Abstract
The infection status of marine fish and cephalopods with Anisakis simplex third stage larva (L3) was studied over a period of 1 year. A total of 2,537 specimens, which consisted of 40 species of fish and 3 species of cephalopods, were purchased from the Cooperative Fish Market in Busan, Korea, from August 2006 to July 2007. They were examined for A. simplex L3 from the whole body cavity, viscera, and muscles. A. simplex L3 were confirmed by light microscopy. The overall infection rate reached 34.3%, and average 17.1 larvae were parasitized per infected fish. Fish that recorded the highest infection rate was Lophiomus setigerus (100%), followed by Liparis tessellates (90%), Pleurogrammus azonus (90%), and Scomber japonicus (88.7%). The intensity of infection was the highest in Gadus macrocephalus (117.7 larvae per fish), followed by S. japonicus (103.9 larvae) and L. setigerus (54.2 larvae). Although abundance of A. simplex L3 was not seasonal in most of the fish species, 10 of the 16 selected species showed the highest abundance in February and April. A positive correlation between the intensity of L3 infection and the fish length was obvious in S. japonicus and G. macrocephalus. It was likely that A. simplex L3 are more frequently infected during the spring season in some species of fish. Our study revealed that eating raw or undercooked fish or cephalopods could still be a source of human infection with A. simplex L3 in Korea.
-
Key words: Anisakis simplex, third stage larva (L3), fish, cephalopod, infection rate, abundance, intensity of infection, seasonality
INTRODUCTION
The third stage larvae (L3) of Anisakis simplex are prevalent in marine fish and cephalopods, and the most important etiologic agent of anisakidosis in humans. The marine fish and cephalopods act as a paratenic or transport host of A. simplex, so that the consumption of raw or insufficiently processed fish and cephalopods can cause anisakidosis.
The prevalence of
A. simplex larvae in fish and cephalopods has been known to be over 80%, although it varies upon fish species and location. Abollo et al. [
1] reported 100% prevalence in 5 species of fish, such as
Prionace glauca,
Belone belone,
Merluccius merluccius,
Lophius piscatorius, and
Scorpaena scrofa, although the overall mean intensity and abundance of
A. simplex L3 varied considerably among the investigated species. Costa et al. [
2] also found that the prevalence of L3 in
Aphanopus carbo,
Scomber japonicas, and
Trachurus picturatus ranged from 97.2% to 62.5% in Madeira, Portugal. The infection rate of
A. simplex in the fish and squids purchased in Bayuquan (Bohai Sea, China) was 63.4% and 14.8%, respectively [
3]. The infection rates of 8 of 19 fish species, however, were higher than 80% [
3]. After Chun et al. [
4] reported that anisakid infection was higher in fish from the southern sea than in those from the eastern sea, there were a few surveys on the prevalence of
A. simplex larvae in marine fish and cephalopods in Korea. Chai et al. [
5] collected an average of 35.6 anisakid larvae per yellow corvina (
Pseudosciaena manchurica), and almost all of them were found infected with
Anisakis type I (=
A. simplex) larvae. See eels (
Astroconger myriaster) and anchovies (
Engraulis japonicus) also showed 58.0% and 6.9% infection rates, respectively [
6-
8].
High prevalence of
A. simplex larvae in fish and cephalopods was realized by frequent occurrence of zoonotic problems due to these larvae [
9-
13]. Im et al. [
10] reported that 107 cases were diagnosed by gastrofiberscopy for 3 years in a local clinic at Cheju-do, Korea. They also demonstrated that important fish species from which the patients became infected were yellow corvina, sea eel, ling, cuttle fish, yellowtail, and others. It has also been reported that ingested larvae can cause allergic responses along with intestinal symptoms [
14,
15]. Moreover, the presence of anisakid larvae is not only applied as biological tags for marine fish, mammals, and invertebrate population studies, but also related to commercially important fish marketing because of the parasite removing costs [
1,
16].
Because of health consciousness, meat consumption in Korea drops remarkably while fish consumption is increasing year by year. This trend will bring about a rise of infectious diseases and is a tribute to parasite infection through marine fish consumption, especially A. simplex larvae. The infection status with A. simplex L3 will be a timely study subject to provide useful information for fish consumers under these circumstances.
