Abstract
Opisthorchis viverrini infection causes inflammation and liver injury leading to periductal fibrosis. Little is known about the pathological alterations in bile canaliculi in opisthorchiasis. This study aimed to investigate bile canalicular alterations in O. viverrini-infected hamsters and to examine the chemopreventive effects of curcumin on such changes. Hamsters were infected with O. viverrini and one group of animals was fed with 1% dietary curcumin supplement. Animals were examined during the acute infection phase, days 21 and 30 post-infection (PI) and chronic infection phase (day 90 PI). Scanning electron microscopy revealed that in the infected group fed with a normal diet, bile canaliculi became slightly tortuous by 30 day PI and more tortuous at day 90 PI. Transmission electron microscopy showed a reduction in microvilli density of canaliculi starting at day 30 PI, with a marked loss of microvilli at day 90 PI. These ultrastructral changes were slightly seen at day 21 PI, which was similar to that found in infected animals fed with 1% curcumin-supplemented diet. Notably, curcumin treatment prevented the reduction of microvilli density, reduced the dilation of bile canaliculi, and decreased the tortuosity of the bile canaliculi relative to non-infected animals on a normal diet at days 30 and 90 PI. These results suggest that curcumin reduces alteration of bile canaliculi and may be a promising agent to prevent the onset of bile duct abnormalities induced by O. viverrini infection.
-
Key words: Opisthorchis viverrini, bile canaliculus, curcumin, hamster liver, ultrastructure
INTRODUCTION
Opisthorchiasis is a parasitic disease caused by the liver fluke,
Opisthorchis viverrini. This medically important fluke is endemic in many tropical countries of Southeast Asia such as Laos, southern Vietnam, Cambodia, and Thailand [
1]. In Thailand, it is highly prevalent in some rural areas of the northeastern region, where people like consuming the flesh of pickled, raw or poorly cooked freshwater fish such as
pla-som,
koi-pla, and
pla-ra [
2]. People are infected with
O. viverrini when they consume these undercooked foods contaminated with the infective metacercariae. The excysted metacercariae then develop into juvenile flukes within the biliary tree of the liver, leading to acute and chronic cholangitis [
1]. Early histological findings in the livers of infected hamsters are infiltration of inflammatory cells and bile duct epithelial hyperplasia. In cases of chronic infection, periductal fibrosis increases over time. These are the major risk factors for cholangiocarcinoma (CCA) [
1,
3,
4].
Ultrastructural investigation of the hepatocytes of
O. viverrini-infected hamsters shows accumulation of intermediate filaments in the adjacent bile duct epithelial cells and in the epithelial lining of the gall bladder. It has been suggested that these findings are related to neoplastic transformation [
5]. Bile canaliculi, the minute anastomosing channels formed by adjacent hepatocytes, collect bile secreted by the hepatocytes. The bile canaliculi merge and form bile ductules, which eventually become the common hepatic duct. The only report on the ultrastructure of rat bile canaliculi studied by scanning electron microscope is that of Motta and Fumagalli [
6]. However, the ultrastructure of bile canaliculi in hamsters infected with
O. viverrini has not yet been investigated.
Curcumin is the principal component found in a well-known herb, turmeric (
Curcuma longa), which is a widely used spice and coloring agent in food. Numerous studies have shown that curcumin possesses potent antioxidant and anti-inflammatory effects [
7-
9]. The prophylactic effect of curcumin on drug-induced liver fibrosis has also been demonstrated [
9,
10]. In hamsters infected with
O. viverrini, curcumin has shown a potential for chemoprevention and treatment by suppression of inflammation-mediated DNA damage [
11] and reduction of periductal fibrosis [
12]. However, it remains unclear whether these chemopreventive effects can prevent the alteration of bile canaliculi, the first channel in the biliary system. The effect of curcumin on bile canaliculi alterations was investigated using scanning and transmission electron microscopy (SEM and TEM).
MATERIALS AND METHODS
Parasite preparation
The metacercariae of
O. viverrini were obtained from cyprinoid fish in an endemic area, Ban Phai, Khon Kaen Province, in northeastern Thailand.
