Abstract
Not only Taenia solium and Taenia saginata, but also Taenia asiatica infects humans. The last species is not included in the evaluation of the specificity of the immunodiagnostic techniques for taeniasis/cysticercosis. There is currently no specific immunodiagnostic method for T. asiatica available. Therefore, due to the fact that molecular techniques (the only tool to distinguish the 3 Taenia species) are normally not employed in routine diagnostic methods, the 2 questions concerning T. asiatica (its definite geographic distribution and its ability to cause human cysticercosis), remain open, turning T. asiatica into the most neglected agent of human taeniasis-cysticercosis.
-
Key words: Taenia asiatica, Taenia solium, Taenia saginata, human taeniasis, cysticercosis
INTRODUCTION
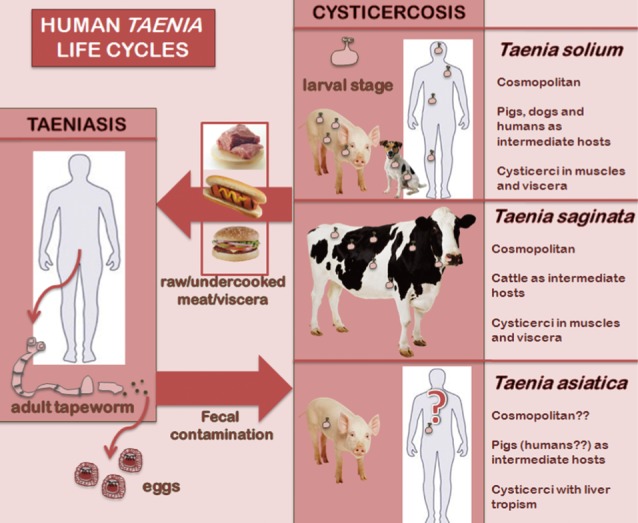
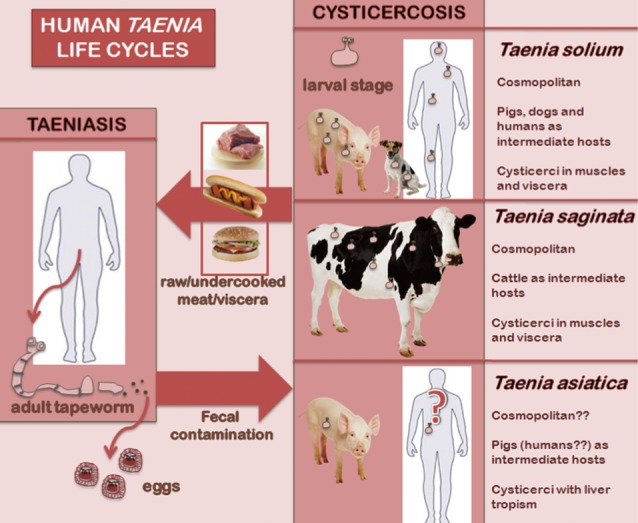
Taenia solium and
Taenia saginata cause human taeniasis, i.e., the intestinal parasitization by the adult stage of both species. Cysticercosis is caused by the larval stage or metacestode of
T. solium in humans, pigs and dogs, and by larvae of
T. saginata in cattle. Taeniasis-cysticercosis is included among the most relevant neglected zoonoses [
1,
2]. Concerning its control, the International Task Force for Disease Eradication (ITFDE) declared taeniasis-cysticercosis "potentially eradicable" in 1993 [
3]. All strategies aimed at the elimination/eradication of taeniasis and cysticercosis are focused on the 2 cosmopolitan
