Abstract
Fishborne trematode (FBT) metacercariae were investigated in fish from 3 Provinces of Lao PDR. Total 242 freshwater fish of 40 species were collected in local markets of Luang Prabang (59 fish of 16 species), Khammouane (81 fish of 19 species), and Saravane (97 fish of 14 species), and each of them was examined by artificial digestion method. Four species of metacercariae (Opisthorchis viverrini, Haplorchis taichui, Haplorchis yokogawai, and Centrocestus formosanus) were detected. O. viverrini was detected in 35 fish (14.5%), and their density was 252 per infected fish (Luang Prabang, 88 metacercariae in 5 fish; Khammouane, 187 in 6 fish; Saravane, 303 in 24 fish). H. taichui was found in 102 fish (42.1%), and their density was 485 per infected fish (Luang Prabang, 260 metacercariae in 38 fish; Khammouane, 1,084 in 23 fish; Saravane, 359 in 41 fish). H. yokogawai was detected in 92 fish (38.0%), and their density was 222 per infected fish (Luang Prabang, 362 metacercariae in 17 fish; Khammouane, 126 in 20 fish; Saravane, 214 in 55 fish). Metacercariae of C. formosanus were found in 8 fish (3.3%), and their density was 3 per infected fish. In the present study, it has been confirmed that FBT metacercariae, in particular, H. taichui, H. yokogawai, and O. viverrini, are highly prevalent in fish from Luang Prabang, Khammouane, and Saravane Province, Lao PDR.
-
Key words: Opisthorchis viverrini, Haplorchis taichui, Haplorchis yokogawai, Centrocestus formosanus, fishborne trematode, prevalence, density, metacercaria, Lao PDR
INTRODUCTION
Fishborne trematode (FBT) infection is a public health problem in some Asian countries, including Lao People's Democratic Republic (Lao PDR), Vietnam, Cambodia, and Thailand, where these flukes provoke remarkable morbidity and cause serious damages to aquaculture. Human infections by FBTs are almost entirely caused by habitual consumption of raw fish containing infective larvae, i.e., metacercariae. These infections are highly localized depending on the food habits of residents and on the presence of susceptible snail hosts. Moreover, FBTs show low host-specificity, and many kinds of reservoir hosts can contribute to the maintanence of the life cycle, making it extremely difficult to control these parasite infections in the epidemiological points of view [
1,
2].
Lao PDR is located in the middle of Southeast Asia, and has the Mekong River flow through the whole country from north to south. Laotian people residing in the Mekong River basin, which occupy a quarter of the territory, have some unique food habits. Some of them like to eat dishes containing raw freshwater fish, and are easily infected with FBT [
3,
4].
It has been reported that many Laotian people are infected with trematodes such as
Opisthorchis viverrini, heterophyids, echinostomatids, and lecithodendriids [
5-
7]. Some investigators have previously reported that FBTs, including
O. viverrini, are prevalent in Lao PDR [
8-
14]. Metacercarial infections were also investigated in fish intermediate hosts from some local areas in Lao PDR by some workers. Schotz et al. [
15] surveyed freshwater fishes from rice fields around Vientiane Municipality and Nam Ngum water reservoir. Freshwater fish from Savannakhet Province and Vientiane Municipality were examined for their metacercarial infections by Rim et al. [
16]. However, studies on the second intermediate hosts of FBT were performed in limited areas and, therefore, studies on more wide areas are needed to know the variety of fish intermediate hosts for FBT in Lao PDR. Therefore, we performed field surveys to determine the infection status of freshwater fish with FBT metacercariae caught in 3 Provinces, i.e., Luang Prabang, Khammouane, and Saravane, in Lao PDR.
MATERIALS AND METHODS
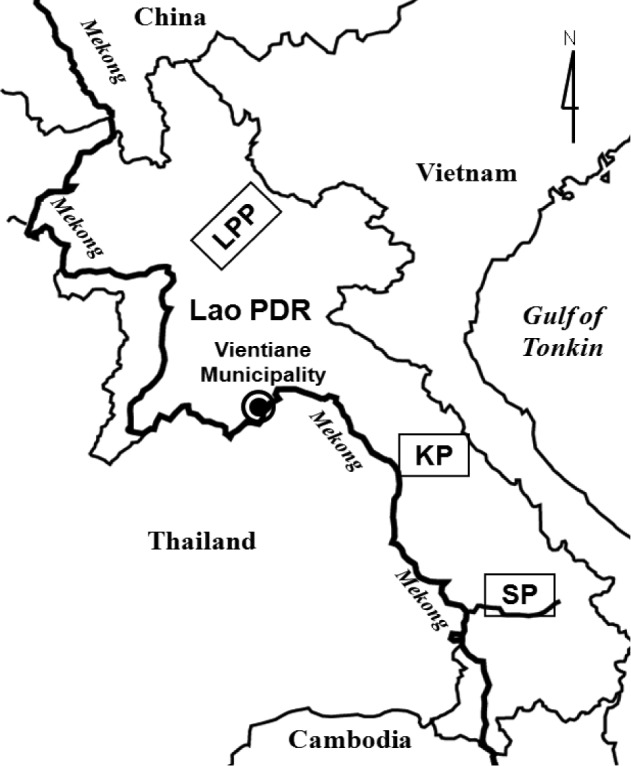
We collected a total of 242 freshwater fish of 40 species in local markets of Khammouane Province (81 fish of 14 species) in March 2003, Saravane Province (97 fish of 13 species) in November 2003, and Luang Prabang (59 fish of 16 species) in February 2004 (
Fig. 1). All collected fish were transferred to the laboratories (on-the-spot local laboratories in Lao PDR and then to the Department of Parasitology, Gyeongsang National University School of Medicine) and identified the fish species with the aid of ichthyologists in Lao PDR and FishBase site in internet (
Table 1) [
17]. Individual fish was finely ground with a mortar with pestle or a grinder, and the ground fish meat was mixed with artificial gastric juice. The mixture was incubated at 36℃ for 2-3 hr. The digested material was filtered through 1×1 mm mesh, and washed with 0.85% saline until the supernatant became clear. The sediment was carefully examined using a stereomicroscope and then the metacercariae of each FBT species were separately collected based on their general features. The collected metacercariae were categorized according to their measurements and morphologic characters, and then the infection rate and the intensity of infection were calculated according to the fish species.
