Abstract
Various Leishmania species were engineered with green fluorescent protein (GFP) using episomal vectors that encoded an antibiotic resistance gene, such as aminoglycoside geneticin sulphate (G418). Most reports of GFP-Leishmania have used the flagellated extracellular promastigote, the stage of parasite detected in the midgut of the sandfly vector; fewer studies have been performed with amastigotes, the stage of parasite detected in mammals. In this study, comparisons were made regarding the efficiency for in vitro G418 selection of GFP-Leishmania amazonensis promastigotes and amastigotes and the use of in vivo G418 selection. The GFP-promastigotes retained episomal plasmid for a prolonged period and G418 treatment was necessary and efficient for in vitro selection. In contrast, GFP-amastigotes showed low retention of the episomal plasmid in the absence of G418 selection and low sensitivity to antibiotics in vitro. The use of protocols for G418 selection using infected BALB/c mice also indicated low sensitivity to antibiotics against amastigotes in cutaneous lesions.
-
Key words: Leishmania amazonensis, green fluorescent protein, macrophage, geneticin
INTRODUCTION
Advances in genetic manipulation with transfection techniques permit the preparation of
Leishmania expressing reporter genes, such as green fluorescent protein (GFP) from jellyfish
Aequorea victoria [
1-
9]. In general, GFP-based methods are simpler and easier to perform in studies of cellular dynamics than non-reporter and other reporter-gene-based methods and can be performed in living samples [
10,
11]. Various
Leishmania species have been transfected with GFP using episomal vectors and then maintained under selective pressure in vitro using antibiotics, including aminoglycoside geneticin sulphate (G418) [
1,
4]. Most reports of GFP-
Leishmania have used the flagellated extracellular promastigote, the stage of parasite detected in the midgut of the sandfly vector and which is absent in the mammalian host [
1,
4-
6,
8,
9,
12], however, few studies have been performed with amastigotes, the stage of parasite exclusively detected in mammals [
2,
3]. The importance lies in the fact that amastigotes reside in macrophages and are the target for chemotherapy and drug screening tests [
9,
13].
In this study, comparisons were made regarding the efficiency for in vitro G418 selection of GFP-
L. amazonensis promastigotes and amastigotes, a species that causes cutaneous and cutaneous metastatic lesions in South American countries [
14,
15]. For the first time, the use of the in vivo G418 selection for GFP-
Leishmania amastigotes is also described.
MATERIALS AND METHODS
Parasites
Leishmania amazonensis (MHOM/BR/75/Josefa strain) were rendered fluorescent as previously described [
1,
16]. Briefly, promastigotes were transfected by electroporation with the gene fragment coding for the C-terminal extension of cysteine proteinase fused to the reporter GFP in the pXG-'GFP
+ vector, a derivative of pX63NEO, developed by Ha and coworkers [
1]. Promastigotes were cultured at 26℃ in Earle 199 medium with 10% fetal calf serum (FCS) (Cultilab, Campinas São Paulo, Brazil), and amastigotes were isolated from footpad lesions of infected BALB/c mice, as previously described [
17]. The experimental protocols were approved by the Animal Ethics Committee of the Universidade Estadual de Campinas, Campinas, São Paulo, Brazil.
Selection of GFP-L. amazonensis
G418 was purchased from Sigma (Sigma, St. Louis, Missouri, USA) and diluted in water at 50 mg/ml. For in vitro selection, GFP-promastigotes and GFP-amastigotes were cultured in 25 cm
2 culture flasks and exposed to different concentrations of G418. Untreated parasites were maintained for each assay. For in vivo selection, BALB/c mice were inoculated with 2×10
7 promastigotes (70-80% GFP-promastigotes positives) subcutaneously in the hind footpad, and at different times postinfection, mice received daily intralesional dose of G418 (40 mg/kg of body weight/day). For each group, mice were administered 3, 5, 6, or 7 doses of G418 or saline only. Control mice received intralesional injections with saline solution. Cultured promastigotes and amastigotes, and amastigotes isolated from lesions, were analyzed by flow cytometry [
17]. The parasites were visualized under a Nikon Eclipse 50
i fluorescence microscope (Nikon, Tokyo, Japan). All images were captured and analyzed with a digital camera (Nikon DXM1200-F) and imaging software (ACT-1, Nikon). The parasite count was determined by optical microscopy.
