Abstract
The present study was performed to trace the decisive evidence for mixed infection of 2 Myxobolus species, M. episquamalis and Myxobolus sp., in the gray mullet, Mugil cephalus, from Korean waters. Mullets with whitish cyst-like plasmodia on their scales were collected near a sewage plant in Yeosu, southern part of Korea, in 2009. The cysts were mainly located on scales and also found in the intestine. The spores from scales were oval in a frontal view, tapering anteriorly to a blunt apex, and measured 7.2 µm (5.8-8.0) in length and 5.3 µm (4.7-6.1) in width. Two polar capsules were pyriform and extended over the anterior half of the spore, measuring 3.5 µm (2.3-4.8) in length and 2.0 µm (1.5-2.2) in width. In contrast, the spores from the intestine were ellipsoidal, 10.4 µm (9.0-11.9) in length and 8.4 µm (7.3-10.1) in width. The polar capsules were pyriform but did not extend over the anterior half of the spore, 3.7 µm (2.5-4.5) in length and 2.2 µm (1.8-2.9) in width. The nucleotide sequences of the 18S rDNA gene of the 2 myxosporean spores from scales and intestine showed 88.1% identity to each other and 100% identity with M. episquamalis and 94.5% identity with M. spinacurvatura from mullet, respectively. By the above findings, it is first confirmed that mullets from the Korean water are infected with 2 myxosporean species, M. episquamalis and Myxobolus sp.
-
Key words: Myxobolus episquamalis, Myxobolus sp., mixed infection, mullet, Mugil cephalus
The mullet, Mugil cephalus, is a cosmopolitan coastal species occurring in tropical, subtropical, and temperate zones of all seas, and has been widely cultivated in several regions such as the Mediterranean, Egypt, Israel, Taiwan, Japan, and Korea. The mullet is an economically important fish in Korea, and more than 17,900 tons were harvested in 2008. It is consumed chiefly as a sliced raw fish in Korea, and also as a dried salted fish roe in several other countries, including Taiwan, Japan, and the southeastern United States.
The mullet is known to be infected with several
Myxobolus species such as
M. muelleri Bütschli, 1882,
M. exiguus Thélohan, 1895,
M. cheni Schulman, 1962,
M. parvus Schulman, 1962,
M. cephalus Iversen, Chitty and Van Meter, 1971,
M. spinacurvatura Maeno, Sorimachi, Ogawa and Egusa, 1990,
M. bizerti Bahri and Marques, 1996,
M. ichkeulensis Bahri and Marques, 1996, and
M. episquamalis Egusa, Maeno and Sorimachi, 1990 [
1-
7]. Of these,
M. episquamalis is considered an important parasitic pathogen because affected fish appear unsightly with many cysts on their scales and cannot be sold.
M. episquamalis was first described from wild mullets in Japan by Egusa et al. [
1] in 1990. Since then, there have been many reports on
M. episquamalis infection in mullet species in Tunisia, Taiwan, Australia, Senegal, and Korea [
5,
6,
8-
11]. All infected fish showed a single infection of
M. episquamalis. However, recently, Kim et al. [
11] reported the possibility of a mixed infection by different
Myxobolus species,
M. episquamalis and
Myxobolus sp., and thus myxosporean spores were observed not only on scales but also in various internal organs, i.e. intestine, pancreas, heart, kidney, stomach, skin, spleen, liver, and gills. However, it has been known that
M. episquamalis is infected only in scales of mullets, and the myxosporean species detected in the internal organs of mullets have not been determined yet. Therefore, we aimed to trace morphologic and genetic evidence to confirm mixed infection with 2
Myxobolus species,
M. episquamalis and
Myxobolus sp., in gray mullets from Korean waters.
Wild gray mullets with white cystic masses on their body surfaces were collected from local fishermen who were harvesting fish near a sewage plant in Yeosu, Korea in October 2009. Eight infected fish (25-43 cm, 310-700 g) were transported on ice and examined for the presence of myxosporeans. Cyst-like plasmodia were carefully removed from infected scales and intestines and squashed to observe the myxosporeans. Fresh spores were photographed and measured according to the guidelines for species description of myxosporeans by Lom and Authur [
12]. The dimensions of the spores were measured from 30 fresh spores. The morphologic characteristics of the spores were compared with those of 16
Myxobolus species found in mullets [
1-
5,
7,
11,
13].