RESULTS
Opisthorchis viverrini metacercariae
O. viverrini metacercariae were detected in 4 fish species (25.0%), i.e.,
Neolissochilus stracheyi,
Cyclocheilichthys apogon,
Hypsibarbus pierrei, and
Cyclocheilichthys furcatus, from Luang Prabang. Their infection rate was 38.5% and the average metacercarial density was 88 per infected fish. Total 6 (31.6%) out of 19 fish of 5 species (26.3%), i.e.,
Onychostoma fusiforme,
Puntius brevis,
Cyclocheilichthys armatus,
Hampala dispar, and
Hypsibarbus wetmorei, from Khammouane were infected with av. 187 metacercariae per fish. A total of 24 fish (42.1%) of 6 species (46.2%), i.e.,
H. dispar,
Puntioplites proctozysron,
Poropuntius dearatus,
P. brevis,
Paralaubuca barroni, and
Cyclocheilichthys enoplos, from Saravane were infected with av. 303 metacercariae per fish. The status of metacercarial infection by surveyed areas and fish species are designated in
Table 2.
Haplorchis taichui metacercariae
H. taichui metacercariae were detected in 10 fish species (62.5%) from Luang Prabang, and their infection rate was 86.4% and the average metacercarial density was 260 per infected fish. Total 23 (79.3%) out of 29 fish of 5 species (26.3%) from Khammouane were infected with av. 1,084 metacercariae per fish. A total of 41 fish (54.7%) of 9 species (64.3%) from Saravane were infected with av. 359 metacercariae per fish. The infection status by surveyed areas and fish species are shown in
Table 3.
Haplorchis yokogawai metacercariae
H. yokogawai metacercariae were detected in 6 fish species (37.5%) from Luang Prabang, and their infection rate was 70.8% and the average metacercarial density was 362 per infected fish. Total 20 (66.7%) out of 30 fish of 5 species (26.3%) from Khammouane were infected with av. 126 metacercariae. A total of 55 fish (74.3%) of 8 species (57.1%) from Saravane were infected with av. 214 metacercariae per fish. The infection status by surveyed areas and fish species are presented in
Table 4.
Centrocestus formosanus metacercariae
Only 1
C. formosanus metacercaria was detected in 1 (25.0%) of 5
Physoschistura meridionalis from Luang Prabang. No metacercariae were detected in fish from Khammouane. Total 7 fish (29.2%) of 3 species (21.4%), i.e.,
Mystacoleucus greenwayi,
P. proctozysron, and
Hypsibarbus pierrei, from Saravane were infected with av. 3 metacercariae per fish. The infection status by surveyed areas and fish species are shown in
Table 5.
DISCUSSION
In the present study, mainly 4 species of FBT metacercariae, i.e.,
O. viverrini,
H. taichui,
H. yokogawai, and
C. formosanus, were detected in freshwater fish from 3 Provinces, i.e., Luang Prabang, Khammouane, and Saravane, in Lao PDR. Schotz et al. [
15] found 5 species of trematode metacercariae,
O. viverrini,
H. taichui,
H. pumilio,
Stellantchasmus falcatus, and
C. formosanus, in freshwater fishes from rice fields around Vientiane Municipality and Nam Ngum water reservoir, Lao PDR [
15]. Rim et al. [
16] also detected 2 (
O. viverrini and
H. taichui) and 4 (
O. viverrini,
H. taichui,
H. yokogawai and
C. formosanus) species of metacercariae in freshwater fish from Savannakhet Province and Vientiane Municipality, respectively [
16]. On the other hand, Schotz et al. [
15] examined 782 freshwater fish of 45 species by the flesh compression method [
15]. Rim et al. [
16] examined 156 freshwater fish of 17 species in Savannakhet and 177 fish of 12 species in Vientiane Municipality. They mainly used the artificial digestion method with the exception of a few fish from Savannakhet which were examined by the flesh compression method [
16]. In the present study, we examined a total of 242 fish of 40 species from Luang Prabang, Khammouane, and Saravane Province by the artificial digestion method.
