Abstract
A loop-mediated isothermal amplification (LAMP) assay allows rapid diagnosis of Toxoplasma gondii infection. In the present study, the LAMP assay was evaluated using blood from both naturally and experimentally infected pigs. The sensitivity of the LAMP assay was compared with that of Q-PCR. Both assays detected T. gondii in the blood of experimentally infected pigs, with 100% agreement. In infected blood samples, the parasite was detected as early as 2 days post-infection and reached a peak in 3-5 days. In 216 field serum samples, the detection rates of LAMP and Q-PCR assays were 6.9% and 7.8%, respectively. This result indicates that the sensitivity of the LAMP assay was slightly lower than that of the Q-PCR assay. However, the LAMP may be an attractive diagnostic method in conditions where sophisticated and expensive equipment is unavailable. This assay could be a powerful supplement to current diagnostic methods.
-
Key words: Toxoplasma gondii, loop-mediated isothermal amplification (LAMP), Q-PCR, pig
Toxoplasma gondii is an obligate intracellular parasite that causes toxoplasmosis and is capable of infecting a variety of mammals and birds.
T. gondii is an important foodborne parasite whose main route of transmission from animals to humans is through the consumption of infected meat [
1]. In some countries, pork is the most common meat consumed, and some ethnic groups consume raw pork; thus, pigs are considered the primary source of human infections with
T. gondii [
2]. In addition, due to gross lesions in infected animals leading to their being condemned at slaughter, expenses associated with treatment, and weight loss associated with clinical diseases, toxoplasmosis is a source of significant economic loss for swine farmers. Therefore, the establishment of a rapid, highly specific, and accurate method for diagnosis of
T. gondii infection is essential to administer appropriate treatment and reduce economic losses.
The diagnosis of toxoplasmosis is based on detection of specific antibodies in serum samples using serological assays, isolation of the parasite by mouse bioassay, or amplification of parasite DNA from biological samples by PCR [
3,
4]. Classical serology methods cannot differentiate between vaccine-induced and infection-induced antibodies [
5] and between past and present infections [
6]. Although the definitive method of diagnosis of
T. gondii infection is isolation of the pathogen from infected tissues, this method is labor-intensive, time-consuming and expensive, and it relies upon the submission of fresh material to the diagnostic laboratory. For these reasons, it is generally impractical in diagnostic situations. PCR (such as Q-PCR) is a useful diagnostic tool to detect the presence of infection indirectly [
7]. Despite these advances, PCR methods are still limited in some regions where sophisticated and expensive equipment is unavailable.
Loop-mediated isothermal amplification (LAMP), which was originally developed by Notomi et al. [
8], is a very sensitive, easy, and less time-consuming method. The advantage of this assay is that LAMP products can easily be detected by the naked eye due to the formation of magnesium pyrophosphate, a turbid white by-product of DNA amplification that accumulates as the reaction progresses [
9]. LAMP products can also be detected by direct fluorescence [
10]. Fluorescent dyes, such as ethidium bromide, SYBR green, and Evagreen, can also be used for visualization of LAMP products under UV light [
11]. LAMP can amplify different types of samples, including purified DNA, heat-treated blood, and blood dried on filter paper [
12,
13]. In addition, Thekisoe et al. [
14] have reported that LAMP reagents are relatively stable even when stored at 25 or 37℃, which supports the use of LAMP in field conditions and resource poor laboratories.
Recently, this method was found to be a powerful diagnostic tool, and LAMP assays targeting the SAG1 gene (SAG1-LAMP), 529-bp repetitive element, B1 gene, SAG2 gene, and
TgOWP of
T. gondii were developed [
6,
15-
19]. The LAMP based on the 529-bp repetitive element was shown to be useful for detection of
T. gondii DNA extracted from veterinary samples [
6,
18]. Kong et al. [
15] also reported that the LAMP based on the 529-bp repetitive element was able to detect
T. gondii DNA in mouse blood samples. The SAG1-LAMP assay was applied to detect the presence of
T. gondii in infected mouse organs. This indicates that the method is attractive for
T. gondii detection in biopsy specimens [
16]. LAMP assays based on SAG1, SAG2, and B1 genes were shown to be useful for detection of
T. gondii DNA extracted from blood samples in humans [
17]. Sotiriadou and Karanis [
19] reported that a LAMP amplification method targeting the
TgOWP and B1 genes is among the most accurate molecular methods as a specific, sensitive, simple, and rapid diagnostic tool for the detection of