The aim of the present work was to determine A. simplex larval infection status, including the infection rate, abundance, intensity, and seasonal variation according to the fish and cephalopod species and their size.
MATERIALS AND METHODS
Refrigerated fish and cephalopods were purchased on a monthly basis at Busan Cooperative Fish Market from August 2006 to July 2007. A total of 2,537 specimens, representing 40 species of fish and 3 species of cephalopods, were examined. The classification of fish and cephalopods was referred to web sites
http://portal.nfrdi.re.kr (National Fisheries Research & Development Institute, Korea) and
http://www.kunsan.ac.kr/fishes (College of Ocean Science and Technology, Kunsan University, Korea). The number of fish and cephalopods investigated in each month was represented in
Table 1.
Each sample of fish was weighed and measured. The whole body cavity and the viscera of each sample were carefully dissected and thoroughly examined for anisakids. Most larvae were examined directly under a light microscope, but some of anisakids were fixed in 70% ethanol and cleared in glycerin for identification of the species.
A. simplex L3 were identified based on the following morphological characters: (1) the shape and the presence of the boring tooth, (2) the shape of the tail and the presence of the mucron, and (3) the shape of the ventriculus [
5,
17]. The infection rate (IR=no. of fish positive/no. of fish examined ×100), abundance (A=total no. of larvae detected/no. of fish examined), and intensity of infection (I=total no. of larvae detected/no. of fish infected) were calculated according to Bush et al. [
18]. To study the correlation between size and infection intensity, we examined 4 specimens in each group of
S. japonicus and
G. macrocephalus, except for 3 specimens of 49-51 cm size group of
G. macrocephalus. We also analyzed seasonal variation of
A. simplex infection among 16 fish hosts secured more than 12 specimens per season. The studied species of fish and cephalopods were summarized in
Table 1. The Student's t-test was employed to determine the statistical significance.
RESULTS
A. simplex L3 infection by fish and cephalopod species
The overall infection rate with
A. simplex L3 was 34.3%, and the mean intensity and abundance were 17.1 and 5.9 larvae, respectively, per fish or cephalopod (
Table 1). There were many fish species which showed higher than 50% infection rate. The highest infection rate observed was 100% in
Lophiomus setigerus, followed by 90% in
Pleurogrammus azonus and
Liparis tessellatus, 88.7% in
S. japonicas, and 84.6% in
Gadus macrocephalus. The lowest infection rates were found in
Mugil cephalus,
Sepia esculenta,
Larimichthys crocea,
Sebastes pachycephalus,
Eptatretus burgeri,
Hemiramphus sajori, and
Liparis kanakai, which ranged from 0.8% to 8.9%.
The intensity of infection was the highest in
G. macrocephalus (117.7 larvae per fish), followed by
S. japonicus (103.9 larvae) and
L. setigerus (54.2 larvae).
A. simplex L3 was not detected in 5 fish species,
Pseudosciaena crocea,
Cynoglossus semilaevis,
Konosirus punctatus,
Oplegnathus fascitus, and
Chromis notata, and 1 cephalopod species, Bleeker's squid
Loligo bleekeri (
Table 1).
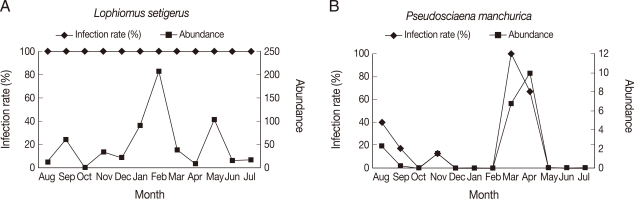
Among the selected 16 species for the study of seasonal dynamics, 10 showed the highest abundance in February and April, including
L. setigerus,
S. japonicus,
S. niphonius,
A. myriaster,
S. inermis,
T. kitaharai,
P. manchurica,
P. sinensis,
E. burgeri, and
L. tanakai.
L. setigerus along with
S. japonicus and
T. kitaharai showed the highest abundance in February (
Fig. 1A).