As the second intermediate hosts for
O. viverrini, 15 species of freshwater fish, i.e.,
Barbonymus gonionotus,
C. armatus,
Cyclocheilichthys repasson,
Esomus metallicus,
H. dispar,
Hampala macrolepidota,
Hypsibarbus lagleri,
Labiobarbus lineatus,
Mystacoleucus marginatus,
Onychostoma elongatum,
Osteochilus hasseltii,
Puntioplites falcifer,
P. proctozystron,
P. brevis, and
Puntius orphoides, have been reported in Thailand [
18,
19] and Lao PDR [
15,
16]. In the present study,
O. viverrini metacercariae were detected in 4 fish species,
C. apogon,
C. furcatus,
H. pierrei, and
N. stracheyi, from Luang Prabang, in 5 fish species,
C. armatus,
H. dispar,
H. wetmorei,
O. fusiforme and
P. brevis from Khammouane, and 6 fish species,
C. enoplos,
H. dispar,
P. barroni,
P. dearatus,
P. proctozysron, and
P. brevis from Saravane Province. Accordingly, 9 fish species,
C. apogon,
C. enoplos,
C. furcatus,
H. pierrei,
H. wetmorei,
N. stracheyi,
O. fusiforme,
P. barroni, and
P. dearatus, are newly recorded as the second intermediate hosts for
O. viverrini by the present study.
The infection rates and intensities of
O. viverrini metacercariae were relatively low in fish from 3 Provinces in the present study. However, 3 particular fish species,
C. apogon from Luang Prabang,
P. brevis from Khammouane, and
H. dispar from Saravane Province, were heavily infected with
O. viverrini metacercariae. Among them,
P. brevis and
H. dispar were already known as highly suitable fish hosts for
O. viverrini in a previous study [
16]. These 3 fish species together with another 2, C. armatus and
O. elongatum, are highly dangerous due to the high intensity of metacercariae when they were eaten raw by humans. The liver flukes, like
O. viverrini, provoke severe pathological changes in the bile duct, such as dilatation, wall thickening, inflammation and mucosal hyperplasia, and the cirrhotic changes of the liver [
18]. Moreover, they act as a risk factor for development of cholangiocarcinoma in humans [
20,
21].
H. taichui metacercariae have been recorded in fish from some Asian counties, i.e., India, China, Tailand, the Philippine, and Lao PDR. They have been detected in 35 fish species, i.e.,
Abbottina rivularis,
Amblypharyngodon mola,
B. gonionotus,
Carassius auratus,
Channa striata,
Chanodichthys dabryi,
Cirrhinus molitorella,
Culter recurviceps,
C. armatus,
C. repasson,
Cyprinus carpio,
H. dispar,
H. macrolepidota,
Hemibarbus maculates,
Hemiculter leucisculus,
Hypophthalmichthys molitrix,
L. leptocheila,
Labeo ariza,
Labeo bata,
Metzia lineata,
M. marginatus,
O. elongatum,
Opsariichythys bidens,
Pseudohemiculter dispar,
P. falcifer,
Puntius binotatus,
P. brevis,
P. orphoides,
Puntius semifasciolatus,
Puntius sophore,
Saurogobio dabryi,
Spratellicypris palata,
Squalidus argentatus, and
Toxabramis houdemeri, in endemic countries [
15,
16,
22-
26]. In the present study,
H. taichui metacercariae were found in 9 fish species,
B. gonionotus,
C. apogon,
C. furcatus,
Henicorhynchus siamensis,
L. rhabdoura,
Mekongina erythrospila,
Physoschistura meridionalis,
Scaphognathops bandanensis, and
Sinibrama melrosei, from Luang Prabang, in 5 fish species,
Crossocheilus oblongus,
C. repasson,
H. lagleri,
O. fusiforme, and
P. brevis, from Khammouane, and 9 fish species,
C. enoplos,
E. metallicus,
H. dispar,
L. rahabdoura,
Mystacoleucus greenwayi,
P. barroni,
P. dearatus,
P. proctozysron, and
P. orphoides, from Saravane Province. Therefore, 13 fish species,
C. oblongus,
C. apogon,
C. enoplos,
C. furcatus,
E. metallicus,
H. lagleri,
H. siamensis,
L. rhabdoura,
M. erythrospila,
M. greenwayi,
P. meridionalis,
S. bandanensis, and
S. melrosei, are added as new second intermediate hosts for
H. taichui by the present study.
The infection status of
H. taichui metacercariae was relatively high in fish from all 3 Provinces. Infection rates of fish from Luang Prabang (86.4%) and Khammouane (79.3%) were very high, and that of fish from Saravane (54.7%) was moderate. Intensities of
H. taichui metacercariae per infected fish were 260, 1,084, and 359 in fish from Luang Prabang, Khammouane, and Saravane Province, respectively. Particularly 3 fish species,
S. bandanensis from Luang Prabang,
H. lagleri from Khammouane, and
H. dispar from Saravane Province, were heavily infected with
H. taichui metacercariae, more than 1,000 in number. On the other hand, Chai et al. [
6] demonstrated that the worm burden of
H. taichui was remarkably high in residents of Saravane Province [
6]. Based on these findings and high infection rates and intensities of
H. taichui metacercariae in the present study, we could speculate that residents in Khammouane and Luang Prabang may be heavily infected with
