Abstract
Lactobacillus species in the female genital tract are thought to act as a barrier to infection. Several studies have demonstrated that lactobacilli can adhere to vaginal epithelial cells. However, little is known about how the adherence of lactobacilli to vaginal epithelial cells affects the acidity, cell viability, or proliferation of the lactobacilli themselves or those of vaginal epithelial cells. Lactobacillus acidophilus was co-cultured with immortalized human vaginal epithelial cells (MS74 cell line), and the growth of L. acidophilus and the acidity of the culture medium were measured. MS74 cell density and viability were also assessed by counting cell numbers and observing the cell attachment state. L. acidophilus showed exponential growth for the first 6 hr until 9 hr, and the pH was maintained close to 4.0-5.0 at 24 hr after culture, consistent with previous studies. The growth curve of L. acidophilus or the pH values were relatively unaffected by co-culture with MS74 cells, confirming that L. acidophilus maintains a low pH in the presence of MS74 cells. This co-culture model could therefore potentially be used to mimic vaginal conditions for future in vitro studies. On the other hand, MS74 cells co-cultured with L. acidophilus more firmly attached to the culture plate, and a higher number of cells were present compared to cells cultured in the absence of L. acidophilus. These results indicate that L. acidophilus increases MS74 cell proliferation and viability, suggesting that lactobacilli may contribute to the healthy environment for vaginal epithelial cells.
-
Key words: Lactobacillus, vaginal epithelial cell, cell viability, cell proliferation
The vaginal mucosa is populated with microflora, with lactobacilli being the dominant microbes. The dominance of lactobacilli over pathogenic anaerobes is positively associated with vaginal health [
1], as evidenced by the strong association between reduced numbers of vaginal lactobacilli and common vaginal disorders, including bacterial vaginosis [
1] and trichomoniasis [
2,
3]. Trichomoniasis is the most common nonviral sexually-transmitted infection worldwide and an apparent contributor to HIV acquisition [
1,
2].
Trichomonas vaginalis has been associated with multiple morbidities, including pelvic inflammatory diseases, preterm birth, cervical cancer, and infertility as well [
1,
2]. Lactobacilli are believed to protect their hosts from urogenital tract infections via various mechanisms. For example, lactobacilli have higher affinity for human uroepithelial cells in vitro and inhibit other pathogenic bacteria from adhering to those cells based on competitive exclusion [
4,
5]. They are also capable of co-aggregating with pathogenic bacteria, which may result in the elimination of pathogens [
6]. Several studies have demonstrated that lactobacilli adhere to vaginal epithelial cells (VECs). However, little is known about the effects of lactobacilli adherence to VECs on either the bacteria themselves or VECs. We therefore investigated the effects of adherence of lactobacilli to in vitro cultured VECs by analyzing the acidity of the culture medium, cell viability, and proliferation in the presence or absence of lactobacilli.
We examined interactions between
Lactobacillus acidophilus and in vitro cultured VECs, because this bacterial strain is frequently recovered from the vagina [
7].
L. acidophilus was grown in 20 ml of MRS broth (Difco Laboratories, Detroit, Michigan, USA) at 37℃ in a 5% CO
2 incubator for 16 to 24 hr. The lactobacilli were then subcultured for another 2 hr to achieve exponential growth [
8]. Immortalized human MS74 cells (kindly provided by Prof. John F. Alderete JF, Texas University, USA) [
9] were cultured at 37℃ in a 5% CO
2 atmosphere in DMEM (WelGene, Daegu, Korea) supplemented with 10% FBS (WelGene) and 0.5% penicillin-streptomycin (GenDEPOT, Barker, Texas, USA) in a humidified atmosphere for 2-3 days. Confluent MS74 cells were harvested and seeded in new culture flasks (1.9×10
6 cells/ml) in a 5% CO
2 atmosphere in the presence or absence of lactobacilli (2.5×10
5 CFU/ml). Then the cells were incubated for different time intervals; 3, 6, 9, 12, and 24 hr. All experiments were performed in triplicate.
An acidic vaginal pH and the presence of lactobacilli are clearly components of the multifaceted antimicrobial defense system that operates in the vaginal fluid, because lactobacilli produce organic acids, including lactic acid [
10]. An alteration in vaginal pH to less acidic values is strongly associated with vaginal flora changes. For example,
Trichomonas,
Chlamydia, and
Neisseria infections are associated with increases in the vaginal pH [
11]. In particular,
T. vaginalis appears to have a deleterious effect on the growth of
L. acidophilus in vitro competitive assay [
16].