T. gondii in water samples.
Early diagnosis of toxoplasmosis is essential for a successful treatment. However, we know very little about the effectiveness of SAG1-LAMP for detection of acute toxoplasmosis in pigs. Therefore, the aim of this study was to utilize both LAMP and Q-PCR protocols to obtain data through amplification of T. gondii DNA from routine blood samples from the pig.
For this study, 15 healthy pigs (7-9-week-old), which were Toxoplasma seronegative as determined by ELISA, were purchased from a pig farm in Lanzhou, China. The pigs in this study were raised on a conventional farm or in experimental isolation units. The experimental protocol was approved by the Ethical Committee of the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China.
Each pig was challenged intraperitoneally with 10
7 tachyzoites of the highly virulent Gansu Jingtai strain (GJS) of
T. gondii. Blood samples were collected by armpit bleeding at days 2, 3, 4, 5, 6, 7, 10, 14, 21, 28, and 35 post-infection (PI) from 15 pigs. DNA from the blood samples for the LAMP and Q-PCR assays was extracted using the Universal Genomic DNA Extraction kit (TaKaRa Biotechnology Co., Ltd, Dalian, China) according to the manufacturer's protocol. DNA from healthy pig blood was extracted in parallel with the experimental samples and used as the negative control. The blood samples collected from each pig on day 5 PI were also used for mouse bioassay. Each sample was inoculated with a volume of 0.5 ml subcutaneously into 5 mice (25-30 g). Impression smears of the lung tissue from the mice that died were stained with Giemsa and examined microscopically. The brain of each mouse that survived 60 days after inoculation was examined microscopically for
T. gondii tissue cysts. Parasites were successfully isolated in all of the blood samples collected on day 5 PI by mouse bioassay. The results were shown in
Table 1.
For the LAMP assay targeting the SAG1 gene primers [
17], B3 (GTAGCAGGACCTTTCGCG), F3 (GCGCAACGAAGACTGTTGA), BIP (TGGCAAGGAATGCACAGACTCGGACCTTAGCTGTCAAGACCG), and FIP (CGCCACTGGGACTGATGGTTAGTGCACCCTCCAGTGGTTC) were used. The LAMP assay was performed as previously described by Lau corrected as Lau et al. [
17]. The LAMP reaction was performed in a total volume of 25 µl containing 2 µl of the extracted DNA, 40
pmol (each) of primers FIP and BIP, 5
pmol (each) of primers B3 and F3, 1 µl of Bst DNA polymerase (NEB) in 2.5 µl of buffer (20 mM Tris-HCl [pH 8.8], 10 mM KCl, 8 mM MgSO
4, 10 mM [NH4]
2SO
4, 0.1% Tween 20), 0.8 M betaine (Sigma, St. Louis, Missouri, USA), and 1.4 mM deoxynucleoside triphosphate (dNTP). The LAMP reaction was incubated for 60 min at 65℃ and inactivated for 2 min at 80℃. The resulting amplicons were detected by visual observations after addition of SYBR green I and analyzed by 1.5% agarose gel electrophoresis, followed by ethidium bromide staining and photography.
SYBR Green I-based Q-PCR targeting the SAG1 gene of
T. gongdii was carried out as previously described by Wang [
20], using the primers 5'-GCCTCATCGGTCGTCAATAA-3' and 5'-CTCCATCTTTCCCGCACAC-3'. The Q-PCR reactions were performed in a 25-µl volume containing 2×SYBR Premix Ex Taq II supplemented with ROX II, primers (10 µM) and 2 µl of DNA template. The cycling parameters were to preheat at 95℃ for 30 sec, followed by 40 cycles of 95℃ for 5 sec and 64℃ for 20 sec. After amplification, the data were analyzed using the 7500 System software.