P. manchurica revealed the highest abundance in April, including
S. niphonius,
A. myriaster,
S. inermis,
P. sinensis,
E. burgeri, and
L. tanakai (
Fig. 1B). No seasonal variation was recognized in the remaining 6 species. Abundance of some hosts was as high as 99.6 in
G. macrocephalus, 92.2 in
S. japonicus, 54.2 in
L. setigerus, 22.1 in
P. azonus, 16.9 in
T. lepturus, 13.1 in
P. indicus, and 11.3 in
S. niphonius all the year round, while the others showed very low rates (0.01-7.2) throughout the year.
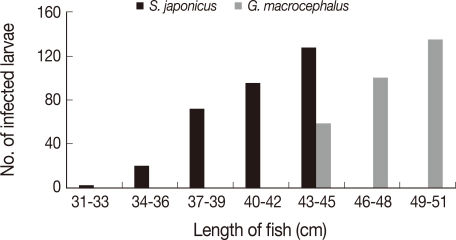
A significant positive correlation was found in
S. japonicus and
G. macrocephalus (
Fig. 2). However, this correlation was not apparent in fish which do not change greatly in their length and weight after they are fully grown.
DISCUSSION
We investigated the occurrence and infection dynamics of A. simplex L3 in 40 species of fish and 3 species of cephalopods. A total of 2,537 fish and cephalopods were purchased from the Busan Cooperative Fish Market. Busan Cooperative Fish market is a suitable place for investigation of marine fish infection since the market is the biggest in fish sales scale in Korea and sales over 3,200 ton fishes a day on commission.
The overall infection rate of
A. simplex L3 in marine fish and cephalopods was 34.3% and the mean infection intensity 17.1 larvae per fish. The infection rate, however, varied greatly depending on the species of fish, ranging from 0% to100%. This result was considered to mean that some species of fish show higher affinity to
A. simplex than others. Fish showing high infection rate exhibit features of flabby flesh and high fat contents. Frog fish (
Lophiomus setigerus) showed the highest infection rate as 100%. They have a very large head with a large mouth that catch a lot of fish at a time. The voracious appetite of the frog fish can be a reason of the high infection rate with
A. simplex larvae. Abollo et al. [
1] also reported 100% infection rate in
Lophius piscatorius in Galician waters, which is similar species to
L. setigerus. Fish species which show especially high infection level were Korean favorite fish, such as
T. lepturus,
S. niphonius,
G. macrocephalus,
Theragra chalcogramma,
P. azonus, and
S. japonicus. These fishes are almost served at table daily. Although the main habitat of the larva is the viscera, health risk is still present due to postmortem migration of larvae and salted fish viscera eating habit of Korean people.
Six fish species, such as P. crocea, C. semilaevis, K. punctatus, O. fasciatus, C. notate, and L. bleekeri, were not infected with A. simplex larvae. S. pachycephalus, E. burgeri, H. sajori, and L. tanakai fish also showed very low infection rates. These fish had several features in common, like a small size, hard flesh, and narrow cavity.
Drawing special attention is the infection status of the sea eel (
Astroconger myriaster), which was suspected as one of the most important fish host for human anisakidosis in Korea [
6,
19]. The present study showed that the infection rate and intensity of the sea eel was 76.5% and 16.2, respectively. These data suggest that the sea eel still could be an important fish host of anisakidosis in Korea. The infection rates of other Korean favorite raw fish species, such as
Sebastes inermis,
Engraulis japonicas, and
Pampus argenteus were also considerably high (42.5-75.6%). Another favorite raw fish,
Mugil cephalus, however, showed a comparatively low rate (8.9%).