H. taichui like Saravane.
Distribution of
H. yokogawai metacercariae has been reported in some Asian countries such as India, Thailand, and Lao PDR. They were found in 28 fish species,
A. mola,
B. gonionotus,
Channa punctatus,
C. molitorella,
C. armatus,
C. repasson,
Cyprinus carpio,
Glossogobius giuris,
H. dispar,
Hypsibarbus wetmorei,
H. lagleri,
Labeo ariza,
L. bata,
L. leptocheila,
Mystus vittatus,
Nandus nandus,
Ompok bimaculatus,
Oreochromis niloticus niloticus,
O. elongatum,
O. hasseltii,
Osteochilus lini,
P. binotatus,
Puntius chola,
Puntius leiacanthus,
P. orphoides,
Puntius sarana,
P. sophore, and
Tilapia zillii [
16,
23,
25,
27-
31]. In the present study,
H. yokogawai metacercariae were detected in 6 fish species,
C. apogon,
C. furcatus,
L. rhabdoura,
M. ectypus,
S. bandanensis, and
S. melrosei, from Luang Prabang, 5 fish species,
C. oblongus,
C. armatus,
C. repasson,
H. lagleri and
O. fusiforme, from Khammouane, and 8 fish species,
C. enoplos,
H. dispar,
H. pierrei,
M. greenwayi,
P. barroni,
P. dearatus,
P. proctozysron, and
P. brevis, from Saravane. Among them, 15 fish species, i.e.,
C. oblongus,
C. apogon,
C. enoplos,
C. furcatus,
H. pierrei,
L. rhabdoura,
M. ectypus,
M. greenwayi,
O. fusiforme,
P. barroni,
P. dearatus,
P. proctozysron, and
P. brevis,
S. bandanensis and
S. melrosei, have never been reported as the second intermediate hosts of
H. yokogawai in the available literature. Therefore, we record aforementioned 15 fish species as new second intermediate hosts for
H. yokogawai.
C. formosanus is distributed in China, Taiwan, Japan, the Philippines, India, and Lao PDR [
15,
16,
32-
34]. Schotz et al. [
15] detected
C. formosanus metacercariae in only 1 fish species,
Esomus longimanus, among 45 fish species examined. Rim et al. [
16] found them in 4 fish species,
C. repasson,
P. brevis,
O. hasseltii, and
C. molitorella, in Vientiane Municipality. In the present study,
C. formosanus metacercariae were detected in 1 fish species,
P. meridionalis, from Luang Prabang, and 3 fish species,
M. greenwayi,
P. proctozysron, and
H. pierrei, from Saravane. Collectively, total 9 fish species,
C. molitorella,
C. repasson,
E. longimanus,
H. pierrei,
M. greenwayi,
O. hasseltii,
P. proctozysron, and
P. brevis, are listed as the second intermediate hosts for
C. formosanus in Lao PDR.
It has been proved that
O. viverrini is more endemic in humans and fish in Vientiane Municipality and Savannakhet Province, and
H. taichui is highly endemic in humans and fish in Saravane Province [
6,
7,
16]. From this study, it is suggested that Luang Prabang and Khammouane are highly endemic areas of intestinal flukes,
H. taichui and
H. yokogawai, rather than
O. viverrini.
ACKNOWLEDGMENTS
We thank Dr. Bounnaloth Insisiengmay, a staff of Department of Hygiene and Prevention, Ministry of Public Health, Vientiane, Ms. Souvanny Phommakone, a technical staff of LAPRec, Ministry of Agriculture and Irrigation, Vientiane, and Mr. Bounthong Sengvilaykham, deputy head in Livestock and Fisheries Section, Division of Agriculture and Forestry of Savannakhet Province, Lao PDR, for their help in collection and identification of fish. We also thank the staff of the Korea Association of Health Promotion, Seoul, the Republic of Korea, who participated in the Korea-Laos Cooperation Project on Parasite Control in Lao PDR (1999-2004).
References
- 1. WHO. Food-borne trematode infections in Asia. Report Joint WHO/FAO Workshop. 2002, Hanoi, Vietnam.
- 2. Chai JY, Murrell KD, Lymbry A. Fishborne parasitic zoonoses: status and issues. Int J Parasitol 2005;35:1233-1254.
- 3. Neveu R. Laos, the horizons travel guide series. Extra Image. 1999, Bangkok, Thailand.
- 4. Lao National Geographic Department. Lao geographic atlas. 2000, Vientiane, Lao PDR.
- 5. Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CY, Hoang EH. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res 2003;91:267-272.
- 6. Chai JY, Park JH, Han ET, Guk SM, Lin A, Kim JL, Sohn WM, Yong TS, Eom KS, Min DY, Hwang EH, Phommasack B, Insisiengmay B, Rim HJ. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol 2005;79:283-289.
- 7. Chai JY, Han EK, Guk SM, Shin EH, Sohn WM, Yong TS, Eom KS, Lee KH, Jeong HG, Ryong YS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH, Rim HJ. High prevalence of liver and intestinal fluke infection among residents of Savannakhet Province in Laos. Korean J Parasitol 2007;45:213-218.