Taenia species,
T. solium and
T. saginata. The morphology, life cycle, epidemiology as well as the pathogenicity of both species are quite different.
T. solium and
T. saginata human infections can be diagnosed through the morphology of gravid proglottids, immunological or molecular techniques. In the case of
T. solium, the pig is the main intermediate host, and therefore humans acquire taeniasis solium through ingestion of raw or undercooked pork, while cattle take this role in the case of
T. saginata, consequently beef is the source of human infection. Concerning pathogenicity, the intestinal adult stage of both
Taenia species usually does not cause relevant symptoms, while only
T. solium may cause another disorder since the eggs of this species can infect humans. The accidental ingestion of eggs released by human tapeworm carriers causes cysticercosis, i.e. the extraintestinal development of
T. solium larvae mainly in muscles, eyes, and the central nervous system (neurocysticercosis). Epileptic seizures are a major manifestation when cysts develop in the brain. Approximately 50,000 infected individuals die annually due to neurocysticercosis [
4].
Since the recommendations of the ITFDE 20 years ago, the scenario of human taeniasis-cysticercosis has changed dramatically as a new species of
Taenia,
T. asiatica, has also been found to infect humans in addition to
T. solium and
T. saginata [
5]. This third species exhibits a
T. saginata-like morphology, but a
T. solium-like life cycle. Also, its liver larval tropism in pigs is worth mentioning (
Fig. 1).
T. asiatica is generally neglected in the global elimination context of human taeniasis-cysticercosis. We assume that T. asiatica remains ignored mainly for 2 reasons; first, its supposedly non-cosmopolitan character, being restricted to Asian countries, and second, its close molecular similarities to T. saginata, suggesting that T. asiatica probably does not cause human cysticercosis since T. saginata eggs do not infect humans.
WHY NOT COSMOPOLITAN?
It is generally believed that
T. asiatica is restricted to Asia mainly for 2 reasons; the particular culinary habits of certain Asian countries and the fact that the species has never been found out of Asia [
6,
7]. The life cycle of
T. asiatica is considered to take place in remote rural areas only, where pigs roam with free access to human feces and where people habitually eat raw liver [
6]. It seems clear that the access of pigs to human feces does not restrict the distribution of
T. asiatica to Asia, since
T. solium, which presents the same life cycle and the same infection route, is present worldwide. Concerning the origin of human infections, there is no doubt that the pork liver is the main source of cysticerci and the habit of eating it raw is not so common in other parts of the world. However, eating undercooked pork liver or dishes containing it, is not restricted to Asia since the pork liver is available in all non-Islamic countries around the world and is used in hundreds of recipes. In addition, in Vietnam, where people not used to eat raw or undercooked pork liver or viscera,
T. asiatica is the most common human
Taenia species [
8]. This point suggests alternative location sites for the cysticerci in pigs, also supported by the fact that
T. asiatica is not a sporadic parasite as it is present in the majority of East and South East Asian countries (Taiwan, Indonesia, Korea, Vietnam, the Philippines, Thailand, and China) and has a prevalence of up to 21% [
9], being more common than
T. solium or
T. saginata [
10,
11]. In addition, it has been suggested that
T. asiatica could have an effect on
T. solium transmission dynamics due to interspecific competition since both species share the same hosts [
12].
T. asiatica has also been detected in Japan [
7] and Lao PDR (non-published data
http://aciar.gov.au/project/AH/2006/161). It is well accepted that
T. solium and
T. saginata circulate around the world, and there are no reasons why globalization should not have affected
T. asiatica, since it is a tapeworm with cosmopolitan hosts with migratory movements which are clearly linked to the spread of neglected diseases [
13]. Precisely, due to the migratory movements from Asia to the rest of the world, it is common to find imported cases of Asiatic parasites in America or Europe.
Clonorchis sinensis and
Opistorchis viverrini, for instance, are frequently found in Asian people in the US [
14].
Diphyllobothrium nihonkaiense originally endemic in Japan, is an emerging parasite in European countries after molecular techniques were used in its diagnosis [
15].