Konosirus punctatus was infected with different species of nematodes except for
A. simplex. We also examined 3 species of cephalopods, including
Todarodes pacificus,
Sepia esculenta, and
L. bleekeri.
T. pacificus showed the highest infection rate, which is eaten raw most frequently.
Chun et al. [
4] investigated the infection rate of
Anisakis-like larvae from 17 species of marine fish from the Yellow Sea and the southern coast of Korea. All of the 313 examined samples were infected with
Anisakis-like larvae. Both prevalence and intensity of the Chun's report were low in
Trachurus japonicus,
Pseudostiaena manchurica,
Trichiurus lepturus and
Liparis tanakai compared to the present study. These differences seem to be caused by the size of fish examined. Our subject fish of
S. japonicus was bigger than those of Chun's (31-43 cm to 24-40 cm). The positive correlation between size and infection level was also found in
G. macrocephalus. There was no difference of infection rate in fish which do not show significant difference in length even after they are grown-up.
Ma et al. [
3] reported the infection status of
A. simplex larvae in marine fish and cephalopods from the Bohai Sea, China. When compared to the present study, infection rate and density of
Scomberomorus niphonius,
Sepia esculenta, and
Cynoglossus semilaevis was similar among each other. But the infection rate of
Lateolabrax japonicus was greatly different although the infection density was high in both reports. These differences can be attributed to many factors, such as collecting sites, fish size or numbers and detecting methods.
To investigate the seasonal variation of larval infection, we examined the 15 species of fish and 1species of cephalopods for a year by month. Ten species of fish showed abundance peak in February or April. But there was no tendency of seasonal variation in other species of fish. Abundance of some hosts was high all the year round, while the others showed very low infection rates throughout the year. Strømnes and Andersen [
20] used the term "spring rise" to describe the significant increase in the abundance of
A. simplex L3 during the springtime of March and April. Seasonal variations in infection levels are considered due to changes in the population of the infected euphausiids in the zooplankton according to increase of water temperature [
21]. Besides, additional supply of eggs and larvae in the study area due to seasonal migration of whales and migration of fish should have deliberated [
20]. The distinct abundance peak of
A. simplex L3 in April and February can be attributed the same factors. But it is not feasible to explain the seasonality of
A. simplex larval infection without considering other important factors such as physicochemical conditions, reproductive circumstances, social conditions, coactive circumstances or combination of these factors in aquatic environments, as Stavn [
22] pointed out.
Our results show that the infection rates of A. simplex L3 were still high in Korean favorite fish, including such raw fish species as sea eel. The current data also reveal information regarding A. simplex L3 infection of some fish in Korean waters, which have never been reported previously. The positive correlation between the host size and infection intensity was obvious in S. japonicus and G. macrocephalus. Some host species exhibited a seasonal pattern of abundance at February and April.
Our results clarified that eating of fish could still be the source of infection with A. simplex L3 of humans in Korea. A. simplex L3 are more likely to be infected during spring time in some species of fish. These results could be significant findings because these fish species are most widely served side dishes of Korean as well as high commercial value. These data of A. simplex L3 larva infection in marine fish and cephalopods will provide information for prevention of anisakidosis and regulation of marine fish distribution process and sales.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-E00037).
References
- 1. Abollo E, Gestal C, Pascual S. Anisakis infestation in marine fish and cephalopods from Galician waters: an updated perspective. Parasitol Res 2001;87:492-499.
- 2. Costa G, Pontes T, Mattiucci S, D'Amélio S. The occurrence and infection dynamics of Anisakis larvae in the black-scabbard fish, Aphanopus carbo, chub mackerel, Scomber japonicus, and oceanic horse mackerel, Trachurus picturatus from Madeira, Portugal. J Helminthol 2003;77:163-166.
- 3. Ma HW, Jiang TJ, Quan FS, Chen XG, Wang HD, Zhang YS, Cui MS, Zhi WY, Jiang DC. The infection status of anisakid larvae in marine fish and cephalopods from the Bohai Sea, China and their taxonomical consideration. Korean J Parasitol 1997;35:19-24.
- 4. Chun SK, Chung BK, Ryu BS. Studies on Anisakis sp. (1) On the infection state of Anisakis-like larvae isolated from various marine fishes. Korean J Fish Aquat Sci 1968;1:1-6. (in Korean).
- 5. Chai JY, Chu YM, Sohn WM, Lee SH. Larval anisakids collected from the yellow corvina in Korea. Korean J Parasitol 1986;24:1-11.