- 8. Sornmani S, Pathammavong O, Bunnag T, Impand P, Intarakhao C, Thirachantra S. An epidemiological survey of human intestinal parasites in Vientiane, Laos. Southeast Asian J Trop Med Public Health 1974;5:541-546.
- 9. Ditrich O, Scholz T, Giboda M. Occurrence of some medically important flukes (Trematoda: Opisthorchiidae and Heterophyidae) in Nam Ngum water reservoir. Southeast Asian J Trop Med Public Health 1990;21:482-488.
- 10. Giboda M, Ditrich O, Scholz T, Viengsay T, Bouaphanh S. Human Opisthorchis and Haplorchis infections in Laos. Trans R Soc Trop Med Hyg 1991;85:538-540.
- 11. Pholsena K, Sayaseng B, Hongvanthong B, Vanisaveth V. The prevalence of helminth infection in Ban Nanin, Laos. Southeast Asian J Trop Med Public Health 1991;22:137-138.
- 12. Kobayashi J, Vannachone B, Xeutvongsa A, Manivong K, Ogawa S, Sato Y, Pholsena K. Prevalence of intestinal parasitic infection among children in 2 villages in Lao PDR. Southeast Asian J Trop Med Public Health 1996;27:562-565.
- 13. Chai JY, Hongvanthong B. A small-scale survey of intestinal helminthic infections among the residents near Pakse, Laos. Korean J Parasitol 1998;36:55-58.
- 14. Kobayashi J, Vannachone B, Sato Y, Manivong K, Nambanya S, Inthakone S. An epidemiological study on Opisthorchis viverrini infection in Lao villages. Southeast Asian J Trop Med Public Health 2000;31:128-132.
- 15. Scholz T, Ditrich O, Giboda M. Larval stages of medically important flukes (Trematoda) from Vientiane Province, Laos. Part I. Metacercariae. Ann Parasitol Hum Comp 1990;65:238-243.
- 16. Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J Parasitol 2008;46:253-260.
- 17. Search FishBase: http://www.fishbase.org/search.php.
- 18. Rim HJ. In Steele JH ed, Opisthorchiasis. CRC Handbook Series in Zoonoses, Section C: Parasitic Zoonoses. 1982, Vol. III (Trematode Zoonoses):Boca Raton, Florida, USA. CRC Press; pp 109-121.
- 19. Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop 2003;88:177-186.
- 20. Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini infected Syrian golden hamsters. Cancer Res 1978;38:4634-4639.
- 21. Vatanasapt V, Tangvoraphonkchai V, Titapant V, Pipitgool V, Viriyapap D, Sriamporn S. A high incidence of liver cancer in Khon Kaen Province, Thailand. Southeast Asian J Trop Med Public Health 1990;21:489-494.
- 22. Pearson JC. The Haplorchis group. A revision of the subfamily Haplorchinae Looss, 1899 (Trematoda: Heterophyidae). I. Parasitology 1964;54:601-676.
- 23. Nath D, Pande BP. Identify of the three heterophyid metacercariae infesting some of the freshwater fishes. Current Sci India 1970;39:325-331.
- 24. Velasquez CC. Observations on some Heterophyidae (Trematoda: Digenea) encysted in Philippine fishes. J Parasitol 1973;59:77-84.
- 25. Pande V, Premvati G. Development of metacercariae of Haplorchis spp. in chicks. Indian J Parasitol 1977;1:165-172.
- 26. Sohn WM, Eom KS, Min DY, Rim HJ, Hoang EH, Yang Y, Li X. Fishborne trematode metacercariae in freshwater fish from Guangxi Zhuang Autonomous Region, China. Korean J Parasitol 2009;47:249-257.
- 27. Pearson JC, Ow-Yang CK. New species of Haplorchis from Southeast Asia, together with keys to the Haplorchis-group of heterophyid trematodes of the region. Southeast Asian J Trop Med Public Health 1982;13:35-60.
- 28. Pandey KC. Studies on metacercariae of freshwater fishes of India. 1. On the morphology of metacercaria of Haplorchis yokogawai (Katsuta, 1932) Chen, 1936. Proc Nat Acad Sci India Sect B 1966;36:437.
- 29. Pande BP, Shukla RP. Metacercarial cyst of Haplorchis pumilio, it's development in experimental mammals and 2 other heterophyid infection of freshwater fishes and their zoonotic significance. Indian J Animal Sci 1972;42:971-978.
- 30. Kliks M, Tantachamrun T. Heterophyid (Trematoda) parasites of cats in North Thailand, with notes on a human case found at necropsy. Southeast Asian J Trop Med Public Health 1974;5:547.
- 31. Fahmy MA, Mandour AM, El-Naffar MK. Successful infection of dogs and cats by Prohemistomum vivax Sonsino, 1893 and Haplorchis yokogawai Katsuta, 1922. J Egyptian Soc Parasitol 1976;6:77.
- 32. Premvati G, Pande V. On Centrocestus formosanus (Nishigori, 1924) Price, 1932 and it's experimental infection in White Leghorn chicks. Jpn J Parasitol 1974;23:79-84.
- 33. Yanohara Y. On analysis of transmission dynamics of trematode infection. 1. Centrocestus formosanus infection in Miyakojima. Jpn J Parasitol 1985;34:55-70.