Imported cases of T. asiatica out of Asia might be relevant as pigs are present in all non-Islamic countries. Clearly, the specific name "asiatica" does not confer to the third human taeniid a cosmopolitan character and it wrongly evokes the impression that the species is confined to Asia. Nevertheless, extensive epidemiologic surveys are required to establish the real burden of this parasite out of Asia. The fact that, until now, T. asiatica has not been found out of Asia is probably a question of misdiagnosis in humans as well as in pigs.
DIAGNOSIS OF T. ASIATICA TAENIASIS
T. asiatica has been misdiagnosed as
T. saginata for more than 200 years in Asia [
16] and the same might currently occur in the rest of the world, since the morphology of
T. asiatica's gravid proglottids is practically indistinguishable from that of
T. saginata.
Human taeniasis can also be diagnosed by immunological techniques [
17]. Among the antibody-detection methods, an immunoblot assay, i.e., immunoelectrotranfer blot (EITB), has been developed for the diagnosis of
T. solium being 100% specific. However, this EITB has not been tested with human sera infected with
T. asiatica for cross-reactivity. Consequently,
T. asiatica may produce a false positive result, being misdiagnosed as
T. solium. The EITB recently designed by Jeon and Eom seems a promising tool to discriminate
T. asiatica carriers since it exhibits a specific band for this species [
18].
Among coproantigen-detection methods (only genus specific), a coproantigen ELISA for
T. solium was tested with
T. asiatica but did not cross-react [
19]. Therefore, this is the only 100% specific immunological method currently available to identify
T. solium carriers, but not
T. asiatica carriers. Even a positive result for
T. solium in this ELISA would not exclude
T. asiatica since dual human infection with
T. solium and
T. asiatica has been reported in Thailand [
10].
Taking into accounts that morphology and immunotechniques are useless when diagnosing T. asiatica, the only tools to avoid misdiagnoses are molecular methods, scarcely used in conventional diagnostic procedures. Therefore, only after employing molecular methods in the long term on a worldwide scale, it may be possible to affirm whether T. asiatica is or is not restricted to Asia.
Unless multiplex PCR [
20] or PCR-RFLP [
8] are used, any
T. saginata-like gravid proglottids expelled by humans around the world could indeed be
T. saginata or
T. asiatica [
10], thus we propose that it should be named
T. saginata/
asiatica taeniasis.
DIAGNOSIS OF T. ASIATICA CYSTICERCOSIS IN PIGS
We face the same problem in the intermediate host since none of the antemortem antibody or antigen detection methods for pig cysticercosis is specific for
T. asiatica. To date, only 1 technique has been tested in pigs with
T. asiatica, namely the EITB which is 100% specific for
T. solium [
21].
T. asiatica cross-reacts even in the most specific serological test for
T. solium cysticercosis. Recently, the high potential of nanobodies (Nbs) as a tool for the diagnosis of
T. solium cysticercosis in pigs has been demonstrated [
22]. Obtained Nbs do not cross-react neither with
T. hydatigena nor with
T. saginata, among other parasites, turning it into a promising tool for a species- specific diagnosis of
T. solium cysticercosis whenever sera of
T. Asiatica-infected pigs do not cross-react in the antigen detection assay employing these Nbs.
Hence, in absence of a specific immunological test for T. Asiatica, only the post-mortem molecular analysis of the extracted cysticerci can be used to assess the species causing pig cysticercosis. As a consequence, T. asiatica may be misdiagnosed as T. solium not only in humans but also in pigs. Therefore, any pig cysticercosis around the world diagnosed only by immunological methods could be produced also by T. asiatica, so we propose that it should be named T. solium/asiatica cysticercosis.
