Abstract
During experimental Eimeria infections in chickens, facilities are often contaminated by fecal oocysts known to be highly resistant to both chemical and enzymatic treatments. Thus, studies using experimental Eimeria infections have been limited due to the difficulty of complete elimination of residual oocysts from both cages and facilities. To overcome this limitation, simple, inexpensive, and disposable cages were constructed from cardboard boxes and tested during experimental Eimeria maxima infections. The cages were used in animal rooms with only a 1.7% evidence of coccidia contamination between adjacent cages. No significant differences in fecal oocyst output and body weight gain were noted between animals housed in disposable cages and animals housed in wire control cages. This cage design is a useful means for preventing oocyst contamination during experimental conditions, suggesting that this disposable cage design could be used for other avian infectious disease studies.
-
Key words: Eimeria maxima, disposable cage, chickens, cardboard
Coccidiosis of chickens, one of the most costly diseases affecting the poultry industry worldwide, is caused by infection with one or more of 7 species of the intracellular protozoan parasite
Eimeria [
1,
2].
Eimeria infections occur when susceptible chickens ingest viable sporulated oocysts from contaminated litter. The ingested oocysts invade the intestinal epithelium in a region-specific manner, causing variant pathogenicity in poultry and ranging from reduced feed conversion, hemorrhagic diarrhea, and weight loss to, at times, mortality.
After at least 2 generations of asexual reproduction, several hundred thousand
Eimeria oocysts can be produced from a single oocyst, and are excreted in feces over several days or weeks [
2-
4]. The oocysts have cyst walls that are highly refractory to environmental extremes and disinfectants. Therefore, the oocysts can be transported mechanically by animals, insects, dust, and contaminated feed, water, and other fomites [
4-
6]. Considering that
Eimeria oocysts are highly infectious, easily transferable, and abundant in the environment [
7], it is difficult to achieve complete elimination of oocysts in experimental facilities. Thus, a prerequisite for investigating
Eimeria spp. is to house infected animals in strict isolation systems to prevent infective oocyst contamination.
A disposable cage, consisting of a disposable cardboard box and reusable plywood, was designed for pathogen studies in small, wild birds [
8]. Subsequently, a variety of isolators or cages have been designed for studies of various avian diseases, including coccidiosis [
9-
13]. However, for experimental studies of
Eimeria infection in chickens, these commercially manufactured cages are expensive and difficult to clean and sterilize properly. To overcome these disadvantages, we designed an economical, simple, and disposable cage, and evaluated its ability to eliminate oocyst contamination during experimental
Eimeria infections in chickens over the course of 2 years.
Male Cobb 500 chickens were reared under Eimeria-free conditions with unlimited access to feed and water. Constant light was provided for the duration of the experiments. All animal experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals established by Gyeongsang National University.
Cages consisted of disposable cardboard boxes (Wonchang box, Jinju, Korea), reusable plastic cups, and wire netting (
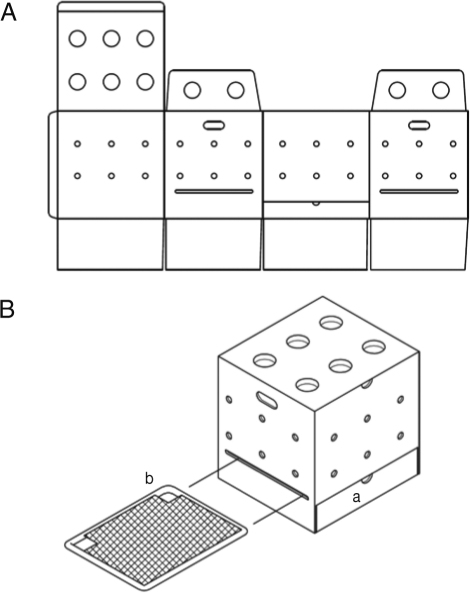
Fig. 1). Cages were 400 mm (W)×370 mm (D)×400 mm (H), which housed 3 chicks for 1 month. Feces fall into the bottom regions of the cages through the wire netting. For collection of excreta, the bottoms of the cages were lined with greaseproof paper that could be removed through region (a) of the cage (
Fig. 1A and 1B). Air and light were supplied freely through the side and upper holes. Cage roofs were designed to be opened for changing of water and feed, which were provided in 250 ml plastic cups located in region (b) of the cages (
Fig. 1B).
To infect Eimeria and assess fecal oocyst production, 4-day-old chickens were orally infected with 1×104 sporulated Eimeria maxima oocysts, and transferred to either wire cages (Farmer Automatic GmbH & Co. KG., Laer, Germany) or disposble cages (3 birds/cage). Fecal materials were collected on days 6-10 post-infection (PI). Eimeria oocysts were isolated from fecal samples by flotation on 6-14% sodium hypochlorite and washed 3 times with PBS. Oocyst counts were assessed using a McMaster counting chamber. Each group consisted of 12 chickens. Total oocyst number per bird was calculated by accounting for fecal sample volumes and the number of birds per cage using the following formula:
Total number of oocysts=oocyst count×dilution factor×(fecal sample volume/counting chamber volume)/number of birds per cage.