- 34. Han ET, Shin EH, Phommakorn S, Sengvilaykham B, Kim JL, Rim HJ, Chai JY. Centrocestus formosanus (Digenea: Heterophyidae) encysted in the freshwater fish, Puntius brevis, from Lao PDR. Korean J Parasitol 2008;46:49-53.
Fig. 1Surveyed areas, Luang Prabang Province (LPP), Khammouane Province (KP), and Saravane Province (SP), in Lao PDR.
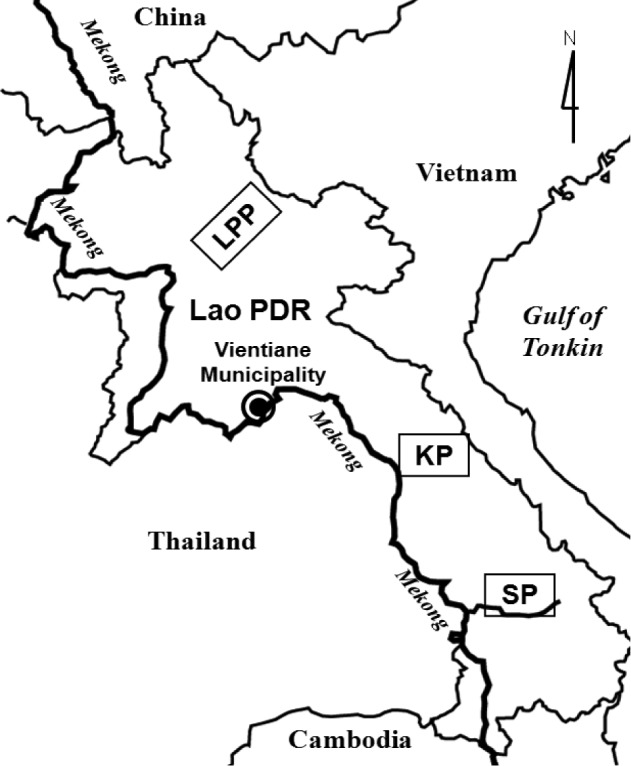
Table 1.Freshwater fish collected from 3 Provinces of Lao PDR
Table 1.
|
Species of fish |
No. of fisha collected from
|
|
Luang Prabang |
Khammouane |
Saravane |
Total |
|
Cyprinidae |
|
|
|
|
|
Barbonymus gonionotus
|
1 |
5 |
3 |
9 |
|
Cosmochilus harmondi
|
- |
5 |
- |
5 |
|
Crossocheilus oblongus
|
- |
6 |
- |
6 |
|
Cyclocheilichthys apogon
|
4 |
- |
- |
4 |
|
Cyclocheilichthys armatus
|
- |
4 |
- |
4 |
|
Cyclocheilichthys enoplos
|
- |
- |
5 |
5 |
|
Cyclocheilichthys furcatus
|
4 |
- |
- |
4 |
|
Cyclocheilichthys repasson
|
2 |
4 |
- |
6 |
|
Esomus longimanus
|
- |
- |
7 |
7 |
|
Esomus metallicus
|
- |
- |
10 |
10 |
|
Hampala dispar
|
- |
2 |
19 |
21 |
|
Henicorhynchus lineatus
|
- |
1 |
- |
1 |
|
Henicorhynchus siamensis
|
4 |
- |
- |
4 |
|
Hypsibarbus lagleri
|
- |
9 |
- |
9 |
|
Hypsibarbus malcolmi
|
- |
5 |
- |
5 |
|
Hypsibarbus pierrei
|
3 |
- |
1 |
4 |
|
Hypsibarbus wetmorei
|
- |
3 |
1 |
4 |
|
Labiobarbus leptocheilus
|
3 |
- |
- |
3 |
|
Labiobarbus siamensis
|
4 |
- |
- |
4 |
|
Lobocheilos rhabdoura
|
1 |
- |
1 |
2 |
|
Mekongina erythrospila
|
10 |
- |
- |
10 |
|
Mystacoleucus ectypus
|
1 |
- |
- |
1 |
|
Mystacoleucus greenwayi
|
- |
- |
16 |
16 |
|
Neolissochilus stracheyi
|
2 |
- |
- |
2 |
|
Onychostoma fusiforme
|
1 |
7 |
- |
8 |
|
Osteochilus vittatus
|
- |
7 |
- |
7 |
|
Oxygaster pointoni
|
- |
10 |
- |
10 |
|
Paralaubuca barroni
|
- |
- |
10 |
10 |
|
Poropuntius dearatus
|
- |
- |
6 |
6 |
|
Puntioplites proctozysron
|
- |
- |
7 |
7 |
|
Puntius brevis
|
- |
3 |
10 |
13 |
|
Puntius orphoides
|
- |
- |
1 |
1 |
|
Rasbora steineri
|
- |
5 |
- |
5 |
|
Scaphognathops bandanensis
|
7 |
- |
- |
7 |
|
Sinibrama melrosei
|
7 |
- |
- |
7 |
|
Clupeidae |
|
|
|
|
|
Tenualosa thibaudeaui
|
- |
4 |
- |
4 |
|
Channidae |
|
|
|
|
|
Channa striata
|
- |
4 |
- |
4 |
|
Hemiramphidae |
|
|
|
|
|
Dermogenys siamensis
|
- |
1 |
- |
1 |
|
Balitoridae |
|
|
|
|
|
Physoschistura meridionalis
|
5 |
- |
- |
5 |
|
Siluridae |
|
|
|
|
|
Kryptopterus bicirrhis
|
- |
1 |
- |
1 |
|
Total |
59 |
86 |
97 |
242 |
Table 2.Infection status of Opisthorchis viverrini metacercariae by species of fish in 3 Provinces of Lao PDR
Table 2.