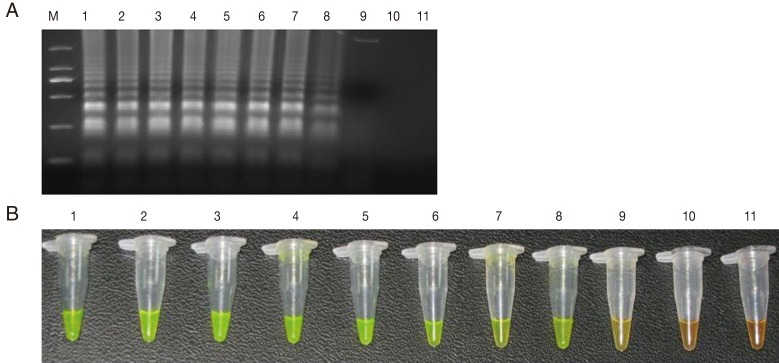
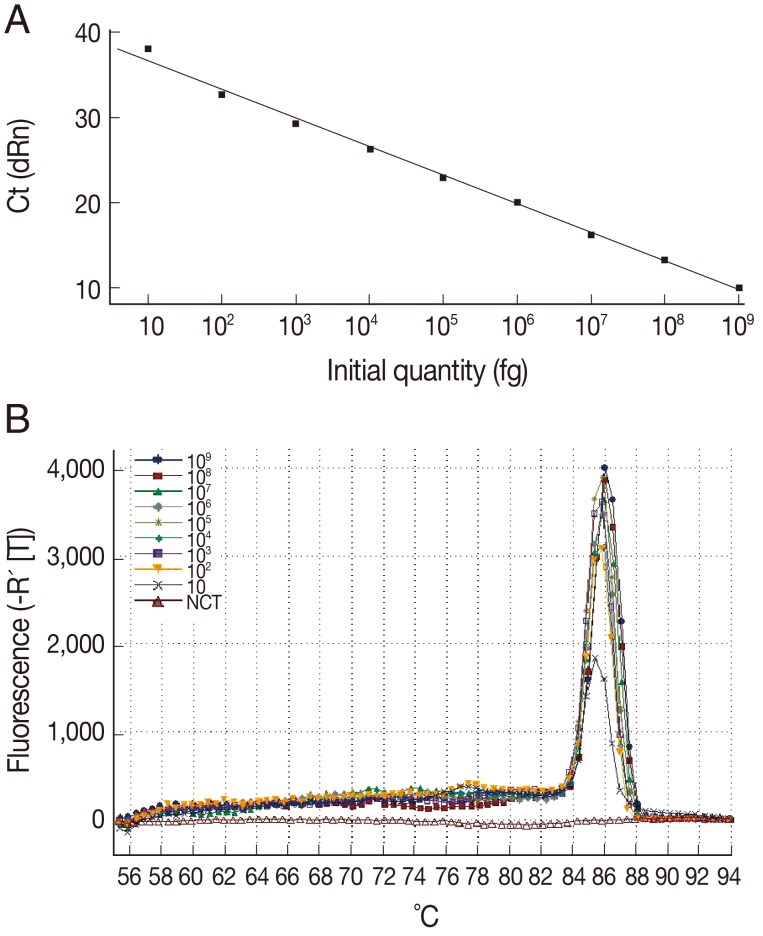
The sensitivity of the LAMP assay in comparison to the Q-PCR assay was determined using the genomic DNA of
T. gondii GJS tachyzoites, which was diluted to contain an amount of DNA equivalent to a range of 0.01-10,000,000 tachyzoites per LAMP and Q-PCR reaction. As shown in
Fig. 1, the detection limit for the LAMP assay was 100 fg, corresponding to 1
T. gondii tachyzoite. For the Q-PCR assay, the slope was -3.370, with an R
2=0.995 and a reaction efficiency of 98%. The standard curve generated using 10-fold dilutions of the
T. gondii tachyzoite DNA was linear over 8 orders of magnitude (10 to 10
9 fg), indicating that accurate measurement of target DNA over a large concentration range is possible with Q-PCR. The detection limit was found to be 10 fg, corresponding to 0.1
T. gondii tachyzoite (
Fig. 2). These results indicated that the sensitivity of the LAMP assay was slightly lower than that of the Q-PCR method for detection of
T. gondii DNA. Lin et al. [
18] previously reported that the relative sensitivity of the LAMP assay targeting a 529-bp repetitive element was slightly lower than that of the Q-PCR method, which is consistent with our results.