HUMAN CYSTICERCOSIS AND T. ASIATICA
WHO/FAO/OIE maintains that
T. asiatica does not cause human cysticercosis due to its molecular similarities with
T. saginata, the species that does not cause this disorder [
23]. However,
T. saginata does not produce pig cysticercosis either but
T. asiatica does, since the pig and not cattle is its intermediate host in spite of this molecular likeness. A recent discovery relates the relevant relationship between intestinal host microflora and the hatching of helminth eggs [
24]. From this point of view, it is comprehensible that
T. saginata eggs, which, in nature, only hatch in the intestine of herbivores, do not infect omnivorous intermediate hosts such as humans. Nevertheless,
T. solium eggs infect only omnivorous intermediate hosts (pigs, humans, and dogs). In nature,
T. asiatica eggs, just as
T. solium, do not infect herbivorous hosts since they only hatch in omnivorous intermediate hosts (pigs and wild boars). It could be a question of the different composition of digestive enzymes or bile acids among different intermediate hosts. Furthermore, in vitro hatching features differ significantly between
T. saginata and
T. asiatica eggs [
25]. Therefore, humans are perfect candidates to occupy a place on the list of
T. asiatica intermediate hosts [
26].
T. asiatica cysticerci show a clear liver tropism in pigs which indicates that
T. asiatica may mainly cause hepatic cysticercosis in humans [
27]. Currently, there is no specific immunological method for
T. asiatica cysticercosis, and, as already mentioned,
T. asiatica cross-reacts even in the most specific serological test for
T. solium cysticercosis. Therefore, the application of serological approaches only can certainly not demonstrate whether
T. asiatica is or is not involved in human cysticercosis cases. We have already suggested the use of liver ultrasonography to investigate the possible presence of cysticerci in patients harbouring
T. saginata-like adults [
28]. Just as in pigs, only the molecular analysis of the extracted cysticerci could assess the species causing human cysticercosis.
CONCLUSIONS
Due to (i) the alomst impossibility to distinguish the morphology of T. asiatica and T. saginata gravid proglottids, (ii) current immunodiagnostic techniques that can not identify T. asiatica (adult/cysticerci) carriers (humans or pigs), and (iii) the fact that molecular techniques (the only tool to distinguish the 3 Taenia species) are normally not employed in routine diagnostic methods, the 2 questions concerning T. asiatica (its definite geographical distribution and its ability to cause human cysticercosis) remain open, turning T. asiatica into the most neglected agent of human taeniasis-cysticercosis. These knowledge gaps should first be assessed before strategies of global taeniasis-cysticercosis elimination are implemented, since T. asiatica is not only a potential candidate to produce human cysticercosis but, as well, a modulating factor in the epidemiology of T. solium.
References
- 1. Maudlin I, Eisler MC, Welburn SC. Neglected and endemic zoonoses. Philos Trans R Soc Lond B Biol Sci 2009;364:2777-2787.
- 2. Hotez PJ, Pecoul B. "Manifesto" for advancing the control and elimination of neglected tropical diseases. PLoS Negl Trop Dis 2010;4:e718.
- 3. Schantz PM, Cruz M, Sarti E, Pawlowski Z. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ 1993;27:397-403.
- 4. Mafojane NA, Appleton CC, Krecek RC, Michael LM, Willingham AL. The current status of neurocysticercosis in Eastern and Southern Africa. Acta Trop 2003;87:25-33.
- 5. Eom KS, Rim HJ. Morphological description of Taenia asiatica sp. n. Korean J Parasitol 1993;31:1-6.
- 6. Ito A, Nakao M, Wandra T. Human taeniasis and cysticercosis in Asia. Lancet 2003;362:1918-1920.
- 7. Eom KS, Jeon HK, Rim HJ. Geographical distribution of Taenia asiatica and related species. Korean J Parasitol 2009;47(suppl):S115-S124.
- 8. Somers R, Dorny P, Geysen D, Nguyen LA, Thach DC, Vercruysse J, Nguyen VK. Human tapeworms in north Vietnam. Trans R Soc Trop Med Hyg 2007;101:275-277.
- 9. Fan PC. Annual economic loss caused by Taenia saginata asiatica taeniasis in East Asia. Parasitol Today 1997;13:194-196.