The GFP-promastigotes and GFP-amastigotes (2×106) were fixed in 1% formaldehyde and washed in PBS. GFP expression was analyzed by flow cytometer FACSCalibur (Becton Dickinson Biosciences, Franklin, New Jersey, USA), and the parasite population was selected using the parameters of size (Forward Scatter-FSC) versus internal complexity (Side Scatter-SSC), using a logarithmic scale.
Spectrofluorometric analyses
The GFP-promastigotes and GFP-amastigotes were serially diluted and incubated on black microplates (Nunc, Thermo Fisher Scientific, Waltham, Massachusetts, USA). The correspondence between the relative intensity of fluorescence to the cell count was analyzed using a spectrofluorimeter (Multi-Detection Microplate Reader- Synergy HT, Bio-Tek, Winooski, Vermont, USA) at 485 nm excitation and 528 nm emission [
1,
18]. Fluorescence is reported in arbitrary fluorescence units and the data represent GFP fluorescence of parasites subtracted out control wells (saline). The wild type
L. amazonensis emitted undetectable levels of GFP fluorescence.
J774 macrophages or peritoneal macrophages from normal BALB/c mice, obtained as previously described [
19], were cultured in Earle 199 medium with 10% FCS. Cells were infected with GFP-promastigotes at a parasite cell ratio of 10:1 for 24 hr at 34℃. After removal of extracellular parasites, the infected cell cultures were incubated at 37℃ for 24 hr and the intracellular forms observed microscopically, as previously described [
19]. Cell cultures were also fixed in 1% formaldehyde diluted in 300 µl PBS at a density of 2×10
6 and analyzed by flow cytometry. For spectrofluorometric analyses, cell cultures were lysed with distilled water and transferred to black microplates to determine fluorescence.
Experiments were repeated at least 3 times and the results are expressed as the mean±SD. The results presented were verified in 3 mice per treatment. Control mice were treated with saline. Data were analyzed by 1-way ANOVA and the Student's t-test (P<0.01).
RESULTS
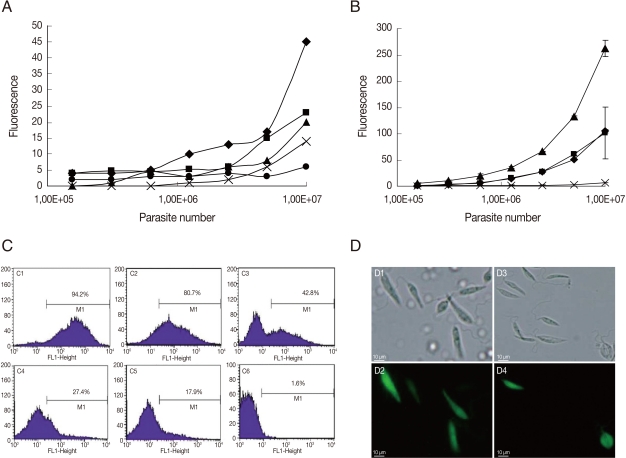
In vitro G418 selection of GFP-L. amazonensis promastigotes
As mentioned previously, promastigotes are currently used for GFP transfection experiments [
1-
8]. The period of episomal plasmid retention and efficiency of G418 selection were analyzed in
L. amazonensis GFP-promastigotes. As seen in
Fig. 1A, the fluorescence intensity was proportional to the number of parasites when serial dilutions of promastigotes cultured for 7, 14, 32, and 41 days without G418 were conducted. When GFP-promastigotes were cultured for 56 days without G418, the fluorescence intensity was not proportional to the number of promastigotes (
Fig. 1A), indicating a low fluorescence signal from parasites cultured for this period. Flow cytometry analysis (
Fig. 1C) verified that the percentage of GFP-promastigotes decreased with time, and 80.7%, 42.8%, 27.4%, 17.9% parasites were GFP-positive after 60, 90, 120, and 210 days without G418 treatment, respectively. In fact, when promastigotes were treated with 1 mg/ml G418, 94.2% were GFP-positive after 5 days (
Fig. 1C), the fluorescence intensity was high and proportional to the number of parasites (
Fig. 1B) and death of G418 non-resistant promastigotes occurred (data not shown). Brightly fluorescence GFP-promastigotes presenting normal morphology were observed by microscopy (
Fig. 1D). Taken together, these results indicate that the fluorescence intensity and the percentage of GFP-positive promastigotes decreased after 56-60 days without G418 selection. In addition, G418 treatment was necessary and efficient for GFP-promastigote selection.