To evaluate the efficiency of the LAMP assay for detecting
T. gondii in blood, it was tested using the blood samples from experimentally infected pigs. All DNA samples were tested using both the LAMP and Q-PCR assays. Parasites were detected in most of the blood samples collected from days 2 to 14 PI, with a peak on days 3 to 5 PI (
Table 1). Parasites could be detected in a few samples collected on days 21 and 35 PI. There was 100% agreement between the results of the LAMP and Q-PCR assays. In addition, the positive samples by mouse bioassay were also positive by LAMP, which suggested that LAMP is reliable for detection of
T. gondii infection. In this study, the SAG1 gene was detected using these 2 assays in most of the blood samples from animals with clinical signs of disease. It is likely that tachyzoites express distinct sets of surface antigens, which may contribute to an immune evasion mechanism that allows
T. gondii to persist [
21]. The SAG1 gene may have potential for providing molecular information regarding the actual state of infection.
In addition, both the LAMP and Q-PCR assays were also used to test 216 field blood samples collected from Gansu province. Fifteen samples were positive by both the LAMP and Q-PCR assays. Two samples were negative according to LAMP but positive according to Q-PCR. This discrepancy was attributed to a lower level of
T. gongdii DNA, which fell below the limit of detection of the LAMP assay. The detection rates of the LAMP and Q-PCR assays in field blood samples were 6.9% (15/216) and 7.9% (17/216), respectively. This result indicated the sensitivity of the LAMP assay by visual inspection using SYBR Green I stain was slightly lower than that of the Q-PCR assay. It is important to take into consideration that Q-PCR is time-consuming and requires a thermal cycler with real-time monitoring and expensive data analysis systems. The LAMP assay has clear advantages over the Q-PCR assay, as it is a practical system that can be used in a standard diagnostic laboratory, particularly in developing countries where the disease is prevalent. In addition, previous reports have demonstrated that the sensitivity of the LAMP method is higher than the conventional PCR or nested PCR methods for detection of
T. gongdii [
6,
17].
In conclusion, although discrepancies between the LAMP and Q-PCR results occur in a minority of samples, the LAMP method can be applied for detection of T. gondii parasites for both experiment infected and field-infected blood samples from pig. Particularly, LAMP may be an attractive diagnostic method in conditions where sophisticated and expensive equipment is not available.
National Special Research Programs for Non-profit Trades (Agriculture)200903036-02
NBCITS, MOACARS-38
National Non-profit Institute Research Grant1610322012026
Notes
-
We have no conflict of interest related with this study.
ACKNOWLEDGMENTS
This investigation was supported by grants from the National Special Research Programs for Non-profit Trades (Agriculture) (200903036-02) and NBCITS, MOA (CARS-38). This investigation was also supported by National Non-profit Institute Research Grant (1610322012026). We thank Prof. Wanxiang Xv for providing the field samples.
References
- 1. Nielsen HV, Lauemøller SL, Christiansen L, Buus S, Fomsgaard A, Petersen E. Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect Immun 1999;67:6358-6363.
- 2. Dubey JP, Baker DG, Davis SW, Urban JF, Shen SK. Persistence of immunity to toxoplasmosis in pigs vaccinated with a non-persistent strain of Toxoplasma gondii. Am J Vet Res 1994;55:982-987.
- 3. Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, Decoster A, Villena I, Jenum PA, Hayde M, Naessens A. Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. Am J Obstet Gynecol 1999;181:843-847.