Body weights of infected chickens were individually measured on days 1-10 PI. To measure the body weight gain of uninfected chickens, 4-day-old chickens were randomly assigned to either wire cages or disposable cages (3 birds/cage). Body weights were individually measured for 32 days, beginning on day 4. Each group consisted of 12 chickens.
The disposable cages used were made of cardboard boxes that can be incinerated to eliminate residual oocysts after experimental studies with
Eimeria spp. (
Fig. 1). Because various
Eimeria spp. oocysts are highly resistant to environmental conditions and disinfectants [
5,
7], extreme temperatures and highly toxic chemicals are necessary to eliminate fecal or contaminated oocysts from cages or isolators following experimental
Eimeria infection studies. Additionally, it is difficult to wash and properly sterilize chicken cages because the cages are bigger than those used for small animals, such as mice and rats. Therefore, studies of experimental chicken coccidiosis have been limited due to the difficulty of preventing facility contamination by
Eimeria oocysts.
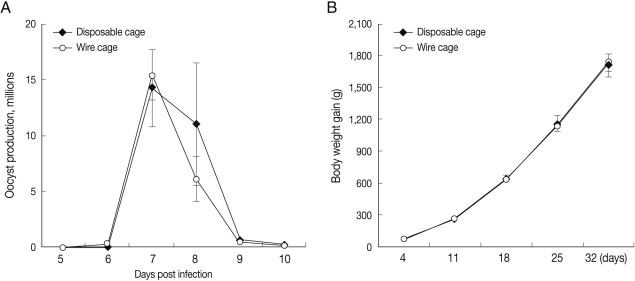
To compare disposable cage conditions with those of the traditional wire cages, we evaluated fecal oocyst shedding and body weight gain, the most reliable disease parameters for measuring the effects of coccidiosis [
14-
16]. No significant differences were noted in fecal oocyst output (
Fig. 2A) or body weight gain (data not shown) between infected chickens hous-ed in either disposable or wire cages. Similarly, there were no differences in body weight gain between uninfected chickens housed in either disposable or wire cages for 1 month with 3 birds per cage (
Fig. 2B). These data imply that the disposable cages can be used for experimental chicken studies with no effect on experimental outcome compared to wire cages.
Considering that 7 different species of
Eimeria can infect chickens and that
Eimeria oocysts are easily transferable by insects, dust, and fomites [
4,
7], contamination can easily occur during
Eimeria experiments without the use of sterile isolators or cages. Although the disposable cages were made from cardboard boxes containing holes (
Fig. 1), coccidia contamination was detected only in 1 cage (1.7%) of adjacent cages in 5 experiments over the course of 2 years. In contrast, coccidia contamination was found in 24.1% of wire cages (
Table 1). The detection limit of fecal examination via microscopy was 50 oocysts per gram of feces. Interestingly, Patton and Sapanski [
12] noted that 22% of trials resulted in unscheduled coccidial infection without the use of isolators.
Little progress has been made in either isolator or cage design for Eimeria studies in chickens to prevent contamination of experimental facilities and cross-contamination between cages [
12,
13]. Therefore, the present study designed a simple, economical, and disposable cage for use in studies of avian coccidiosis that can prevent or greatly decrease oocyst contamination of tools and study environments.
ACKNOWLEDGEMENTS
This study was supported by the Technology Development Program for Agriculture and Forestry (108168-3), the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
References
- 1. Lee BH, Kim WH, Jeong J, Yoo J, Kwon YK, Jung BY, Kwon JH, Lillehoj HS, Min W. Prevalence and cross-immunity of Eimeria species on Korean chicken farms. J Vet Med Sci 2010;72:985-989.
- 2. Sharman PA, Smith NC, Wallach MG, Katrib M. Chasing the golden egg: Vaccination against poultry coccidiosis. Parasite Immunol 2010;32:590-598.
- 3. Min W, Dalloul RA, Lillehoj HS. Application of biotechnological tools for coccidian vaccine development. J Vet Sci 2004;5:279-288.
- 4. Morris GM, Gasser RB. Biotechnological advances in the diagnosis of avian coccidiosis and the analysis of genetic variation in Eimeria. Biotechnol Adv 2006;24:590-603.
- 5. Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. Diseases of Poultry. 2008, 12th ed. Oxford, UK. Blackwell Publishing Co; pp 1068-1085.
- 6. Stotish RL, Wang CC, Meyenhofer M. Structure and composition of the oocyst wall of Eimeria tenella. J Parasitol 1978;64:1074-1081.
- 7. Tomley F. Techniques for isolation and characterization of apical organelles from Eimeria tenella sporozoites. Methods 1997;13:171-176.
- 8. Kale HW 2nd, Jennings WL. A disposable cage for studies of pathogens in small wild birds. Am J Trop Med Hyg 1970;19:733-734.
- 9. Cooper DM, Timms JR. The rearing and maintenance of breeding chickens in isolators. 2. Plastic tent isolators. Avian Pathol 1972;1:59-64.