- 10. Anantaphruti MT, Yamasaki H, Nakao M, Waikagul J, Watthanakulpanich D, Nuamtanong S, Maipanich W, Pubampen S, Sanguankiat S, Muennoo C, Nakaya K, Sato MO, Sako Y, Okamoto M, Ito A. Sympatric occurrence of Taenia solium, T. saginata, and T. Asiatica, Thailand. Emerg Infect Dis 2007;13:1413-1416.
- 11. Jeon HK, Kim KH, Chai JY, Yang HJ, Rim HJ, Eom KS. Sympatric distribution of three human Taenia tapeworms collected between 1935 and 2005 in Korea. Korean J Parasitol 2008;46:235-241.
- 12. Conlan JV, Vongxay K, Fenwick S, Blacksell SD, Thompson RCA. Does interspecific competition have a moderating effect on Taenia solium transmission dynamics in Southeast Asia? Trends Parasitol 2009;25:398-403.
- 13. Aagaard-Hansen J, Nombela N, Alvar J. Population movement: a key factor in the epidemiology of neglected tropical diseases. Trop Med Int Health 2010;15:1281-1288.
- 14. Fried B, Abruzzi A. Food-borne trematode infections of humans in the United States of America. Parasitol Res 2010;106:1263-1280.
- 15. Arizono N, Yamada M, Nakamura-Uchiyama F, Ohnishi K. Diphyllobothriasis associated with eating raw Pacific salmon. Emerg Infect Dis 2009;6:866-870.
- 16. Eom KS. What is Asian Taenia? Parasitol Int 2006;55(suppl):S137-S141.
- 17. Deckers N, Dorny P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol 2010;26:137-144.
- 18. Jeon HK, Eom KS. Immunoblot patterns of Taenia asiatica taeniasis. Korean J Parasitol 2009;47:73-77.
- 19. Guezala MC, Rodriguez S, Zamora H, Garcia HH, Gonzalez AE, Tembo A, Allan JC, Craig PS. Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am J Trop Med Hyg 2009;81:433-437.
- 20. Yamasaki H, Allan JC, Sato MO, Nakao M, Sako Y, Nakaya K, Qiu D, Mamuti W, Craig PS, Ito A. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol 2004;42:548-553.
- 21. Pilcher JB, Tsang VC, Gilman RH, Rhodes ML, Pawlowski ZS. Further evidence of 100% specificity in a recently developed Taenia solium (cysticercosis) immunoblot assay. Am J Trop Med Hyg 1991;45(suppl 3):131.
- 22. Deckers N, Saerens D, Kanobana K, Conrath K, Victor B, Wernery U, Vercruysse J, Muyldermans S, Dorny P. Nanobodies, a promising tool for species-specific diagnosis of Taenia solium cysticercosis. Int J Parasitol 2009;39:625-633.
- 23. Dorny P, Brandt J, Geerts S. Murrell KD. Detection and diagnosis. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/Cysticercosis. 2005, Paris. OIE, WHO & FAO; pp 45-55.
- 24. Hayes KS, Bancroft AJ, Goldrick M. Portsmouth C, Roberts IS, Grencis RK. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 2010;328:1391-1394.
- 25. Ito A, Fan PC, Chung WC, Suzuki M. Cross protections against Taenia taeniaeformis in rats vaccinated with non-viable oncospheres of Asian Taenia or T. saginata. J Helminthol 1994;68:83-85.
- 26. Galán-Puchades MT, Fuentes MV. Taenia asiatica intermediate hosts. Lancet 2004;363:660.
- 27. Galán-Puchades MT, Fuentes MV. Human cysticercosis and larval tropism of Taenia asiatica. Parasitol Today 2000;16:174.
- 28. Teresa Galán-Puchades MT, Fuentes MV. The usefulness of ultrasound diagnosis specifically in taeniasis. Gut 2009;58:465.
Fig. 1Life cycles of the 3 human Taenia tapeworms.