DNA was extracted from the plasmodia on scales and the intestine using an AccuPrep Genomic DNA Purification kit (Bioneer, Daejeon, Korea) following the manufacturer's instructions. Universal primers A (5'-ACCTGGTTGATCCTGCCAGT-3') (primer 1) and B (5'-TGATCCTTCTGCAGGTTCACCTAC-3') (primer 6) were used as described by Sogin [
14] to amplify the 18S rDNA gene. The DNA templates were amplified by initial denaturation at 95℃ for 5 min, followed by 30 cycles of denaturation at 94℃ for 30 sec, annealing at 55℃ for 30 sec, extension at 72℃ for 1 min 30 sec, and a final extension at 72℃ for 5 min. The PCR products were analyzed by 1.5% agarose gel electrophoresis and purified using a QIAquick Gel Extraction kit (Qiagen, Valencia, California, USA). The amplified products were subjected to nucleotide sequencing analysis using ABI PRISM dye terminator sequencing chemistry (Applied Biosystems, Foster City, California, USA) with the above mentioned PCR primers and another primer set from the inserted gene (Myxo-interF: 5'-ATGTTGGTTCCGTCATGGGG-3' and Myxo-interR: 5'-GGATTGGCCTGACAGATCAC-3'), according to the manufacturer's instructions. The resulting sequences were assembled with Genetyx Win Ver. 5.1 software. The sequence of the 18S rDNA gene was submitted to GenBank (no. KC733437 and KC733438) and compared with 7
Myxobolus species that infect mullets [
5,
11,
13]. In addition, multiple alignment was constructed using the Clustal X [
15] to infer the genetic relationship among 26
Myxobolus species, including
Tetracapsuloides bryosalmonae (out-group species) with a neighbor-joining algorithm. The final phylogenetic tree was drawn with the MEGA4 program [
16].
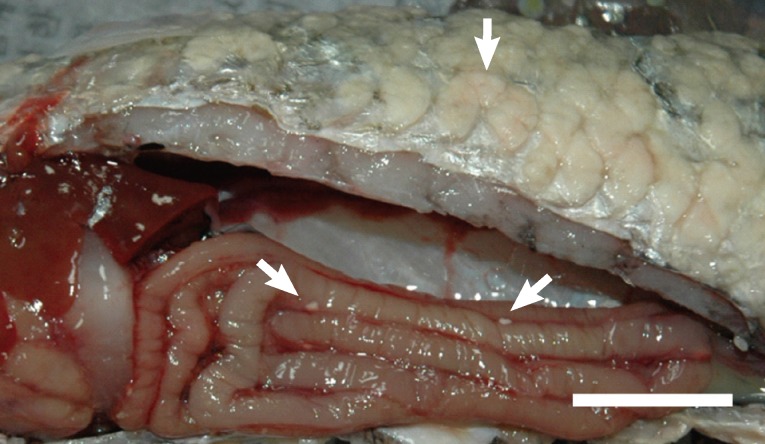
Wild gray mullets with whitish cyst-like plasmodia on their scales were found near a sewage plant in Yeosu, southern part of Korea, in October 2009. The cysts were mainly located on the scales but were also found in the intestine (
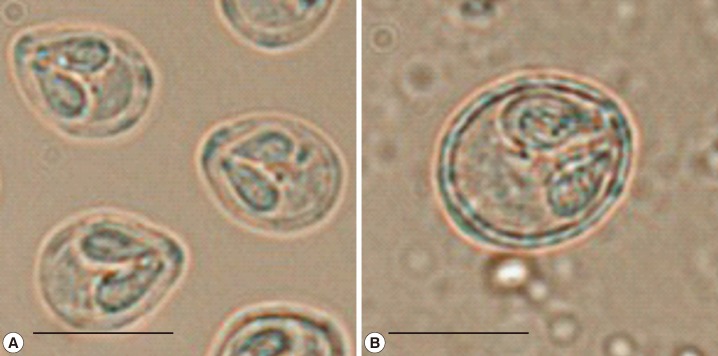
Fig. 1). No organs were infected except the scales, tail fin, and intestine, but all fish (eight fish) showed these signs. Mature spores were found within the cysts on the scales and intestine (
Fig. 2). Spores from scales were oval in a front view, tapered anteriorly to a blunt apex, and measured 7.2 µm (5.8-8.0) in length and 5.3 µm (4.7-6.1) in width (
Table 1). Two polar capsules were pyriform and extended over the anterior half of the spore, measuring 3.5 µm (2.3-4.8) in length and 2.0 µm (1.5-2.2) in width. In contrast, the spores from intestine were ellipsoidal, the length being 10.4 µm (9.0-11.9) and the width 8.4 µm (7.3-10.1). The polar capsules were pyriform but not extended over the anterior half of the spore, 3.7 µm (2.5-4.5) in length and 2.2 µm (1.8-2.9) in width.