O. viverrini metacercariae were identified and isolated from naturally infected fish by 0.25% pepsin-HCl digestion as described previously [
12]. All selected viable
O. viverrini cysts were used to infect hamsters.
Sixty adult male Syrian golden hamsters (
Mesocricetus auratus), 4-6 weeks old and weighing 100-150 g each, were obtained from the animal center at Khon Kaen University. All animals were housed under conventional conditions and fed water and a murine diet (CP-SWT, Thailand) ad libitum before starting the experiment. The experimental animals were randomly divided into 4 groups of 15 hamsters each. Uninfected control animals were fed either a normal diet (group 1) or a 1% curcumin-supplemented diet (group 2).
O. viverrini-infected animals (50 metacercariae each by intragastric intubation) were also fed either a normal diet (group 3) or a 1% curcumin-supplemented diet (group 4). A diet supplemented with this concentration of curcumin (final concentration<1%, approximately 65 mg/day) which has the highest prevention effect on liver injury due to
O. viverrini infection. Curcumin (purity 97%, Merck-Schuchardt, Hohenbrunn, German) supplemented diet was prepared as described previously [
11,
13]. Five animals from each group were sacrificed under deep anesthesia using diethyl ether on days 21, 30, and 90 post-infection (PI). This study was approved by the Animal Ethics Committee of Khon Kaen University, Khon Kaen, Thailand (AEKKU 32/2553).
Liver tissues were taken from the peripheral area and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer solution. All tissue were rinsed for several times in 0.1 M phosphate buffer then post fixed in 1% OsO4. Dehydration was carried out with a graded series of acetone concentrations. Samples for SEM were then critical point dried, carefully fragmented with forceps, mounted on a metal stub, coated with gold, and examined using a JSM-6460 LV scanning electron microscope (JEOL, Tokyo, Japan). For TEM, the dehydrated liver tissues were infiltrated and embedded in Epon 812 resin. Semi-thin sections (1 µm thick) were stained with 2% toluidine blue. Ultrathin sections of the selected areas were cut, picked up on copper grids, and stained sequentially with uranyl acetate and lead citrate. All specimens were photographed using a JEM-1230 transmission electron microscope (JEOL, Tokyo, Japan).
To evaluate the width of bile canaliculi, scanning electron micrographs (×10,000) of canaliculi were taken from 5 randomly selected regions, and data analysis was performed using SMile View version 2.03. The density of microvilli in bile canaliculi was evaluated based on 5 TEM electron micrographs from each animal, using Digital Micrograph software (Gatan, Inc., Pleasanton, California, USA). The data were evaluated using a scoring system with the following criteria: grade 3+, densely packed microvilli; grade 2+, 25% reduction of microvilli density; grade 1+, 50% reduction of microvilli density; grade 0, 75% or higher reduction of microvilli density.
Statistical analysis
To compare the width of bile canalicular lumina and density of microvilli, the χ2 tests were used. Statistical analysis was performed using SPSS version 15 (SPSS, Chicago, Illinois, USA). A P-value less than 0.05 was considered statistically significant.