- 4. Pelloux H, Weiss J, Simon J, Muet F, Fricker-Hidalgo H, Goullier-Fleuret A, Ambroise-Thomas P. A new set of primers for the detection of Toxoplasma gondii in amniotic fluid using polymerase chain reaction. FEMS Microbiol Lett 1996;138:11-15.
- 5. Wang YH, Li XR, Wang GX, Yin H, Cai XP, Fu BQ, Zhang DL. Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitol Int 2011;60:105. 107.
- 6. Zhang H, Thekisoe OM, Aboge GO, Kyan H, Yamagishi Y, Inoue N, Nishikawa Y, Zakimi S, Xuan X. Toxoplasma gondii: Sensitive and rapid detection of infection by loop-mediated isothermal amplification (LAMP) method. Exp Parasitol 2009;122:47-50.
- 7. Switaj K, Master A, Skrzypczak M, Zaborowski P. Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin Microbiol Infect 2005;11:170-176.
- 8. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 2000;28:E63.
- 9. Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 2001;289:150-154.
- 10. Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 2008;3:877-882.
- 11. Qiao YM, Guo YC, Zhang XE, Zhou YF, Zhang ZP, Wei HP, Yang RF, Wang DB. Loop-mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol Lett 2007;29:1939-1946.
- 12. Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol 2003;41:5517-5524.
- 13. Poon LLM, Wong BWY, Ma EHT, Chan KH, Chow LMC, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS. Sensitive and inexpensive molecular test for Falciparum Malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem 2006;52:303-306.
- 14. Thekisoe OMM, Bazie RSB, Coronel-Servian AM, Sugimoto C, Kawazu S, Inoue N. Stability of loop-mediated isothermal amplification (LAMP) reagents and its amplification efficiency on crude trypanosome DNA templates. J Vet Med Sci 2009;71:471-475.
- 15. Kong QM, Lu SH, Tong QB, Lou D, Chen R, Zheng B, Kumagai T, Wen LY, Ohta N, Zhou XN. Loop-mediated isothermal amplification (LAMP): Early detection of Toxoplasma gondii infection in mice. Parasit Vectors 2012;5:2.
- 16. Krasteva D, Toubiana M, Hartati S, Kusumawati A, Dubremetz JF, Widada JS. Development of loop-mediated isothermal amplification (LAMP) as a diagnostic tool of toxoplasmosis. Vet Parasitol 2009;162:327-331.
- 17. Lau YL, Meganathan P, Sonaimuthu P, Thiruvengadam G, Nissapatorn V, Chen Y. Specific, sensitive, and rapid diagnosis of active toxoplasmosis by a loop-mediated isothermal amplification method using blood samples from patients. J Clin Microbiol 2010;48:3698-3702.
- 18. Lin ZB, Zhang YL, Zhang HS, Zhou YZ, Cao J, Zhou JL. Comparison of loop-mediated isothermal amplification (LAMP) and real-time PCR method targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Vet Parasitol 2012;185:296-300.
- 19. Sotiriadou I, Karanis P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT). Diagn Microbiol Infect Dis 2008;62:357-365.
- 20. Wang YH. The study of antigenic epitopes from Toxoplasma gondii excreted/secreted antigens and surface protein 1. Chinese Academy of Agricultural Sciences; 2011, pp 19-20.
- 21. Kim SK, Boothroyd JC. Stage specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J Immunol 2005;174:8038-8048.
Fig. 1Sensitivity of the LAMP assay using agarose gel electrophoresis (A) and SYBR Green I stain (B). M: DNA marker; 1-10 are the reaction results from a 10-fold serial dilution of T. gondii tachyzoite genomic DNA (equivalent to a range of 0.01-10,000,000 tachyzoites per LAMP reaction). 11: negative control.