The results of GFP-amastigotes in vitro selection is shown in
Fig. 2. The amastigotes were taken from mouse lesions and cultured with G418. The number of parasites decreased with time of G418 treatment; after 19 hr, death was induced in 34%, 42%, and 62% of amastigotes treated with 200 µg/ml, 500 µg/ml, and 1 mg/ml, respectively (
Fig. 2A). The fluorescence intensity was proportional to the number of parasites when serial dilutions were conducted on GFP-amastigotes treated with G418, 500 µg/ml and 1 mg/ml (
Fig. 2B). Although a small population of GFP-positive amastigotes (20%) was detected by flow cytometry in parasites treated with G418, 1 mg/ml (
Fig. 2C), very bright fluorescence was observed in these amastigotes (
Fig. 2D). A comparison of results (
Fig. 2) with GFP-promastigotes selection (
Fig. 1) indicated that in vitro G418 selection was less effective with amastigotes.
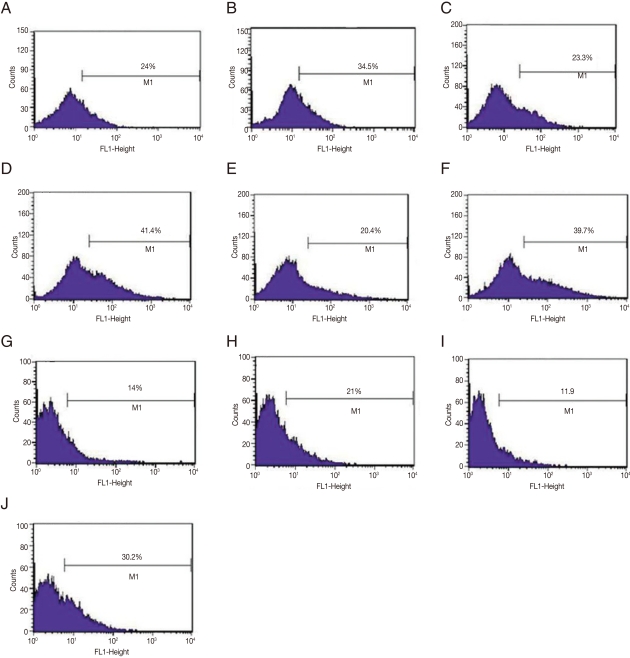
Since amastigotes are the intracellular stage in mammalian hosts and show an exuberant growth in the lesions induced by
L. amazonensis in susceptible mice [
17], in vivo selection of GFP-amastigotes was evaluated. Mice were infected with GFP-promastigotes and intralesional injections were administered at different postinfection times (
Fig. 3). It should be noted that the stability of GFP expression in amastigotes was low, since only 24.0%, 20.4-23.3%, and 11.9-14.0% of the lesion derived-amastigotes from untreated G418 mice were GFP-positive after 15, 30, and 90 days of infection, respectively (
Fig. 3). Most of the treatment courses resulted in a modest increase in GFP-positive amastigotes; for example, administration of 5 doses of G418 after 30 days postinfection compared with saline treatment, resulted in 41.4% positive GFP-positive vs 23.3% GFP-positive amastigotes, respectively (
Fig. 3C, D). Another treatment was administered, increasing the number of G418 doses to 6, and resulted in more efficient selection of GFP-amastigotes (30.2% GFP-positive amastigotes isolated from lesions G418-treated vs 11% GFP-positive amastigotes from lesions treated with saline); a 3-fold increase in the number of GFP-positive amastigotes (
Fig. 3I, J).