RESULTS
Normal ultrastructure of the bile canaliculi of hamsters
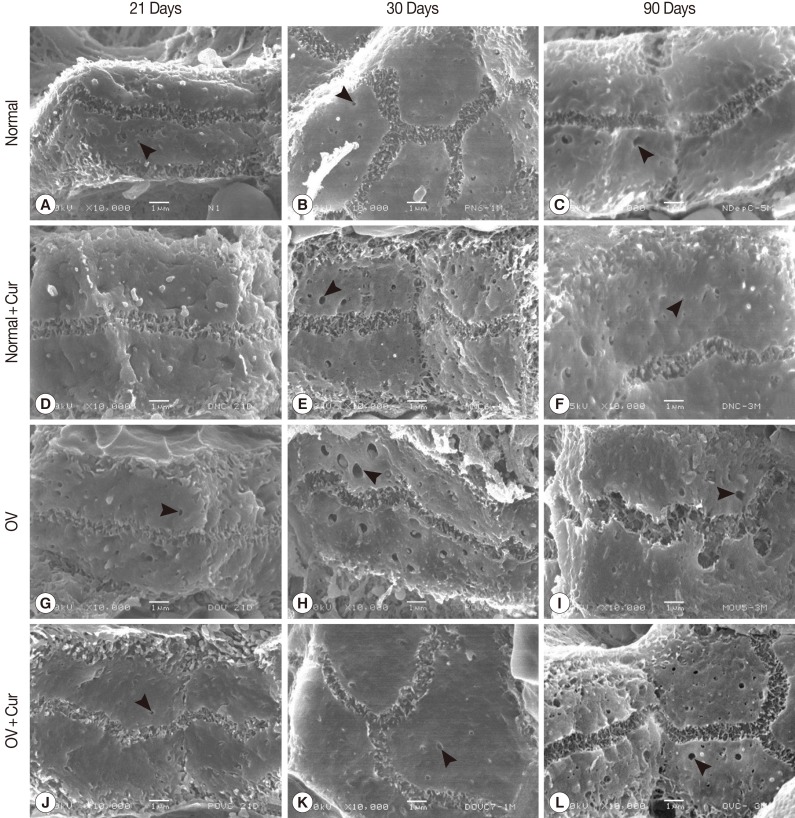
SEM examination revealed that non-infected hamsters fed with a normal diet or a 1% curcumin-supplemented diet showed similar ultrastructure of bile canaliculi on days 21, 30 and 90 PI (
Fig. 1A-F). They appeared as 3-dimensional interconnected networks of narrow tubular structures formed by the opposing surfaces of adjacent hepatocytes. Numerous microvilli projected from the wall into the lumen of the bile canaliculi throughout their entire length. In addition, several pits or small knob like indentations, and some blebs were observed on the surface of the hepatocytes. The widths of canalicular lumina in all non-infected hamsters were not significantly different on days 21, 30, and 90 PI.
O. viverrini-infected hamsters fed with a normal diet exhibited a similar appearance of the ultrastructure of bile canaliculi to that found in non-infected animals by day 21 PI (
Fig. 1G). A slightly tortuous course of the bile canaliculi was observed in infected animals on day 30 PI (
Fig. 1H). The most obvious abnormal appearance of bile canaliculi was found on day 90 PI, with a tortuous course and many lateral sacculations being evident. Microvilli were few and appeared as stud-like structures protruding from the walls of the bile canaliculi (
Fig. 1I).
Chemopreventive effect of curcumin
When
O. viverrini-infected hamsters were fed with a 1% curcumin-supplemented diet (group 4), the morphology of bile canaliculi examined on days 21, 30, and 90 PI showed long, closely packed microvilli (
Fig. 1J-L), very similar to those seen in control group animals. The lumina of bile canaliculi were relatively dilated at 90 days PI in
O. viverrini-infected hamsters, but not so significantly compared with the other treatment groups (
Table 1).