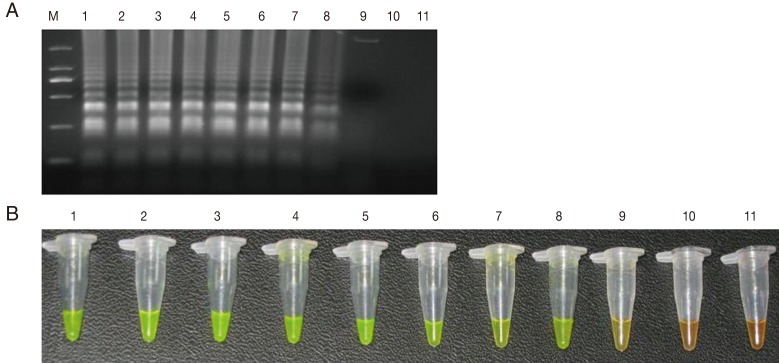
Fig. 2Sensitivity and specificity of the Q-PCR assay for T. gondii. (A) Standard curve of Q-PCR for T. gondii. (B) Dissociation curve of Q-PCR for detecting T. gondii. The assay standard curve was generated from a 10-fold serial dilution of a known concentration of T. gondii tachyzoite genomic DNA (equivalent to a range of 0.1-10,000,000 tachyzoites per Q-PCR reaction).
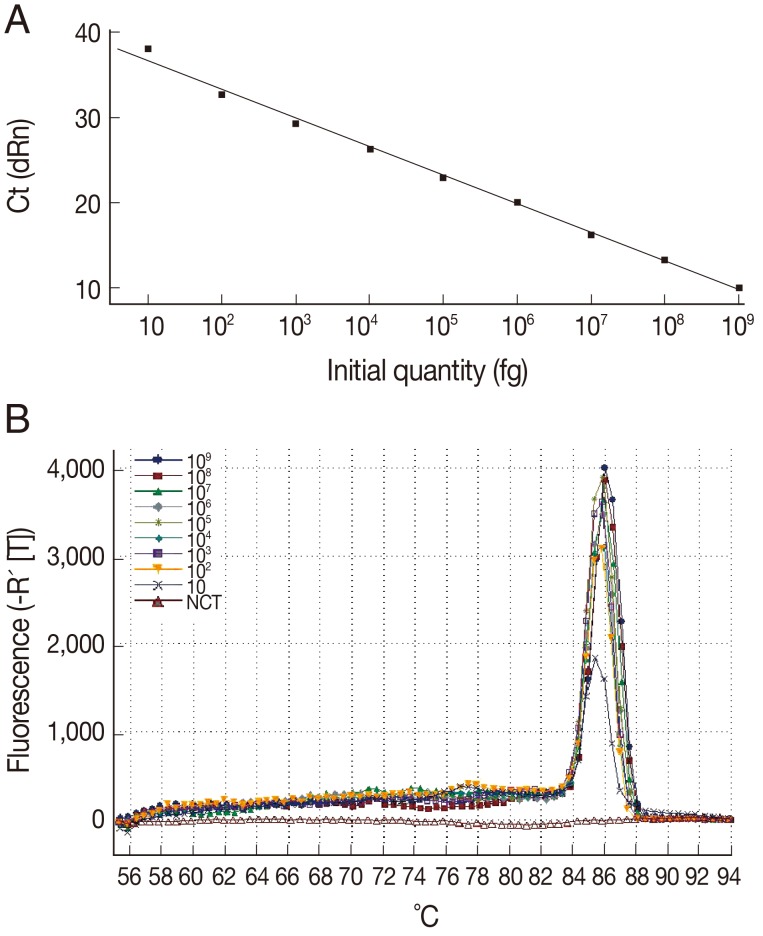
Table 1.Detection of T. gondii in blood from experimentally infected pigs by LAMP and Q-PCR
Table 1.
|
Assays |
Blood samples collected from 15 pigs at different time points (day PI)a
|
Control samplesb
|
|
Day PI |
2 |
3 |
4 |
5c
|
6 |
7 |
10 |
14 |
21 |
28 |
35 |
|
|
LAMP |
5/15 |
14/15 |
15/15 |
15/15 |
13/15 |
10/15 |
8/15 |
6/15 |
3/15 |
1/15 |
0/15 |
0/15 |
|
O-PCR |
5/15 |
14/15 |
15/15 |
15/15 |
13/15 |
10/15 |
8/15 |
6/15 |
3/15 |
1/15 |
0/15 |
0/15 |