- 6. Chai JY, Cho SR, Kook J, Lee SH. Infection status of the sea eel (Astroconger myriaster) purchased from the Noryangjin fish market with anisakid larvae. Korean J Parasitol 1992;30:157-162.
- 7. Kim KH, Joo KH, Rim HJ. A study about infection state of anisakis larvae and parasitic helminths in salmon (Onchorhynchus keta) and sea trout (Oncorhynchus masou) caught from Taepo port, Kangwon Do. Korean J Rural Med 1990;15:27-32.
- 8. Song SB, Lee SR, Chung HH, Han NS. Infection status of anisakid larvae in anchovies purchased from local fishery market near southern and eastern sea in Korea. Korean J Parasitol 1995;33:95-99.
- 9. Bouree P, Paugam A, Petithory JC. Anisakidosis: report of 25 cases and review of the literature. Comp Immunol Microbiol Infect Dis 1995;18:75-84.
- 10. Im KI, Shin HJ, Kim BH, Moon SI. Gastric anisakiasis cases in Cheju-do, Korea. Korean J Parasitol 1995;33:179-186.
- 11. Noh JH, Kim B, Kim SM, Ock M, Park MI, Goo JY. A case of acute gastric anisakiasis provoking severe clinical problems by multiple infections. Korean J Parasitol 2003;41:97-100.
- 12. Kim SG, Jo YJ, Park YS, Kim SH, Song MH, Lee HH, Kim JS, Ryou JW, Joo JE, Kim DH. Four cases of gastric submucosal mass suspected as anisakiasis. Korean J Parasitol 2006;44:81-86.
- 13. Yoon SW, Yu JS, Park MS, Shim JY, Kim HJ, Kim KW. CT findings of surgically verified acute invasive small bowel anisakiasis resulting in small bowel obstruction. Yonsei Med J 2004;45:739-742.
- 14. Alonso A, Daschner A, Moreno-Ancillo A. Anaphylaxis with Anisakis simplex in the gastric mucosa. N Engl J Med 1997;337:350-351.
- 15. Arrieta I, del Barrio M, Vidarte L, del Pozo V, Pastor C, Gonzalez-Cabrero J, Cárdaba B, Rojo M, Mínguez A, Cortegano I, Gallardo S, Aceituno E, Palomino P, Vivanco F, Lahoz C. Molecular cloning and characterization of an IgE-reactive protein from Anisakis simplex: Ani s 1. Mol Biochem Parasitol 2000;107:263-268.
- 16. Mattiucci S. Parasites as biological tags in population studies of demersal and pelagic fish species. Parassitologia 2006;48:23-25.
- 17. Smith JW. Anisakis simplex (Rudolphi, 1809, det. Krabbe, 1878) (Nematoda: Ascaridoidea): morphology and morphometry of larvae from euphausiids and fish, and a review of the life-history and ecology. J Helminthol 1983;57:205-224.
- 18. Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997;83:575-583.
- 19. Lee EJ, Kim YC, Jeong HG, Lee OJ. The Mucosal changes and influencing factors in upper gastrointestinal anisakiasis: analysis of 141 cases. Korean J Gastroenterol 2009;53:90-97.
- 20. Strømnes E, Andersen K. Spring rise of whaleworm (Anisakis simplex; Nematoda, Ascaridoidea) third-stage larvae in some fish species from Norwegian waters. Parasitol Res 2000;86:619-624.
- 21. Andersen K. Hysterothylacium aduncum (Rudolphi, 1862) infection in cod from the third- and fourth-stage larvae as well as worms. Parasitol Res 1993;79:67-72.
- 22. Stavn RH. The horizontal-vertical distribution hypothesis: Langmuir circulations and Daphnia distributions. Limnol Oceanogr 1971;16:453-466.
Fig. 1Seasonal variation of Anisakis simplex L3 infection in Lophiomus setigerus (A) and Pseudosciaena manchurica (B). Three species of fish, including L. setigerus, showed the highest infection level in February and 7 species of fish, including P. manchurica, reached their peaks in April.