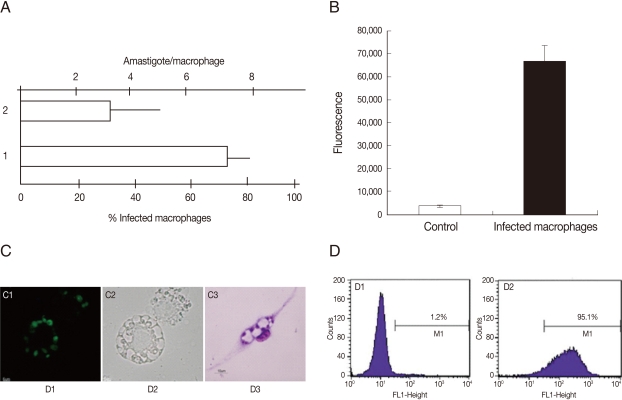
To examine the ability of G418 selected parasites to infect cells, the infection level and fluorescence signal of macrophage cultures were evaluated. As shown in
Fig. 4A, the infection level in murine peritoneal macrophages incubated with GFP-promastigotes was high, around 70% of infected cells and 3 amastigotes per cell. The GFP-parasite infected macrophages also revealed a strong fluorescence signal (
Fig. 4B) and using flow cytometry, a high percentage of GFP-positive macrophages were detected after incubation with GFP-promastigotes (95.1%) (
Fig. 4D). Light and fluorescence microscopy demonstrated the ability of GFP-
L. amazonensis to infect macrophages (
Fig. 4C). Similar results were obtained when macrophages were infected with GFP-amastigotes and the J774 cell line was used instead of peritoneal macrophages (data not shown). The in vitro and in vivo infectivities of GFP-
L. amazonensis were similar to those of wild
L. amazonensis for both promastigotes or amastigotes (data not shown).
DISCUSSION
Although many studies have used GFP-
Leishmania to screen antileishmanial compounds and analyze parasite-host interaction mechanisms, current understanding of G418 selective pressure on promastigotes and amastigotes is poor [
1-
9]. The tendency to silence transgene expression over time is a characteristic of transgenic cells and consequently, antibiotic selection results in a proliferative advantage for the subset of cells expressing antibiotic resistance gene [
10]. In this study, monitoring the stability of GFP expression over a period of 210 days indicated fluorescence decreased in promastigotes cultured 60 days without G418, i.e., GFP-promastigotes retained episomal plasmid for a prolonged period. Reports have referred to the β-galactosidase activity of recombinant
L. amazonensis promastigotes for 30 days without selective pressure [
6] and to the episomal expression of GFP in hamster ovary cells transfected with GFP and cultured 24 days without G418 [
20]. The stability of GFP expression in amastigotes is low, since only 24% of amastigotes derived from murine lesions were GFP-positive after 15 days of infection. The reasons contributing to low retention of episomal plasmid in amastigotes remain unknown; epigenetic effects, such as DNA methylation, have been implicated in this phenomenon in some mammalian cell lines [
21] and need to be evaluated in
Leishmania.
Next, G418 selection for GFP-
L. amazonensis was evaluated. Analysis of the results indicated that amastigote ability to be selected for GFP expression in vitro was poor compared with promastigotes. The best results were obtained with 1 mg/ml G418 (20% of positive GFP-amastigotes vs 94% of positive GFP-promastigotes). Following these experiments, our group evaluated whether it was possible to select GFP-amastigotes in vivo using mice lesions. Such a selection system would be useful in circumstances in which it is not possible to use an in vitro selection system. Again, analysis of the results indicated that amastigote ability to be selected in vivo was poor (the percentage of positive GFP-amastigotes reached a maximum of 41.4%), indicating that G418 selection is not effective with this parasite form. No report of the use of in vivo systems to test G418 selection in
Leishmania is available for comparison with our results; however, Murphy and coworkers [
22] selected high numbers of transfected
Trypanosoma brucei inoculating G418 in infected mice and concluded that the extracellular trypanosome blood form was highly sensitive to G418. The reasons for the high level of sensitivity of
T. brucei trypanosome compared to
L. amazonensis amastigote are unclear, but may be due to the problem of intracellular antibiotic delivery to the parasite and/or the acidic pH of the parasitophorous vacuoles housing amastigotes, since G418 selection is pH-dependent [
23].