|
Species of fish |
No. of fish examined |
No. (%) of fish infected |
No. of metacercariae detected
|
|
Total |
Range |
Average |
|
Fish from Luang Prabang |
|
|
|
|
|
|
Neolissochilus stracheyi
|
2 |
2 (100) |
4 |
1-3 |
2.0 |
|
Cyclocheilichthys apogon
|
4 |
1 (25.0) |
430 |
- |
430.0 |
|
Hypsibarbus pierrei
|
3 |
1 (33.3) |
4 |
- |
4.0 |
|
Cyclocheilichthys furcatus
|
4 |
1 (25.0) |
3 |
- |
3.0 |
|
Subtotal |
13 |
5 (38.5) |
441 |
1-430 |
88.2 |
|
Fish from Khammouane |
|
|
|
|
|
|
Onychostoma fusiforme
|
7 |
1 (14.3) |
65 |
- |
65.0 |
|
Puntius brevis
|
3 |
1 (33.3) |
988 |
- |
988 |
|
Cyclocheilichthys armatus
|
4 |
1 (25.0) |
1 |
- |
1.0 |
|
Hampala dispar
|
2 |
2 (100) |
49 |
20-29 |
24.5 |
|
Hypsibarbus wetmorei
|
3 |
1 (33.3) |
20 |
- |
20.0 |
|
Subtotal |
19 |
6 (31.6) |
1,123 |
1-988 |
187.2 |
|
Fish from Saravane |
|
|
|
|
|
|
Hampala dispar
|
19 |
16 (84.2) |
7,243 |
4-1,480 |
452.7 |
|
Puntioplites proctozysron
|
7 |
3 (42.9) |
8 |
1-6 |
2.7 |
|
Poropuntius dearatus
|
6 |
2 (33.3) |
10 |
3-7 |
5.0 |
|
Puntius brevis
|
10 |
1 (10.0) |
1 |
- |
1.0 |
|
Paralaubuca barroni
|
10 |
1 (10.0) |
1 |
- |
1.0 |
|
Cyclocheilichthys enoplos
|
5 |
1 (20.0) |
6 |
- |
6.0 |
|
Subtotal |
57 |
24 (42.1) |
7,269 |
1-1,480 |
302.9 |
|
Total |
89 |
35 (39.3) |
8,833 |
1-1,480 |
252.4 |
Table 3.Infection status of Haplorchis taichui metacercariae by species of fish in 3 Provinces of Lao PDR
Table 3.
|
Species of fish |
No. of fish examined |
No. (%) of fish infected |
No. of metacercariae detected
|
|
Total |
Range |
Average |
|
Fish from Luang Prabang |
|
|
|
|
|
|
Scaphognathops bandanensis
|
7 |
7 (100) |
7,095 |
22-3,965 |
1,013.6 |
|
Henicorhynchus siamensis
|
4 |
4 (100) |
45 |
7-17 |
11.3 |
|
Mekongina erythrospila
|
10 |
9 (90.0) |
1,317 |
57-308 |
146.3 |
|
Physoschistura meridionals
|
5 |
4 (80.0) |
7 |
1-4 |
1.8 |
|
Cyclocheilichthys apogon
|
4 |
3 (75.0) |
828 |
92-526 |
276.0 |
|
Cyclocheilichthys furcatus
|
4 |
3 (75.0) |
35 |
1-20 |
11.7 |
|
Sinibrama melrosei
|
7 |
5 (71.4) |
62 |
1-41 |
12.4 |
|
Mystacoleucus ectypus
|
1 |
1 (100) |
208 |
- |
208.0 |
|
Barbonymus gonionotus
|
1 |
1 (100) |
275 |
- |
275.0 |
|
Lobocheiios rhabdoura
|
1 |
1 (100) |
9 |
- |
9.0 |
|
Subtotal |
44 |
38 (86.4) |
9,881 |
1-3,965 |
260.0 |
|
Fish from Khammouane |
|
|
|
|
|
|
Hypsibarbus lagleri
|
9 |
9 (100) |
23,838 |
1,149-4,709 |
2,649 |
|
Onychostoma fusiforme
|
7 |
6 (85.7) |
787 |
5-625 |
131.2 |
|
Crossocheilus oblongus
|
6 |
5 (83.3) |
34 |
3-14 |
6.8 |
|
Cyclocheilichthys repasson
|
4 |
1 (25.0) |
82 |
- |
82.0 |
|
Puntius brevis
|
3 |
2 (66.7) |
185 |
31-154 |
92.5 |
|
Subtotal |
29 |
23 (79.3) |
24,926 |
3-4,709 |
1,083.7 |
|
Fish from Saravane |
|
|
|
|
|
|
Mystacoleucus greenwayi
|
16 |
16 (100) |
5,394 |
8-1,625 |
337.1 |
|
Hampala dispar
|
19 |
4 (21.1) |
6,128 |
16-6,050 |
1,532 |
|
Puntioplites proctozysron
|
7 |
5 (71.4) |
875 |
2-863 |
175.0 |
|
Poropuntius dearatus
|
6 |
5 (83.3) |
1,113 |
82-608 |
222.6 |
|
Paralaubuca barroni
|
10 |
4 (40.0) |
61 |
1-58 |
15.3 |
|
Cyclocheilichthys enoplos
|
5 |
3 (60.0) |
1,038 |
14-986 |
346.0 |
|
Esomus metalllcus
|
10 |
2 (20.0) |
2 |
- |
1.0 |
|
Lobocheilos rahabdoura
|
1 |
1 (100) |
91 |
- |
91.0 |
|
Puntius orphoides
|
1 |
1 (100) |
3 |
- |
3.0 |
|
Subtotal |
75 |
41 (54.7) |
14,705 |
1-6,050 |
358.7 |
|
Total |
148 |
102 (68.9) |
49,512 |
1-6,050 |
485.4 |
Table 4.Infection status of
Haplorchis yokogawai metacercariae
a by species of fish in 3 Provinces of Lao PDR
Table 4.