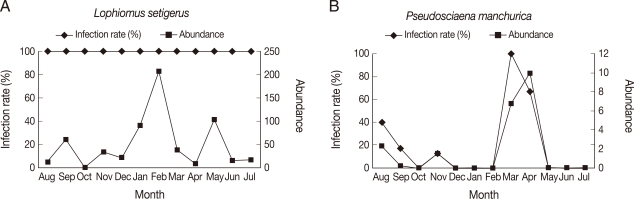
Fig. 2Positive correlations between the length of fish and infection intensity of Anisakis simplex L3 in Scomber japonicus and Gadus macrocephalus.
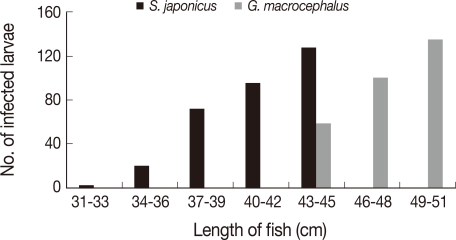
Table 1.Infection status of Anisakis simplex L3 in fish and cephalopods purchased from the Cooperative fish market in Busan, Korea
Table 1.
|
Species |
No. of fish positive |
Length (cm)a
|
Weight (g)b
|
IR (%)c
|
Id
|
Ae
|
|
Lophiomus setigerusf
|
53 (53) |
35.7 ± 4.0 |
566.5 ± 217.7 |
100.0 |
54.2 |
54.2 |
|
Liparis tessellates
|
10 (9) |
43.3 ± 5.7 |
957.1 ± 357.8 |
90.0 |
8.1 |
7.2 |
|
Pleurogrammus azonus
|
30 (27) |
40.3 ± 4.0 |
630.2 ± 184.8 |
90.0 |
24.6 |
22.1 |
|
Scomber japonicusf
|
53 (47) |
35.4 ± 2.9 |
469.2 ± 128.5 |
88.7 |
103.9 |
92.2 |
|
Gadus macrocephalus
|
13 (11) |
46.2 ± 2.7 |
1,091.4 ± 159.6 |
84.6 |
117.7 |
99.6 |
|
Scomberomorus niphoniusf
|
48 (40) |
45.7 ± 3.1 |
539.0 ± 108.2 |
83.3 |
13.2 |
11.3 |
|
Theragra chalcogramma
|
40 (33) |
45.7 ± 4.8 |
582.6 ± 183.3 |
82.5 |
6.6 |
5.4 |
|
Clupea pallasii valenciennesf
|
68 (54) |
28.3 ± 2.1 |
168.8 ± 50.0 |
79.4 |
5.4 |
4.3 |
|
Astroconger myriasterf
|
68 (52) |
53.1 ± 8.9 |
238.3 ± 172.4 |
76.5 |
14.3 |
10.9 |
|
Sebastes inermisf
|
78 (59) |
21.6 ± 1.7 |
173.4 ± 41.8 |
75.6 |
5.4 |
4.1 |
|
Platycephalus indicus
|
32 (24) |
41.8 ± 4.7 |
455.2 ± 151.7 |
75.0 |
17.5 |
13.1 |
|
Trichiurus lepturus
|
47 (31) |
78.4 ± 7.8 |
281.5 ± 99.6 |
66.0 |
25.6 |
16.9 |
|
Engraulis japonicas
|
49 (30) |
13.6 ± 1.2 |
15.9 ± 4.9 |
61.2 |
1.6 |
1.0 |
|
Trachurus japonicusf
|
111 (64) |
23.4 ± 4.7 |
129.1 ± 85.3 |
57.7 |
8.8 |
5.1 |
|
Tanakius kitaharaif
|
79 (41) |
25.8 ± 2.7 |
156.7 ± 55.0 |
51.9 |
2.6 |
1.4 |
|
Scombrops boops
|
13 (6) |
25.4 ± 4.0 |
191.7 ± 35.2 |
46.2 |
8.0 |
3.7 |
|
Miichthys miiuy
|
11 (5) |
37.9 ± 4.2 |
421.6 ± 212.3 |
45.5 |
2.4 |
1.1 |
|
Arctoscopus japonicus
|
86 (38) |
19.9 ± 3.3 |
59.3 ± 24.0 |
44.2 |
1.1 |
0.5 |
|