To analyze the effects of co-culture on the ability of
L. acidophilus to maintain acidity, pH of the conditioned media was measured before and 3, 6, 9, 12, and 24 hr after culture using a pH meter (MP 230, Mettler Toledo, AG, Greifensee, Switzerland). The pH of the culture medium of
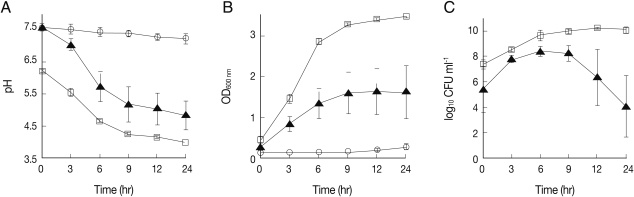
L. acidophilus decreased rapidly from 6.2 to 4.6 over the first 6 hr and was then maintained at 3.8 until the end of the incubation period (
Fig. 1A). Consistent with the previous report [
12], these pH changes corresponded to the maximum growth rate of the bacterium, as shown in
Fig. 1B and 1C. Likewise, the pH value of media from MS74 cells co-cultured with
L. acidophilus also decreased profoundly from 7.6 to 5.7 for the first 6 hr, consistent with the change in growth rate of the bacterium (
Fig. 1B, 1C). This pH value measured in the media from co-cultures is similar to the value measured in healthy vaginas. In contrast, there was no change in the pH of the media from MS74 cells cultured alone, as expected (
Fig. 1A). These results confirm that
L. acidophilus maintains a low pH in the presence of MS74 cells. This co-culture model could therefore potentially be used to mimic vaginal conditions in future in vitro studies.
The growth of
L. acidophilus was also analyzed by measuring the optical density at 600 nm (OD
600 nm) and assessing the number of colony forming units (CFUs) for each designated culture time point. The OD
600 nm of cultured media collected from culture flasks was measured using a Beckman spectrophotometer (Beckman, Fullerton, California, USA). To determine the number of viable microorganisms, culture suspensions were diluted 10
4- and 10
6-fold in sterile distilled water, and then 100 µl of these diluted culture suspensions were spread onto MRS agar plates. The number of growing bacterial colonies was counted after 48 hr incubation.
L. acidophilus cultured alone showed an exponential increase in OD
600 nm from 0.4 to 3.0 for the first 6 hr after culture, and the number of CFUs also reached a maximum value (5×10
9 CFU/ml) during the same period of time (
Figs. 1B, 1C). Likewise, the OD
600 nm of
L. acidophilus co-cultured with MS74 cells increased to 1.3 after the first 6 hr of incubation and reached 1.6 at the end of the incubation (
Fig. 1B). Although the OD
600 nm value for
L. acidophilus co-cultured with MS74 cells was half that obtained for
L. acidophilus cultured alone, the bacteria numbers increased over time under both culture conditions in a similar manner. However, the number of CFUs after co-culture increased from 3×10
5 to 3×10
8 CFU/ml over the first 6 hr, decreased after 9 hr, and was at 1.3×10
4 CFU/ml at the end of the culture (
Fig. 1C). The discrepancy in OD
600 nm values may partly be explained by metabolic wastes produced by the co-cultured MS74 cells.