The infection site and morphologic characteristics of the spores from the scales in this study were almost similar to those reported previously for
M. episquamalis in Korea by Kim et al. [
11], although the spores were smaller than those of
M. episquamalis reported in Japan by Egusa et al. [
1] (
Table 1). Moreover, the species differed from that of the intestine because of the more oval and smaller shaped spores. The infection site and morphologic characteristics of the spores from the intestine were similar to those of
M. muelleri (spore size; 10.5±0.51 µm length, 8.5±0.51 µm width, and polar capsule; 4.5±0.31 µm length, 2.5±0.21 µm width) and differed from 15 previously reported
Myxobolus species in mullets:
M. bizerti,
M. cephalus,
M. hannensis,
M. ichkeulensis,
M. raibauti, and
M. rohdei have larger spores;
M. episquamalis,
M. goensis,
M. parvus,
M. exiguus, and
M. supamattayai have smaller spores;
M. chiungchowensis,
M. goreensis, and
M. spinacurvatura have more rounded spores; and
M. mugilii has smaller width spores.
The 18S rDNA sequences of the 2 myxosporean spores from the scales and intestine were analyzed and named Scales-
Myxobolus and Intestine-
Myxobolus, respectively. The 18S rDNA sequences showed 88.1% identity to each other (
Table 2). The nucleotide sequences of the Scales-
Myxobolus showed 100% identity with
M. episquamalis, but 82.2-88.8% identity with 6
Myxobolus species (
M. bizerti,
M. spinacurvatura,
M. exiguus,
M. ichkeulensis,
M. supamattayai, and
M. muelleri) infecting mullets. In contrast, the nucleotide sequences of the Intestine-
Myxobolus showed 94.5% identity with
M. spinacurvatura, but 81-93% identity with the sequences of 6 other
Myxobolus species. A phylogenetic tree based on the published 18S rDNA sequences of 26
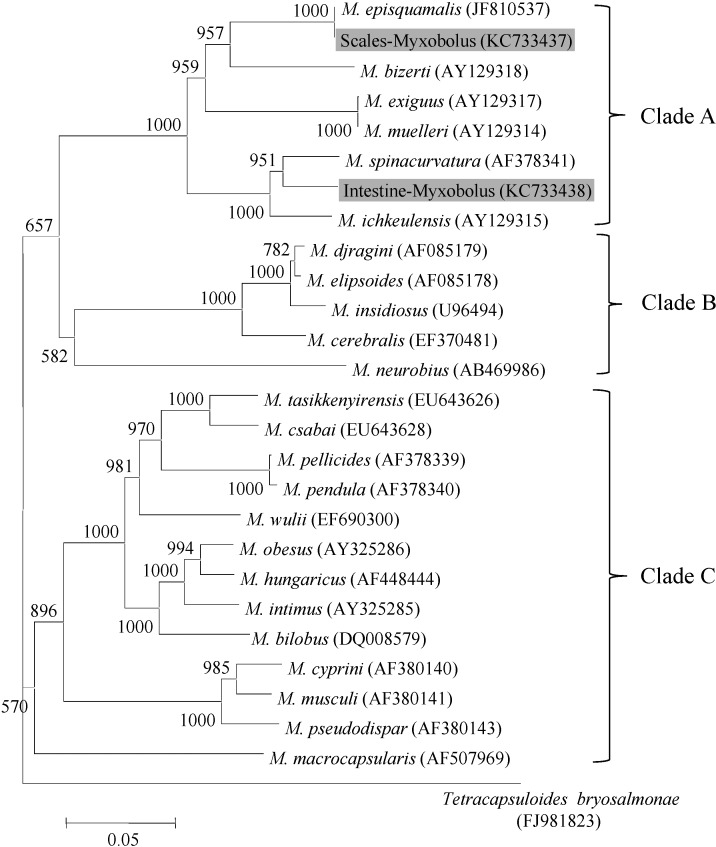
Myxobolus species, including the
Myxobolus species in this study, revealed 3 main clades (
Fig. 3): Six species (
M. bizerti,
M. spinacurvatura,
M. episquamalis,
M. exiguus,
M. muelleri, and
M. ichkeulensis) corresponded to a monophyletic group (clade A). The marine
Myxobolus (clade A) was separated from the other
Myxobolus clades (B and C), which are freshwater fish parasites. Scales-
Myxobolus and Intestine-
Myxobolus belonged to clade A and were situated in the same location as
M. episquamalis and near
M. spinacurvatura, respectively. These results confirmed that the myxosporean species from the scales was
M. episquamalis. However, the myxosporean species from the intestine did not correlate with any other