Doederleinia berycoidesf
|
165 (73) |
18.6 ± 3.0 |
100.2 ± 52.8 |
44.2 |
2.1 |
0.9 |
|
Seriola quinqueradiata
|
34 (15) |
39.2 ± 6.0 |
642.9 ± 250.8 |
44.1 |
5.6 |
2.5 |
|
Pampus argenteusf
|
87 (37) |
20.8 ± 4.0 |
133.3 ± 62.4 |
42.5 |
1.0 |
0.4 |
|
Pagrus major
|
17 (7) |
30.8 ± 4.5 |
477.7 ± 275.4 |
41.2 |
2.6 |
1.1 |
|
Takifugu chinensis
|
16 (6) |
30.1 ± 4.2 |
516.4 ± 223.0 |
37.5 |
1.0 |
0.4 |
|
Pseudosciaena manchuricaf
|
106 (36) |
23.3 ± 3.5 |
152.4 ± 196.9 |
34.0 |
9.2 |
3.1 |
|
Semicossyphus reticulates
|
54 (14) |
23.2 ± 4.2 |
210.5 ± 67.8 |
25.9 |
1.0 |
0.3 |
|
Platyrhina sinensisf
|
48 (11) |
33.8 ± 8.6 |
324.7 ± 248.9 |
22.9 |
0.4 |
0.1 |
|
Todarodes pacificusf,g
|
82 (18) |
52.4 ± 6.0 |
340.3 ± 102.1 |
22.0 |
14.1 |
2.5 |
|
Stephanolepis cirrhifer
|
24 (5) |
20.8 ± 2.2 |
118.0 ± 36.3 |
20.8 |
1.4 |
0.3 |
|
Paralichthys olivaceus
|
47 (9) |
28.1 ± 6.1 |
279.2 ± 210.5 |
19.1 |
1.0 |
0.2 |
|
Ilisha elongate
|
18 (3) |
41.8 ± 4.1 |
480.4 ± 99.0 |
16.7 |
1.3 |
0.2 |
|
Mugil cephalus
|
45 (4) |
38.4 ± 8.6 |
480.5 ± 342.9 |
8.9 |
1.0 |
0.02 |
|
Sepia esculentag
|
36 (3) |
30.7 ± 8.2 |
302.7 ± 103.3 |
8.3 |
1.0 |
0.08 |
|
Larimichthys crocea
|
28 (2) |
27.9 ± 1.2 |
226.1 ± 28.2 |
7.1 |
4.0 |
0.3 |
|
Sebastes pachycephalus
|
39 (1) |
27.2 ± 1.5 |
277.8 ± 67.3 |
2.6 |
1.0 |
0.03 |
|
Eptatretus burgerif
|
70 (1) |
39.0 ± 9.0 |
82.8 ± 55.1 |
1.4 |
1.0 |
0.01 |
|
Hemiramphus sajorif
|
178 (2) |
25.2 ± 3.3 |
37.3 ± 17.8 |
1.1 |
1.0 |
0.01 |
|
Liparis tanakaif
|
126 (1) |
33.0 ± 1.0 |
158.5 ± 21.3 |
0.8 |
1.0 |
0.01 |
|
Pseudosciaena crocea
|
74 (11) |
28.9 ± 2.1 |
203.7 ± 60.7 |
0 |
0 |
0 |
|
Cynoglossus semilaevis |
66 (4) |
33.4 ± 3.8 |
173.1 ± 65.7 |
0 |
0 |
0 |
|
Konosirus punctatus
|
174 (7) |
20.6 ± 3.5 |
82.0 ± 49.1 |
0 |
0 |
0 |
|
Loligo bleekerig
|
69 (1) |
37.7 ± 7.5 |
116.1 ± 30.6 |
0 |
0 |
0 |
|
Oplegnathus fasciatus
|
13 (0) |
18.5 ± 1.6 |
148.3 ± 23.5 |
0 |
0 |
0 |
|
Chromis notate
|
32 (0) |
13.3 ± 1.6 |
36.2 ± 15.7 |
0 |
0 |
0 |