In conclusion, analysis of the results of the present study showed that L. amazonensis GFP-promastigotes retained episomal plasmid for a prolonged period (i.e., 60 days) and G418 treatment is necessary and efficient for GFP-promastigote selection. In addition, the analyses of amastigotes showed that the intracellular parasite presented low retention of episomal plasmid in the absence of G418 selection and low levels of sensitivity to G418. The development of high level regulatable expression systems to boost transgenic protein levels in Leishmania amastigotes will be essential to advance the studies of infection dynamics in vitro and in vivo.
ACKNOWLEDGMENTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
References
- 1. Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol 1996;77:57-64.
- 2. Misslitz A, Mottram JC, Overath P, Aebischer T. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol 2000;107:251-261.
- 3. Sereno D, Roy G, Lemesre JL, Papadopoulou B, Ouellette M. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob Agents Chemother 2001;45:1168-1173.
- 4. Kamau SW, Grimm F, Hehl AB. Expression of green fluorescent protein as a marker for effects of antileishmanial compounds in vitro. Antimicrob Agents Chemother 2001;45:3654-3656.
- 5. Chan MM, Bulinski JC, Chang KP, Fong D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol Res 2003;89:266-271.
- 6. Okuno T, Goto Y, Matsumoto Y, Otsuka H, Matsumoto Y. Applications of recombinant Leishmania amazonensis expressing egfp or the beta-galactosidase gene for drug screening and histopathological analysis. Exp Anim 2003;52:109-118.
- 7. Singh N, Gupta R, Jaiswal AK, Sundar S, Dube A. Transgenic Leishmania donovani clinical isolates expressing green fluorescent protein constitutively for rapid and reliable ex vivo drug screening. J Antimicrob Chemother 2009;64:370-374.
- 8. Varela M RE, Muñoz DL, Robledo SM, Kolli BK, Dutta S, Chang KP, Muskus C. Leishmania (Viannia) panamensis: An in vitro assay using the expression of GFP for screening of antileishmanial drug. Exp Parasitol 2009;122:134-139.
- 9. Singh N, Dube A. Short report: fluorescent Leishmania: application to anti-leishmanial drug testing. Am J Trop Med Hyg 2004;71:400-402.
- 10. Kain SR. Green fluorescent protein (GFP): Applications in cell-based assays for drug discovery. Drug Discov Today 1999;4:304-312.
- 11. Mehta SR, Huang R, Yang M, Zhang XQ, Kolli B, Chang KP, Hoffman RM, Goto Y, Badaro R, Schooley RT. Real-time in vivo green fluorescent protein imaging of a murine leishmaniasis model as a new tool for Leishmania vaccine and drug discovery. Clin Vaccine Immunol 2008;15:1764-1770.
- 12. Roy G, Dumas C, Sereno D, Wu Y, Singh AK, Tremblay MJ, Ouellette M, Olivier M, Papadopoulou B. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol 2000;110:195-206.
- 13. Shimony O, Jaffe CL. Rapid fluorescent assay for screening drugs on Leishmania amastigotes. J Microbiol Methods 2008;75:196-200.
- 14. Grimaldi G Jr, Tesh RB. Leishmaniases of the new world: Current concepts and implications for future research. Clin Microbiol Rev 1993;6:230-250.
- 15. Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004;27:305-318.
- 16. Rossi-Bergmann B, Lenglet A, Bezerra-Santos CR, Costa-Pinto D, Traub-Czeko YM. Use of fluorescent Leishmania for faster quantitation of parasite growth in vitro and in vivo. Mem Inst Oswaldo Cruz 1999;94:74.
- 17. Arrais-Silva WW, Pinto EF, Rossi-Bergmann B, Giorgio S. Hyperbaric oxygen therapy reduces the size of Leishmania amazonensis-induced soft tissue lesions in mice. Acta Trop 2006;98:130-136.
- 18. Boeck P, Bandeira Falcão CA, Leal PC, Yunes RA, Filho VC, Torres-Santos EC, Rossi-Bergmann B. Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg Med Chem 2006;14:1538-1545.
- 19. Colhone MC, Arrais-Silva WW, Picoli C, Giorgio S. Effect of hypoxia on macrophage infection by Leishmania amazonensis. J Parasitol 2004;90:510-515.
- 20. Naumov GN, Wilson SM, MacDonald IC, Schmidt EE, Morris VL, Groom AC, Hoffman RM, Chambers AF. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J Cell Sci 1999;112:1835-1842.