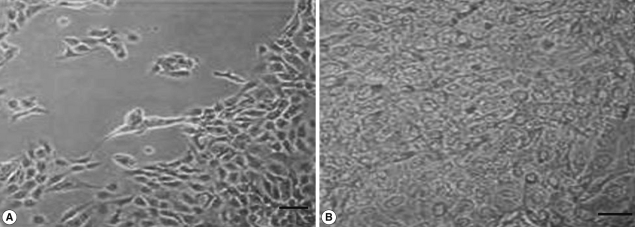
We also analyzed the effects of
L. acidophilus on MS74 cells by observing their cellular attachment to the culture plate and counting the number of viable cells. MS74 cells were cultured in T75 cell culture flasks (SPL lifesciences, Pocheon, Korea) in the presence or absence of
L. acidophilus. After 24 hr culture, the culture flask was examined under a microscope (×400) (TS100, Nikon, Tokyo, Japan). As shown in
Fig. 2, MS74 cells co-cultured with
L. acidophilus were more firmly attached to the plate and were more confluent than were those cultured in the absence of
L. acidophilus; several detached areas were observed on the latter plate (
Fig. 2A). The cells were harvested by incubation with 10% trypsin-EDTA solution (GenDEPOT), and then washed twice and resuspended with PBS via centrifugation at 2,000 g at 4℃ for 30 min. Viable cells were counted by staining the cells with trypan blue followed by visualization under a microscope using a hemocytometer (Superior MarienFeld, Bad Mergentheim, Germany). Differences between groups were analyzed by the Student's
t-test using SPSS 17.0 software for Windows (SPSS Inc., Chicago, Illinois, USA). Significance was accepted at
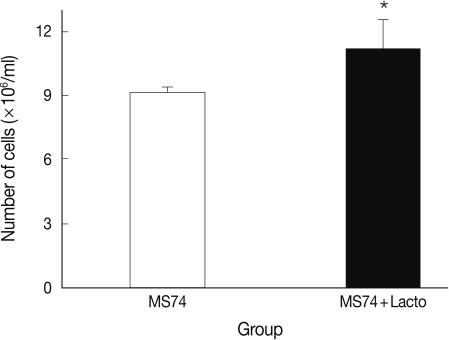
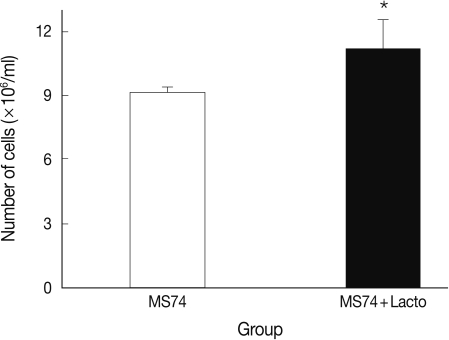
P<0.05. More MS74 cells were present in co-cultures with
L. acidophilus than in cultures that did not contain
L. acidophilus (1.1×10
7 cells/ml versus 9×10
6 cells/ml, respectively) (
P<0.05) (
Fig. 3).
It has been suggested that close interdigitations formed between
T. vaginalis and vaginal epithelia may cause mechanical damage to the cells and contribute to microhemorrhages and inflammation observed in the vaginal epithelium of patients with trichomoniasis [
15]. Adhesion of lactobacilli results in the formation of a bacterial film on the vaginal epithelium and may contribute to the exclusion of pathogens from the vaginal mucosa. In addition, lactobacilli may promote a healthy vaginal epithelial environment. In other words, VECs may be protected by the adherence of lactobacilli, and the vaginal ecosystem may be more resistant to pathogens because of the presence of lactobacilli.
The ability of
L. acidophilus to survive and proliferate in the presence of VECs indicates that this system could be used in in vitro studies to mimic the vaginal environment. The co-culture system described in this study could therefore be a valuable tool for investigating pathogenesis or for finding compounds that can protect against common vaginal disorders, including bacterial vaginosis and trichomoniasis. Previous studies have shown that the numbers of vaginal lactobacilli are reduced in the presence of a
Trichomonas infection, and vice versa [
13,
14].
T. vaginalis can phagocytose lactobacilli, while lactobacilli slow the growth of
T. vaginalis in in vitro culture [
15]. Because high numbers of lactobacilli and a low pH are characteristic of a healthy human vagina only, but not in animals [
16], our in vitro model could be used to study the possible roles of
Trichomonas virulence factors in reducing the number of lactobacilli as well as any direct harmful effects of the virulence factors on the VECs. Although
T. vaginalis is usually curable, it has evolved and adjusted to the host environment in order to survive as any other parasite, and standard treatment-resistant trichomoniasis has been implicated in an increasing number of refractory cases [
17]. This in vitro culture system could contribute to investigation of alternative curative therapies in a number of refractory cases.
Vaginal floras are dynamic, and the level of lactobacilli used in the present study may not reflect the level of lactobacilli present in the vagina of women. Further clarification of this relationship could improve our understanding of Trichomonas and may have implications for the treatment of trichomoniasis. This study showed that lactobacilli increase MS74 cell proliferation and cell viability. These results suggest that lactobacilli contribute to a healthy environment for VECs, thereby facilitating resistance to pathogens, although the exact mechanisms still need to be clarified. Our model co-culture system can potentially be used to identify the factors that maintain a healthy vaginal micro-biota.
ACKNOWLEDGEMENTS
We thank Hankyu Choi and Min-Young Seo for preparing cells and technical support. This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF 531-2008-1-E00043 and KRF 2009-0074679).
