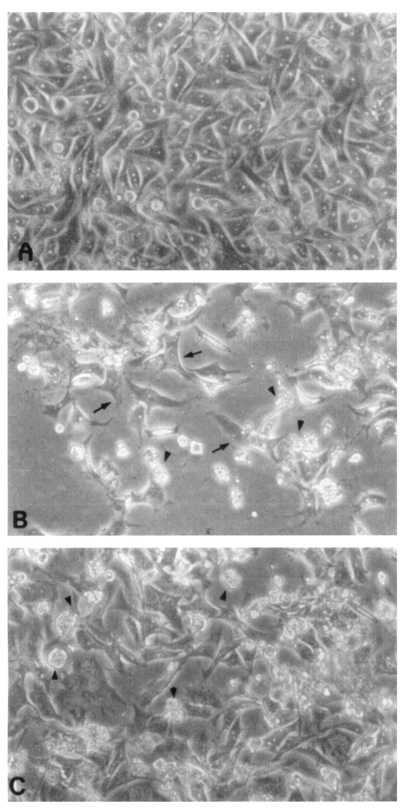
CHO cells co-cultured with
N. fowleri trophozoites (group I) for 24 hrs showed severe morphological destruction (
Fig. 2B). On the contrary, CHO cells cocultured with
N. fowleri trophozoites and anti-Nfa1 polyclonal antibody (group II) showed less destruction than above experimental group I (
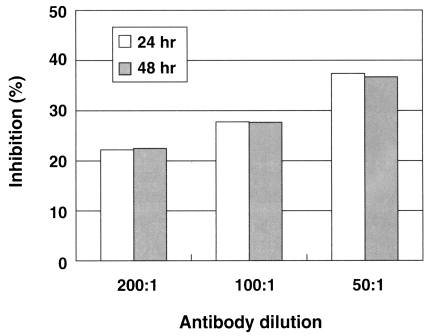
Fig. 2C). In the LDH release assay results, when an anti-Nfa1-antibody (1:200 dilution) was added in the co-cultivating system, group I showed 55.3% cytotoxicity and group II showed 43.7% in the LDH release assay 24 hrs postincubation. After 48 hrs of incubation, group I showed 59.0% cytotoxicity, and group II showd 48.0% (
Table 1). When an anti-Nfa1-antibody (1:100 dilution) was added in the co-cultivating system, group I showed 50.6% cytotoxicity 24 hrs post-incubation, and group II showed 39.3% (
Table 1). After 48 hr of incubation, group I showed 53.3% cytotoxicity and group II 41.3%, respectively (
Table 1). When an anti-Nfa1 antibody (1:50 dilution) was added in the co-cultivating system, group I showed 54.3% cytotoxicity 24 hrs post-incubation, and group II showed 34.0%. After 48 hrs of incubation, group I showed 66.3% cytotoxicity, and group II showed 42.0% (
Table 1). Consequently, the addition of an anti-Nfa1 polyclonal antibody showed a decreasing effect of in vitro cytotoxicity of
N. fowleri in a dose-dependent manner (
Fig. 3).
DISCUSSION
PAME caused by
N. fowleri is an acute progressive illness with the olfactory neuroepithelium as the major route of invasion for the
N. fowleri (
John DT, 1982). Concerned with host-tissue invasion, the adherence of the amoeba to host cells is the most important step in the mechanism of pathogenicity of
N. fowleri, a specific pseudopodial projection, called an amoebastome, is formed (
Marciano-Carbral, 1988). Other cytotoxic toxins and cytolytic proteinases have been proposed that amoeba destroy target cells (
John, 1982;
Marciano-Cabral, 1988;
Ma et al., 1990).
In the previous research, we cloned an antigenic gene,
nfa1, by using the immune and the infected sera and produced a recombinant 13.1 kDa protein that had a strong immunoreactivity against both the immune and the infected sera (
Shin et al., 2001). The amino acid sequence and the secondary structure of the
nfa1 gene were related to those of the hemerythrin (Hr) or myohemerythrin (myoHr) gene of some marine invertebrates (
Negri et al., 1994). The
nfa1 gene encoding myoHr-like protein was firstly cloned from amoeba, and its genetic relationship indicated a separate cluster from myoHr genes that were reported previously (
Shin et al., 2001). In another study, trophozoites of
N. fowleri in cultivating system and in mouse brain tissue infected experimentally with
N. fowleri were well immunostained, as results of immunohistochemistry of the Nfa1 protein (
Cho et al., 2003). The immunolocalization study of the Nfa1 protein revealed that this protein was abundant in pseudopodia and around food vacuoles of
N. fowleri trophozoites (
Cho et al., 2003). This suggests that the Nfa1 protein is required for food ingestion and amoeba movement.
It was reported that highly virulent amoeba exhibited faster movement than weakly virulent amoeba (
Cline et al, 1986). Based on the Nfa1 protein localization on the pseudopodia of
N. fowleri in this study, the proliferation and the cytotoxicity of
N. fowleri against CHO target cells were observed by adding an anti-Nfa1 antibody in the co-cultivation system to elucidate the antibody effect. It was shown that anti-Nfa1 antibody inhibited the proliferation of
N. fowleri trophozoites in a dose-dependent manner. In addtion,
N. fowleri destroyed the CHO cells severely and showed a high cytotoxicity in the LDH release assay, but the cytotoxicity was decreased when an anti-Nfa1 antibody was added in a dose-dependent manner.
As iron-containing heme compounds like myoHr are essential for infectivity, invasion or destruction of the host tissue and anti-bacterial function (
Negri et al, 1994;
Raner et al., 1997), detailed biological roles of the Nfa1 protein need to be found in future studies. Moreover, further studies need to determine whether the Nfa1 protein is related to in vivo amoebic pathogenicity.