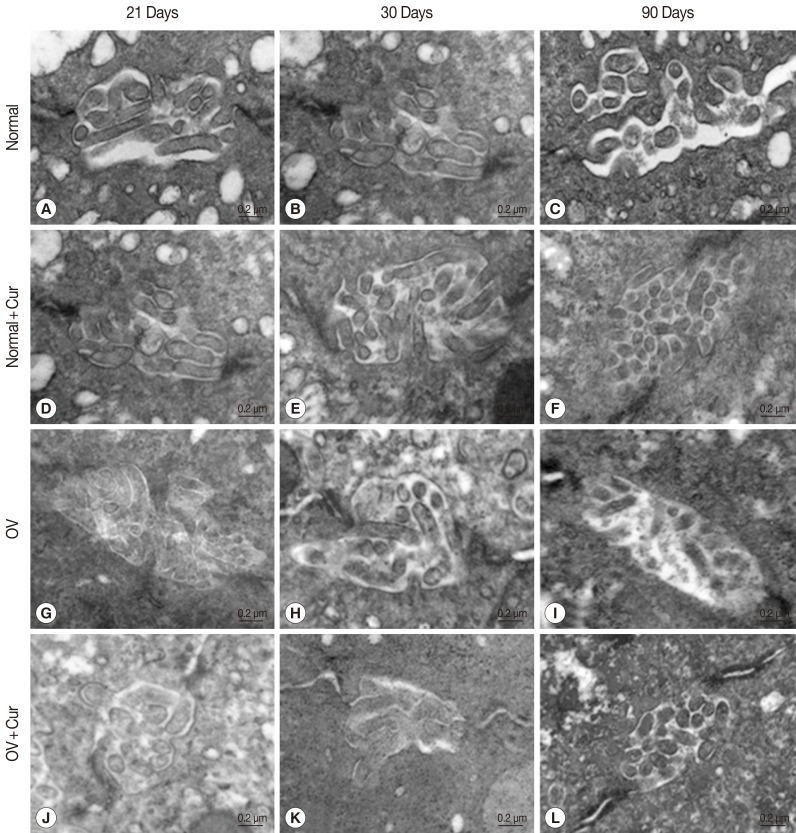
The TEM results for bile canaliculi in all experimental animal groups closely corresponded with the results of SEM. Both non-infected groups fed with a normal or a 1% curcumin-supplemented diet showed similar density of microvilli in bile canaliculi throughout the period examined (
Fig. 2A-F).
Opisthorchis viverrini-infected hamsters fed with a normal diet showed normal density of microvilli in the bile canalicular lumen only on days 21 and 30 PI (
Table 1;
Fig. 2G, H), whereas the density of microvilli in the bile canalicular lumen markedly decreased on day 90 PI (
Table 1;
Fig. 2I).
O. viverrini-infected hamsters fed with a 1% curcumin-supplemented diet exhibited a normal density of microvilli in the bile canalicular lumen at all time periods of the study (
Fig. 2J-L).
DISCUSSION
Previous studies of chronic opisthorchiasis and its associated pathological changes such as periductal fibrosis have focused on the bile ductules [
14]. However, little is known about the pathological alterations in bile canaliculi in the early phase of opisthorchiasis. We found that bile canaliculi were slightly changed by day 30 during acute infection, and there was a marked decrease in numbers of bile canalicular microvilli in the chronic stage of infection (day 90 PI). The similar finding of dilated bile canaliculi with a few short microvilli were often observed in cirrhotic and hyperplastic foci in rat livers [
15,
16]. In addition, dilation and loss of microvilli in bile canaliculi as pathological effects caused by excessive feeding of Tower rapeseed oil were observed in liver of the piglets [
17]. Moreover, abnormalities of canalicular microvilli have been reported in intrahepatic cholestasis before and after chenodeoxycholic acid therapy in patients with cholelithiasis [
18] and with cystic fibrosis [
19].
The present observations corresponded to the previous report that dilation of bile ducts and thickening of periductal fibrosis in
O. viverrini-infected hamsters progressed with time [
12]. Our results showed the chemopreventive effect of curcumin against ultrastructural changes in bile canaliculi in
O. viverrini-infected hamsters. Curcumin may have anti-inflammatory effect [
8,
11] leading to prevent bile canaliculi alteration during acute infection.
O. viverrini infection induces inflammation surrounding the bile duct lumen in hamsters. Accumulation of inflammation is predominantly seen on days 21 and 30 PI and decreases thereafter on day 90 PI [
20]. During the acute phase on days 21 and 30 PI, increased oxidative/nitrative stress induces inflammation-mediated liver injury [
21]. Sustained damage leads to obvious bile canalicular abnormalities in the chronic phase of infection on day 90 PI (
Figs. 1,
2). Curcumin has many beneficial properties, e.g., anti-inflammation, anti-oxidant, and anti-oxidative stress [
7-
9]. Furthermore, curcumin reduces oxidative/nitrative stress and prevents liver injury by balancing the oxidant and anti-oxidant system in
O. viverrini-infected hamsters [
11]. The plasma membranes of liver cells have an important role in formation of vesicles and vacuoles in the pericanalicular region [
22]. Minimization of hepatocyte injury by curcumin may prevent bile canalicular abnormalities.