References
- 1. Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
- 2. Torok MR, Miller WC, Hobbs MM, Macdonald PD, Leone PA, Schwebke JR, Sena AC. The association between Trichomonas vaginalis infection and level of vaginal lactobacilli, in nonpregnant women. J Infect Dis 2007;196:1102-1107.
- 3. Ryu JS, Min DY. Trichomonas vaginalis and trichomoniasis in the Republic of Korea. Korean J Parasitol 2006;44:101-116.
- 4. Chan RC, Reid G, Irvin RT, Bruce AW, Costerton JW. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun 1985;47:84-89.
- 5. Velraeds MM, van der Mei HC, Reid G, Busscher HJ. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates. Appl Environ Microbiol 1996;62:1958-1963.
- 6. Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis 1990;12:856-872.
- 7. Sobel JD. Vaginitis and vaginal flora: Controversies abound. Curr Opin Infect Dis 1996;9:42-47.
- 8. Juárez T, de L, de R, Nader-Macías ME. Estimation of vaginal probiotic lactobacilli growth parameters with the application of the Gompertz model. Can J Microbiol 2002;48:82-92.
- 9. Klumpp DJ, Forrestal SG, Karr JE, Mudge CS, Anderson BE, Schaeffer AJ. Epithelial differentiation promotes the adherence of type 1-piliated Escherichia coli to human vaginal cells. J Infect Dis 2002;186:1631-1638.
- 10. Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother 1987;31:1231-1233.
- 11. Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol 2011;204:120. e1-e5.
- 12. Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 2001;185:375-379.
- 13. Jírovec O, Petrů M. Trichomonas vaginalis and trichomoniasis. Adv Parasitol 1968;6:117-188.
- 14. Soszka S, Kuczynska K. Influence of Trichomonas vaginalis on the physiological flora of the vagina. Wiad Parazytol 1977;23:39-45.
- 15. Rendón-Maldonado JG, Espinosa-Cantellano M, González-Robles A, Martínez-Palomo A. Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp Parasitol 1998;89:241-250.
- 16. McGrory T, Garber GE. Mouse intravaginal infection with Trichomonas vaginalis and role of Lactobacillus acidophilus in sustaining infection. Infect Immun 1992;60:2375-2379.
- 17. Waters LJ, Dave SS, Deayton JR, French PD. Recalcitrant Trichomonas vaginalis infection-a case series. Int J STD AIDS 2005;16:505-509.
Fig. 1Analysis of pH, optical density (OD600 nm), and number of colony forming units (CFUs) from cultures of L. acidophilus with or without MS74 cells. To assess the ability of L. acidophilus to maintain a low pH under different culture conditions, L. acidophilus was cultured with or without MS74 cells in the basal medium. The pH measurements were performed 0, 3, 6, 9, 12, and 24 hr after incubation. (A) Measurements of the pH of bacterial suspensions before and after culture, (B) Measurements of the OD600 nm of bacterial suspensions, (C) Measurements of CFUs to assess the viability of L. acidophilus. CFUs are presented as logarithmic values. MS74 cells, immortalized human vaginal epithelial cells; OD600 nm, optical density; CFU, colony forming unit;-○-, MS74 cells alone;-▴-, MS74 cells co-cultured with L. acidophilus;-□-, L. acidophilus alone. Each value represents the mean±SD from 3 independent experiments, with each condition tested in triplicate.

Fig. 2Microscopic findings of MS74 cells cultured with or without L. acidophilus after a 24-hr incubation. (A) MS74 cells cultured without L. acidophilus. (B) MS74 cells co-cultured with L. acidophilus (×400).
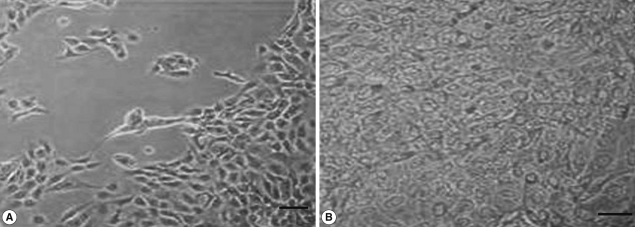
Fig. 3Viable cell numbers of MS74 cells cultured with or without L. acidophilus after a 24-hr incubation. MS74, MS74 cells alone; MS74 + Lacto, MS74 cells co-cultured with L. acidophilus. Each value represents the mean±SD from 3 independent experiments. Statistical analysis between groups was done using the Student's t-test, *P=0.046.