|
Species of fish |
No. of fish examined |
No. (%) of fish infected |
No. of metacercariae detected
|
|
Total |
Range |
Average |
|
Fish from Luang Prabang |
|
|
|
|
|
|
Scaphognathops bandanensis
|
7 |
6 (85.7) |
1,525 |
4-788 |
254.2 |
|
Cyclocheilichthys apogon
|
4 |
3 (75.0) |
4,037 |
183-2,874 |
1,345.7 |
|
Cyclocheilichthys furcatus
|
4 |
3 (75.0) |
443 |
19-312 |
147.7 |
|
Sinibrama melrosei
|
7 |
3 (42.9) |
21 |
1-17 |
7.0 |
|
Mystacoleucus ectypus
|
1 |
1 (100) |
128 |
- |
128.0 |
|
Lobocheiios rhabdoura
|
1 |
1 (100) |
4 |
- |
4.0 |
|
Subtotal |
24 |
17 (70.8) |
6,158 |
1-2,874 |
362.2 |
|
Fish from Khammuan |
|
|
|
|
|
|
Hypsibarbus lagleri
|
9 |
9 (100) |
1,387 |
67-274 |
154.1 |
|
Onychostoma fusiform
|
7 |
6 (85.7) |
898 |
18-553 |
149.7 |
|
Crossocheilus oblongus
|
6 |
1 (16.7) |
2 |
- |
2.0 |
|
Cyclocheilichthys armatus
|
4 |
3 (75.0) |
201 |
- |
201.0 |
|
Cyclocheilichthys repasson
|
4 |
1 (25.0) |
29 5 |
- |
179.7 |
|
Subtotal |
30 |
20 (66.7) |
2,517 |
2-553 |
125.9 |
|
Fish from Salavan |
|
|
|
|
|
|
Mystacoleucus greenwayi
|
16 |
16 (100) |
6,002 |
6-975 |
375.1 |
|
Hampala dispar
|
19 |
8 (42.1) |
1,249 |
5-1,011 |
156.1 |
|
Puntioplites proctozysron
|
7 |
6 (85.7) |
210 |
5-120 |
35.0 |
|
Poropuntius dearatus
|
6 |
6 (100) |
1,507 |
173-408 |
251.2 |
|
Paralaubuca barroni
|
10 |
9 (90.0) |
143 |
1-75 |
15.9 |
|
Cyclocheilichthys enoplos
|
5 |
5 (100) |
2,516 |
47-2,210 |
503.2 |
|
Puntius brevis
|
10 |
4 (40.0) |
102 |
1-92 |
25.5 |
|
Hypsibarbus pierrei
|
1 |
1 (100) |
13 |
- |
13.0 |
|
Subtotal |
74 |
55 (74.3) |
11,742 |
1-2,210 |
213.5 |
|
Total |
128 |
92 (71.9) |
20,417 |
1-2,874 |
221.9 |
Table 5.Infection status of Centrocestus formosanus metacercariae by species of fish in 2 Provinces of Lao PDR
Table 5.
|
Species of fish |
No. of fish examined |
No. (%) of fish infected |
No. of metacercariae detected
|
|
Total |
Range |
Average |
|
Fish from Luang Prabang |
|
|
|
|
|
|
Physoschistura meridionalis
|
5 |
1 (25.0) |
1 |
- |
1.0 |
|
Fish from Saravane |
|
|
|
|
|
|
Mystacoleucus greenwayi
|
16 |
4 (25.0) |
17 |
1-8 |
4.3 |
|
Puntioplites proctozysron
|
7 |
2 (28.6) |
4 |
1-3 |
2.0 |
|
Hypsibarbus pierrei
|
1 |
1 (100) |
1 |
- |
1.0 |
|
Subtotal |
24 |
7 (29.2) |
22 |
1-8 |
3.1 |
|
Total |
29 |
8 (27.6) |
23 |
1-8 |
2.9 |