Alternatively, the chemopreventive effect of curcumin may inhibit fibrogenesis leading to prevent bile canaliculi alteration during chronic infection. This hypothesis may be supported by the previous finding that curcumin reduces fibrosis during long-term treatment (day 90 PI) but not during acute stage treatment (days 21 and 30 PI) in hamsters infected with
O. viverrini [
12]. During the chronic phase, increased periductal fibrosis [
14,
23] and increased worm maturation with time may cause bile duct obstruction. In this situation, bile canaliculi present striking deviations from the normal, such as dilation and distortion of bile canaliculi including loss of microvilli. Most of these morphological abnormalities closely resemble those found in cholestatic states [
24]. Also, in certain circumstances, the chemical effects of the metabolic products and other substances released by the liver flukes [
4] may possibly directly affect bile canalicular abnormalities. Moreover, the liver membrane may be exposed, not only from toxic substances secreted by parasites, but also from bile acid [
25] and bile flooding [
26]. Increased bile acid such as cholic acid during long-term infection leads to damage of hepatocytes [
25], which may affect the bile canaliculi including loss of density of canalicular microvilli. The apical or canalicular plasma membrane is important in secretory and reabsorptive processes of bile production [
27]. Thus, ultrastructural changes of bile canaliculi may affect the mechanism of primary bile formation and defective hepatobiliary transport capacity. Because curcumin has been shown to reduce fibrosis during long-term treatment in hamsters infected with
O. viverrini [
12], obstruction of the bile duct may be ameliorated. Such conditions may improve bile components and bile flow [
28] by induction of bile duct proliferation [
29] and dilation of bile canaliculi. Alternatively, dilation and other ultrastructural canalicular changes are perhaps due to the marked liver hypertrophy and fatty liver [
30] induced by
O. viverrini during chronic infection. Curcumin may stimulate bile flow [
28], because bile flow is rapidly and markedly reduced in hepatic inflammation. Therefore, reduction of inflammation induced by
O. viverrini after curcumin treatment may be accompanied by improvement in morphological changes in bile canaliculi and prevention of the loss of microvilli in the bile canalicular lumen.
In conclusion, O. viverrini infection induces bile canaliculi alteration and curcumin prevents these changes in the liver of hamsters. The chemopreventive effect of curcumin may be due to anti-inflammation effect at acute infection and inhibit fibrosis at chronic infection leading to reduce bile canaliculi abnormalities. However, the molecular mechanisms underlying bile flow, bile synthesis, and bile uptake require further investigation. We suggest that curcumin is a promising agent for early prevention of bile duct abnormalities resulting from opisthorchiasis.
TRFRTA5580004
ACKNOWLEDGMENTS
This work was generously supported by: an Invitation Research Grant from the Faculty of Medicine, Khon Kaen University, Thailand; the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS); and the National Research University Project, Khon Kaen University. We also thank Miss Nual-anong Narkkong at the Central Instrumentation Unit, Faculty of Science, Mahasarakham University, Mahasarakham, Thailand, for technical support. We extend thanks to TRF Senior Researcher Scholar Grant (RTA5580004) to Professor Wanchai Maleewong. We wish to acknowledge the support of the Khon Kaen University Publication Clinic, Research, and Technology Transfer Affairs, Khon Kaen University, for their assistance.
References
- 1. The International Agency for Research on Cancer. Opisthorchis viverrini and Clonorchis sinensis. IARC, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 100B, A Review of Human Carcinogens: Biological Agents. Lyon, France. IARC; 2012, pp 341-370.
- 2. Prasongwatana J, Laummaunwai P, Boonmars T, Pinlaor S. Viable metacercariae of Opisthorchis viverrini in northeastern Thai cyprinid fish dishes-as part of a rational program for control of O. viverrini-associated cholangiocarcinoma. Parasitol Res 2013;112:1323-1327.
- 3. Prakobwong S, Yongvanit P, Hiraku Y, Pairojkul C, Sithithaworn P, Pinlaor P, Pinlaor S. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer 2010;127:2576-2587.