Myxobolus spp. previously reported to infect mullets or another species among the genus
Myxobolus. By the above findings, it is first confirmed that mullets were mixed-infected with 2 myxosporean species,
Myxobolus episquamalis and
Myxobolus sp. In addition, the
Mxobolus sp. infection is first found in the mullet, even though its species was not identified in the present study.
Since
M. episquamalis was first described in wild mullets in 1990 [
1], many
M. episquamalis infections have been reported in several countries [
5,
6,
8-
11]. All infected fish showed a single infection with
M. episquamalis. In this study, an interesting feature of mixed infection with 2 myxosporean species,
Myxobolus episquamalis and
Myxobolus sp. has been found in mullets. The influence of the mixed infection on the host is unknown. Further researches will be performed to identify the pathological properties between the single and mixed infections.
ACKNOWLEDGMENT
This research was supported by the Technology Development Program for Fisheries, Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea.
References
- 1. Egusa S, Maeno Y, Sorimachi M. A new species of Myxozoa, Myxobolus episquamalis sp. nov. infecting the scales of the mullet, Mugil cephalus L. Fish Pathol 1990;25:87-91.
- 2. Maeno Y, Sorimachi M, Ogawa K, Egusa S. Myxobolus spinacurvatura sp. n. (Myxosporea: Bilvalvulida) parasitic in deformed mullet, Mugil cephalus. Fish Pathol 1990;25:37-41.
- 3. Landsberg JH, Lom J. Taxonomy of the genera of the Myxobolus/Myxosoma group (Myxobolidae: Myxosporea), current listing of species and revision of synonyms. Syst Parasitol 1991;18:165-186.
- 4. Lom J, Dyková I. Studies on protozoan parasites of Australian fishes: III. Species of genus Myxobolus Bütschli, 1882. Eur J Protistol 1994;30:431-439.
- 5. Bahri S, Andree KB, Hedrick RP. Morphological and phylogenetic studies of marine Myxobolus spp. from mullet in Ichkeul Lake, Tunisia. J Eukaryot Microbiol 2003;50:463-470.
- 6. Bahri S, Marques A. Myxosporean parasites of the genus Myxobolus from Mugil cephalus in Ichkeul Iagoon, Tunisia: description of two new species. Dis Aquat Org 1996;27:115-122.
- 7. Eiras JC, Molnár K, Lu YS. Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea: Myxobolidae). Syst Parasitol 2005;61:1-46.
- 8. Lin CL, Ho JS. Myxobolus episquamalis (Myxosporea) occurring on the scales of the mullet, Liza macrolepis, cultured in Taiwan. J Fish Soc Taiwan 1997;24:193-200.