Finally, this study elucidates that the Nfa1 protein is a pathogen-related protein concerned with the pathogenesis of N. fowleri, especially with the amoebic proliferation and in vitro cytotoxicity.
Notes
-
This study was supported by a grant (No. R01-2000-000-00077-0) from the Korea Science & Engineering Foundation and in part by a grant from the Ajou University School of Medicine in 2000.
References
- 1. Cho MS, Jung SY, Park S, et al. Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic Naegleria fowleri. Clin Diagn Lab Immunol 2003;10:954-959.
- 2. Cline M, Carchman R, Marciano-Cabral F. Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J Protozool 1986;33:10-13.
- 3. Culbertson CG. The pathogenicity of soil amoebas. Annu Rev Microbiol 1971;25:231-254.
- 4. Im K, Shin HJ. Acanthamoeba sohi, n. sp., a pathogenic Korean isolate YM-4 from a freshwater fish. Korean J Parasitol 2003;41:181-188.
- 5. John DT. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol 1982;36:101-123.
- 6. Kollars TM Jr, Wilhelm WE. The accurence of antibodies to Naegleria species in wild mammals. J Parasitol 1996;82:73-77.
- 7. Ma P, Visvesvara GS, Martinez AJ, et al. Naegleria and Acanthamoeba infections: Review. Rev Infect Dis 1990;12:490-513.
- 8. Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev 1988;52:114-133.
- 9. Negri A, Tedeschi G, Bonomi F, Zhang JH, Kurtz DM Jr. Amino-acid sequences of the alpha- and betasubunits of hemerythrin from Lingula reevii. Biochim Biophys Acta 1994;1208:277-285.
- 10. Raner GM, Martins LJ, Ellis WR Jr. Functional role ofleucine-103 in myohemerythrin. Biochemistry 1997;36:7037-7043.
- 11. Shin HJ, Cho MS, Jung SY, et al. Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of Naegleria fowleri. J Eukaryot Microbiol 2001;48:713-717.
- 12. Willaert E. Isolement et culture in vitro des amibes de genre Naegleria. Ann Soc Belg Med Trop 1971;51:701-708.
Fig. 1Proliferation of Naegleria fowleri trophozoites cocultured with an anti-Nfa1 polyclonal antibody. Box indicates the dilution ratio of the anti-Nfa1 polyclonal antibody.
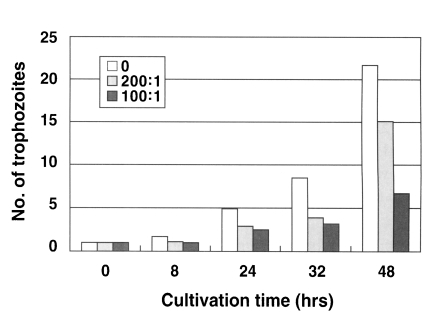
Fig. 2Microscopic findings of CHO cells (arrows) incubated in EMEM (A), with Naegleria fowleri trophozoites (arrow heads) (B), or with N. fowleri and an anti-Nfa1 antibody (C) 48 hrs post-cultivation (× 200).
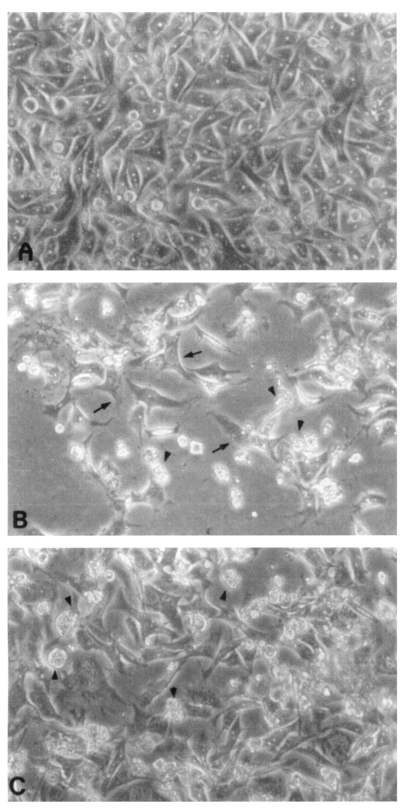
Fig. 3A graphic view showing the addition of the anti-Nfa1 polyclonal antibody inhibited the cytotoxicity of Naegleria fowleri against CHO cells. Box indicates the time post-incubation.
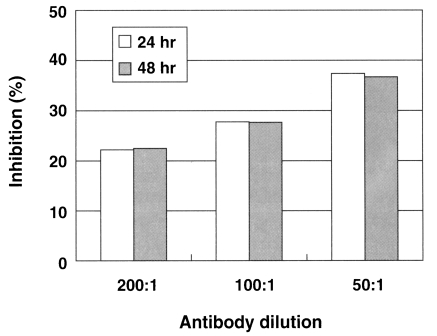
Table 1.Cytotoxicity (%) of Naegleria fowleri against CHO cells by LDH release assay
Table 1.
|
Groups |
1:200 dilutiona)
|
1:100 dilution
|
1:50 dilution
|
|
24 hrsb)
|
48 hrs |
24 hrs |
48 hrs |
24 hrs |
48 hrs |
|
CHO cells + trophozoites |
55.3 ± 0.57 |
59.0 ± 2.00 |
50.6 ± 4.16 |
53.3 ± 3.51 |
54.3 ± 3.21 |
66.3 ± 5.51 |
|
CHO cells + trophozoites + anti-Nfa1 antibody |
43.7 ± 1.52 |
48.0 ± 1.00 |
39.3 ± 3.05 |
41.3 ± 3.21 |
34.0 ± 3.61 |
42.0 ± 6.24 |