- 4. Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med 2007;4:e201.
- 5. Adam R, Hinz E, Sithithaworn P, Pipitgool V, Storch V. Ultrastructural hepatic alterations in hamsters and jirds after experimental infection with the liver fluke Opisthorchis viverrini. Parasitol Res 1993;79:357-364.
- 6. Motta P, Fumagalli G. Structure of rat bile canaliculi as revealed by scanning electron microscopy. Anat Rec 1975;182:499-513.
- 7. Allam G. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 2009;214:712-727.
- 8. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 2009;14:141-153.
- 9. Wu SJ, Tam KW, Tsai YH, Chang CC, Chao JC. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. Am J Chin Med 2010;38:99-111.
- 10. Shu JC, He YJ, Lv X, Ye GR, Wang LX. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. J Nat Med 2009;63:415-420.
- 11. Pinlaor S, Yongvanit P, Prakobwong S, Kaewsamut B, Khoontawad J, Pinlaor P, Hiraku Y. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant-antioxidant status in hamsters infected with Opisthorchis viverrini. Mol Nutr Food Res 2009;53:1316-1328.
- 12. Pinlaor S, Prakobwong S, Hiraku Y, Pinlaor P, Laothong U, Yongvanit P. Reduction of periductal fibrosis in liver fluke-infected hamsters after long-term curcumin treatment. Eur J Pharmacol 2010;638:134-141.
- 13. Prakobwong S, Khoontawad J, Yongvanit P, Pairojkul C, Hiraku Y, Sithithaworn P, Pinlaor P, Aggarwal BB, Pinlaor S. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer 2011;129:88-100.
- 14. Prakobwong S, Pinlaor S, Yongvanit P, Sithithaworn P, Pairojkul C, Hiraku Y. Time profiles of the expression of metalloproteinases, tissue inhibitors of metalloproteases, cytokines and collagens in hamsters infected with Opisthorchis viverrini with special reference to peribiliary fibrosis and liver injury. Int J Parasitol 2009;39:825-835.
- 15. Ogawa H, Itoshima T, Ukida M, Ito T, Kiyotoshi S, Kitadai M, Hattori S, Mizutani S, Kita K, Tanaka R, et al. Scanning electron microscopy of experimentally induced sequential alterations of rat liver hyperplastic nodules. Scan Electron Microsc 1984;369-374.
- 16. Tsuda N, Matsui O. Cirrhotic rat liver: reference to transporter activity and morphologic changes in bile canaliculi--gadoxetic acid-enhanced MR imaging. Radiology 2010;256:767-773.
- 17. Cullen C, Singh A, Shahidi E. Ultrastructure of liver from piglets fed Tower rapeseed oil. Histol Histopathol 1996;11:27-33.
- 18. Phillips MJ, Fisher RL, Anderson DW, Lan SP, Lachin JM, Boyer JL. Ultrastructural evidence of intrahepatic cholestasis before and after chenodeoxycholic acid therapy in patients with cholelithiasis: the national cooperative gallstone study. Hepatology 1983;3:209-220.
- 19. Lugo-Olivieri CH, Soyer PA, Fishman EK. Cystic fibrosis: spectrum of thoracic and abdominal CT findings in the adult patient. Clin Imaging 1998;22:346-354.
- 20. Pinlaor S, Sripa B, Sithithaworn P, Yongvanit P. Hepatobiliary changes, antibody response, and alteration of liver enzymes in hamsters re-infected with Opisthorchis viverrini. Exp Parasitol 2004;108:32-39.
- 21. Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, Murata M, Sripa B, Sithithaworn P, Kawanishi S. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide 2004;11:175-183.
- 22. Balázs M, Juházs J. Electron microscopic study on the injurious effects of chlorpromazine on rat liver cells. Exp Pathol (Jena) 1975;11:25-37.
- 23. Bhamarapravati N, Thammavit W, Vajrasthira S. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am J Trop Med Hyg 1978;27:787-794.