- 9. Rothwell JT, Virgona JL, Callinan RB, Nicholls PJ, Langdon JS. Occurrence of cutaneous infections of Myxobolus episquamalis (Myxozoa: Myxobolidae) in sea mullet, Mugil cephalus L, in Australia. Aust Vet J 1997;75:349-352.
- 10. Diamanka A, Fall M, Diebakate C, Faye N, Toguebaye BS. Identification of Myxobolus episquamalis (Myxozoa, Myxobolidae) in flathead mullet Mugil cephalus (Pisces, Teleostei, Mugilidae) from the coast of Senegal (eastern tropical Atlantic Ocean). Acta Adriat 2008;49:19-23.
- 11. Kim WS, Kim JH, Jang MS, Jung SJ, Oh MJ. Infection of wild mullet (Mugil cephalus) with Myxobolus episquamalis in Korea. Parasitol Res 2013;112:447-451.
- 12. Lom J, Arthur JR. A guideline for the preparation of species descriptions on Myxosporea. J Fish Dis 1989;12:151-156.
- 13. U-taynapun K, Penprapai N, Bangrak P, Mekata T, Itami T, Tantikitti C. Myxobolus supamattayai n. sp. (Myxosporea: Myxobolidae) from Thailand parasitizing the scale pellicle of wild mullet (Valamugil seheli). Parasitol Res 2011;109:81-91.
- 14. Sogin ML. Amplification of Ribosomal RNA Genes for Molecular Evolution Studies. In Innis MA, Gelfand DH, Sninsky JJ, White TJ eds, PCR protocols: a guide to methods and applications. San Diego, CA. Academic Press; 1990.
- 15. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997;25:4876-4882.
- 16. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007;24:1596-1599.
Fig. 1Cysts on the scales and intestine of Mugil cephalus. Bar= 2 cm.

Fig. 2Spores from cysts on the scales (A) and intestine (B). Bar=7 µm.

Fig. 3Molecular phylogenetic tree showing the genetic relationships among 26 Myxobolus species based on the 18S rDNA gene nucleotide sequence. Bootstrap values for 1,000 replicates are shown at major nodes of the tree. The distance marker refers to the expected number of substitutions per site.

Table 1.Comparison of spore characteristics between Myxobolus species infecting mullet
Table 1.
|
Species |
Host |
Localization |
Spore (pm)
|
Polar capsule (pm)
|
|
Length |
Width |
Length |
Width |
|
M. bizerti Bahri and Marques, 1996 |
Mugii cephaius
|
Gills |
14.2 (14.0-14.5) |
14.2 (14.0-14.5) |
6.5 (6.0-7.0) |
5.8 (5.5-6.0) |
|
M. cephaius Landsberg and Lom, 1991 |
M. cephaius
|
Brain meninges, gill arches |
14.1 (14.0-15.0) |
11.0 (10.0-11.0) |
4.7 (4.0-5.0) |
3.2 (3.0-4.0) |
|
M. chiungchowensis Chen in Chen and Ma, 1998 |
M. cephaius
|
Intestine |
10.8 (10.2-11.8) |
10.5 (9.6-11.0) |
6.0 (5.6-6.2) |
3.6 (3.4-3.8) |
|
M. episquamaiis
|
Egusa et al., 1990 |
M. cephaius
|
Scales |
8.6 (7.5-9.5) |
6.8 (6.0-7.5) |
4.4 (3.8-5.0) |
2.2 (2.0-3.0) |
|
Kim et al., 2012 |
|
|
7.0 (6.2-7.6) |
5.2 (4.0-6.2) |
3.5 (2.5-4.5) |