- 10. Dennett DP, Bagust TJ. Application of a flexible-film isolator for rearing specific pathogen-free chickens and investigating poultry pathogens. Avian Pathol 1979;8:289-300.
- 11. Forder RE, Firth GA, Tivey DR, Howarth GS, Hughes RJ. A small-scale, low-cost isolation system for the incubation and rearing of low bacterial load chicks as a model to study microbial-intestinal interactions. Lab Anim 2008;42:185-192.
- 12. Patton WH, Sapanski WB. An isolator for the collection of feces from chickens infected with coccidia. Lab Anim Sci 1974;24:924-928.
- 13. Timms JR, Cooper DM, Millard BJ, Powell PC. An isolator for avian disease research. Lab Anim 1979;13:101-105.
- 14. Chapman HD, Matsler PL, Muthavarapu VK, Chapman ME. Acquisition of immunity to Eimeria maxima in newly hatched chickens given 100 oocysts. Avian Dis 2005;49:426-429.
- 15. Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult Sci 2003;82:62-66.
- 16. Lillehoj HS, Trout JM. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev 1996;9:349-360.
Fig. 1Disposable cage design schematic with plan view (A) and oblique view (B). Region (a) is for collection of excreta. Region (b) shows the locations of the water or feed cups.
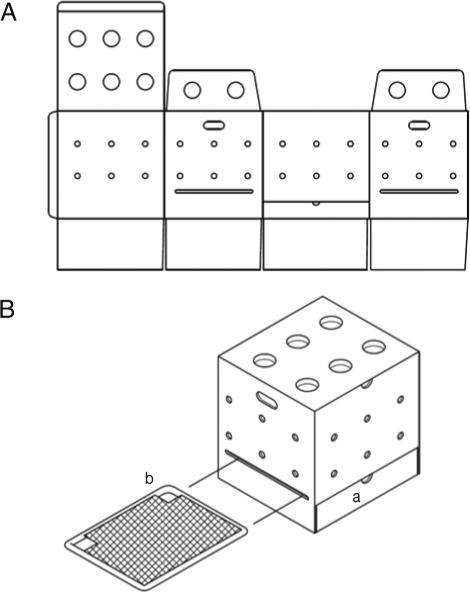
Fig. 2Comparison of fecal oocyst shedding and body weight gain between chickens housed in either disposable or wire cages. (A) Four-day-old chickens were orally infected with 1 × 104 sporulated Eimeria maxima oocysts and housed in either wire or disposable cages (3 birds/cage). Fecal materials were collected on days 6-10 post-infection, and the number of oocysts per bird was assessed. (B) Four-day-old chickens were randomly assigned to either wire or disposable cages (3 birds/cage). Body weight was individually measured for 32 days beginning 4 days after cage assignment. Bars represent the mean±SD of 12 chickens. Means and SDs were calculated by Student's t-test and considered significant at P<0.05.
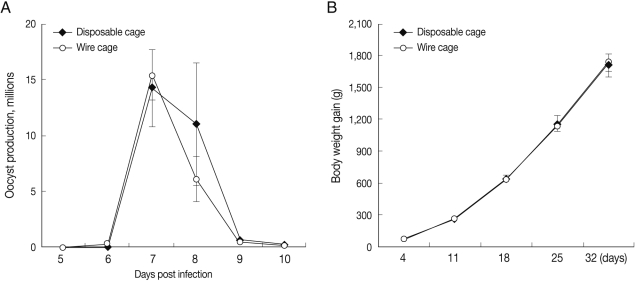
Table 1.Results of trials housing chickens in either wire or box cages
Table 1.
|
Type of cage |
No. of trialsa
|
No. of used cagesb (uninfected/infected) |
No. of contaminated cages (%) |
|
Wire |
6 |
54/194 |
13 (24.1) |
|
Box |
5 |
60/196 |
1 (1.7) |
Citations
Citations to this article as recorded by

- Different strategies for producing naturally soluble form of common cytokine receptor γ chain
Jipseol Jeong, Woo H. Kim, Cherry P. Fernandez, Suk Kim, Yong-Hwan Kim, Hyung-Kwan Jang, Hyun S. Lillehoj, Hee-Jong Woo, Wongi Min
Developmental & Comparative Immunology.2015; 48(1): 13. CrossRef - Chicken IL-17F: Identification and comparative expression analysis in Eimeria-infected chickens
Woo H. Kim, Jipseol Jeong, Ae R. Park, Dongjean Yim, Yong-Hwan Kim, Kwang D. Kim, Hong H. Chang, Hyun S. Lillehoj, Byung-Hyung Lee, Wongi Min
Developmental & Comparative Immunology.2012; 38(3): 401. CrossRef - Identification and Comparative Expression Analysis of Interleukin 2/15 Receptor β Chain in Chickens Infected with E. tenella
Jipseol Jeong, Woo H. Kim, Jeongmi Yoo, Changhwan Lee, Suk Kim, Jae-Hyeon Cho, Hyung-Kwan Jang, Dong W. Kim, Hyun S. Lillehoj, Wongi Min, Ivan Cruz Moura
PLoS ONE.2012; 7(5): e37704. CrossRef