- 24. Scoazec JY, Hassan N, Feldmann G. Bile canalicular alterations in hepatocyte nodules induced by 3'-methyl-4-dimethylaminoazobenzene in the rat: morphological clues on their pathogenesis and relevance to the neoplastic process. Carcinogenesis 1990;11:1119-1125.
- 25. Lefkowitch JH, Feng-Chen KC, Sklar JA, Poh-Fitzpatrick MB. Cholic acid amelioration of light and electron microscopic hepatic lesions in experimental protoporphyria. Hepatology 1983;3:399-406.
- 26. Li FC, Huang GT, Lin CJ, Wang SS, Sun TL, Lo SY, Lo W, Chiou LL, Dong CY, Lee HS. Apical membrane rupture and backward bile flooding in acetaminophen-induced hepatocyte necrosis. Cell Death Dis 2011;2:e183.
- 27. Esteller A. Physiology of bile secretion. World J Gastroenterol 2008;14:5641-5649.
- 28. Deters M, Siegers C, Muhl P, Hänsel W. Choleretic effects of curcuminoids on an acute cyclosporin-induced cholestasis in the rat. Planta Med 1999;65:610-613.
- 29. Boonjaraspinyo S, Boonmars T, Aromdee C, Puapairoj A, Wu Z. Indirect effect of a turmeric diet: enhanced bile duct proliferation in Syrian hamsters with a combination of partial obstruction by Opisthorchis viverrini infection and inflammation by N-nitrosodimethylamine administration. Parasitol Res 2011;108:7-14.
- 30. Ohta M, Marceau N, French SW. Pathologic changes in the cytokeratin pericanalicular sheath in experimental cholestasis and alcoholic fatty liver. Lab Invest 1988;59:60-74.
Fig. 1Scanning electron micrographs of bile canaliculi in non-infected (A-F) and O. viverrini-infected hamsters (G-L) fed with a normal diet and a 1% curcumin-supplemented diet in acute (days 21 and 30) and chronic (day 90) phases. A protective effect of curcumin against alteration of bile canaliculi was observed on day 90 PI in O. viverrini-infected hamsters fed with a 1% curcumin-supplemented diet (L). Several pits (arrowheads) were observed on the lateral surfaces of the hepatocytes.

Fig. 2Transmission electron micrographs of bile canaliculi in non-infected (A-F) and O. viverrini-infected hamsters (G-L) fed with a normal diet and a 1% curcumin-supplemented diet in acute (day 21 and day 30) and chronic (day 90) phases. In O. viverrini-infected hamsters fed with a normal diet, few microvilli were observed within the canalicular lumen on day 90 PI (I). The density of microvilli in the bile canalicular lumen in O. viverrini-infected hamsters fed with a 1% curcumin-supplemented diet for the same period (L) was similar to that found in the control group (F).

Table 1.The width of bile canaliculi and density of microvilli in normal and Opisthorchis viverrini-infected hamsters with and without 1% curcumin-supplemented diet
Table 1.
|
Experimental groups |
Width of BC by SEM (µm, mean ± SD)
|
Microvilli density by TEM (mean ± SD)
|
|
Day 21 |
Day 30 |
Day 90 |
Day 21 |
Day 30 |
Day 90 |
|
Normal |
0.72 ± 0.07 |
0.90 ± 0.16 |
0.87 ± 0.12 |
2.75 ± 0.50 |
2.75 ± 0.50 |
2.75 ± 0.50 |
|
Normal+Cur |
0.79 ± 0.10 |
0.79 ± 0.09 |
0.80 ± 0.14 |
2.67 ± 0.58 |
2.75 ± 0.62 |
2.56 ± 0.73 |
|
OV |
0.80 ± 0.12 |
0.91 ± 0.26 |
0.90 ± 0.17 |
2.78 ± 0.67 |
2.75 ± 0.46 |
0.80 ± 0.84a
|
|
OV+Cur |
0.87 ± 0.20 |
0.78 ± 0.14 |
0.82 ± 0.18 |
2.40 ± 1.34 |
2.90 ± 0.32 |
2.57 ± 0.76b
|