2.0 (1.6-2.3) |
|
M. goensis Eiras and D’Souza, 2004 |
M. cephaius
|
Gill rakers |
9.7 (9.5-10.5) |
6.6 (6.0-7.5) |
5.3 (4.5-6.0) |
2.4 (2.0-3.0) |
|
M. goreensis Fall et al., 1997 |
M. cephaius
|
Gills |
10.9 (10.0-13.0) |
10.9 (10.0-13.0) |
4.1 (4.0-5.0) |
3.1 (2.0-4.0) |
|
M. hannensis Fall et al., 1997 |
M. cephaius
|
Gill arches, gill lamellae |
13.9 (13.0-15.0) |
13.9 (13.0-15.0) |
8.9 (7.0-9.0) |
5.7 (5.0-6.0) |
|
M. ichkeuiensis Bahri and Marques, 1996 |
M. cephaius
|
Gill arches |
13.5 (13.0-14.0) |
12.5 (12.0-13.0) |
5.5 (5.0-6.0) |
4.2 (4.0-4.3) |
|
M. mugiiii Haldar et al., 1996 |
M. cephaius
|
Gills |
11.7 (8.1-16.3) |
5.5 (4.0-7.3) |
6.1 (2.4-8.1) |
2.7 (1.6-4.0) |
|
M. parvus Shulman, 1962 |
M. cephaius
|
Gills |
6.5-7.0 |
5.5-6.0 |
3.8-4.2 |
2.0 |
|
M. raibauti Fall et al., 1997 |
M. cephaius
|
Liver |
15.3 (14.0-16.0) |
12.1 (12.0-13.0) |
5.9 (5.0-6.5) |
3.6 (3.0-4.0) |
|
M. rohdei Lom and Dyková, 1994 |
M. cephaius
|
Kidney interstitium |
11.0 (9.8-11.8) |
8.9 (8.4-9.1) |
4.3 (3.7-5.0) |
2.8 (2.5-3.1) |
|
M. spinacurvatura Maeno et al., 1990 |
M. cephaius
|
Mesentery, brain, spleen |
10.5-12.5 |
9.0-11.0 |
3.5-5.0 |
2.5-3.5 |
|
M. exiguus Bahri et al., 2003 |
Liza ramada
|
Intestine |
9.5±0.51 |
7.5±0.51 |
3.5±0.13 |
2.25±0.26 |
|
M. mueiieri Bahri et al., 2003 |
L. ramada
|
Mesenteric vessels |
10.5±0.51 |
8.5±0.51 |
4.5±0.31 |
2.5±0.21 |
|
M. supamattayai U-taynapun et al., 2010 |
Vaiamugii seheii
|
Scales |
6.6 (6.2-7.0) |
6.5 (6.2-6.7) |
3.5 (3.4-3.6) |
2.0 (1.9-2.2) |
|
Present work |
M. cephaius
|
Scales |
7.2 (5.8-8.0) |
5.3 (4.7-6.1) |
3.5 (2.3-4.8) |
2.0 (1.5-2.2) |
|
|
Intestine |
10.4 (9.0-11.9) |
8.4 (7.3-10.1) |
3.7 (2.5-4.5) |
2.2 (1.8-2.9) |
Table 2.Pairwise comparisons (%) of nucleotide sequence identities of the 18S rDNA gene among 9 Myxobolus species infecting mullet
Table 2.
|
Myxobolus species |
Scales-Myxobolus (KC733437) |
Intestine-Myxobolus (KC733438) |
M. episquamalis (JF810537) |
M. bizerti (AY129318) |
M. spinacurvatura (AF378341) |
M. exiguus (AY129317) |
M. ichkeulensis (AY129315) |
M. supamattayai (HQ166720) |
M. muelleri (AY129314) |
|
Scales-Myxobolus |
100 |
88.1 |
100 |
88.8 |
86.8 |
86.8 |
86.7 |
82.2 |
86.8 |
|
Intestine-Myxobolus |
|
100 |
88.1 |
85.6 |
94.5 |
84.5 |
93.0 |
81.0 |
84.6 |
|
M. episquamaiis
|
|
|
100 |
88.8 |
86.8 |
86.8 |
86.7 |
82.2 |
86.8 |
|
M. bizerti
|
|
|
|
100 |
84.9 |
85.1 |
85.1 |
82.3 |
85.0 |
|
M. spinacurvatura
|
|
|
|
|
100 |
84.9 |
93.4 |
82.0 |
85.0 |
|
M. exiguus
|
|
|
|
|
|
100 |
84.3 |
83.5 |
99.7 |
|
M. ichkeuiensis
|
|
|
|
|
|
|
100 |
82.7 |
84.4 |
|
M. supamattayai
|
|
|
|
|
|
|
|
100 |
83.4 |
|
M. mueiieri
|
|
|
|
|
|
|
|
|
100 |