- 21. Wade-Martins R, Frampton J, James MR. Long-term stability of large insert genomic DNA episomal shuttle vectors in human cells. Nucleic Acids Res 1999;27:1674-1682.
- 22. Murphy NB, Muthiani AM, Peregrine AS. Use of an in vivo system to determine the G418 resistance phenotype of bloodstream-form Trypanosoma brucei brucei transfectants. Antimicrob Agents Chemother 1993;37:1167-1170.
- 23. Appel KF, Wolff AM, Arnau J. A multicopy vector system for genetic studies in Mucor circinelloides and other zygomycetes. Mol Genet Genomics 2004;271:595-602.
Fig. 1GFP-L. amazonensis promastigote fluorescence analysis. (A) The fluorescence signal plotted against promastigote counts. Data at 7 days (♦); 14 days (▪); 32 days (▴); 41 days (X) and 56 days of parasite culture (•). (B) The fluorescence signal plotted against the number of promastigotes treated with 100 µg/ml (♦), 150 µg/ml (▪) and 1 mg/ml (▴) of G418 or untreated (X). Fluorescence is reported in arbitrary fluorescence units. (C) Flow cytometry analyses of GFP-promastigotes 5 days (C1), 60 days (C2), 90 days (C3), 120 days (C4), 210 days (C5) after G418 selection and wild L. amazonensis promastigotes (C6). (D) Microscopic images of GFP-promastigotes. Phase contrast images of G418 selected GFP-promastigotes (D1) and non-selected GFP-promastigotes (D3). Fluorescence images of G418 selected GFP-promastigotes (D2) and non-selected GFP-promastigotes (D4). Magnification 400×.

Fig. 2GFP-L. amazonensis amastigote fluorescence analysis. (A) The number of GFP-amastigotes in vitro treated with 200 µg/ml (♦), 500 µg/ml (▴) and 1 mg/ml (▪) of G418. (B). The fluorescence signal plotted against the number of amastigotes cultured in vitro treated with 200 µg/ml (♦), 500 µg/ml (▪) and 1 mg/ml (▴) of G418. Fluorescence is reported in arbitrary fluorescence units. (C) Flow cytometry analyses of wild L. amazonensis amastigotes (C1), non-selected GFP-amastigotes (C2) and G418 selected GFP-amastigotes (C3). (D) Microscopic images of GFP-amastigotes. Phase contrast images of non-selected GFP-amastigotes (D2); 200 µg/ml G418 selected GFP-amastigotes (D4); 500 µg/ml G418 selected GFP-amastigotes (D6); 1 mg/ml G418 selected GFP-amastigotes (D8); Fluorescence images of non-selected GFP-amastigotes (D1); 200 µg/ml G418 selected GFP-amastigotes (D3); 500 µg/ml G418 selected GFP-amastigotes (D5); 1 mg/ml G418 selected GFP-amastigotes (D7). Magnification 400×.

Fig. 3Flow cytometry analyses of GFP-amastigotes after in vivo G418 selection. Flow cytometry of murine lesion derived GFP-amastigotes after: 15 days infection and 7 doses of saline treatment (A); 15 days infection and 7 doses of G418 treatment (B); 30 days infection and 5 doses of saline treatment (C); 30 days infection and 5 doses of G418 treatment (D); 30 days infection and 9 doses of saline treatment (E); 30 days infection and 9 doses of G418 treatment (F); 90 days infection and 3 doses of saline treatment (G); 90 days infection and 3 doses of G418 treatment (H); 90 days infection and 6 doses of saline treatment (I); 90 days infection and 6 doses of G418 treatment (J). G418 and saline were administered as described in Materials and Methods.

Fig. 4Infection of murine macrophages with GFP-L. amazonensis. (A) Number of amastigotes per macrophage and % of infected macrophages after infection with GFP-promastigotes. (B) The fluorescence signal of noninfected macrophages (control) and infected macrophages. Fluorescence is reported in arbitrary fluorescence units. (C) Microscopic images of infected macrophages. Fluorescence image (C1); Phase contrast image (C2) and Giemsa stained image (C3). Magnification 400×. (D) Flow cytometry of noninfected macrophages (D1) and infected macrophages (D2).