Abstract
Plasmodium falciparum malaria is a major public health problem in Thailand due to the emergence of multidrug resistance. The understanding of genetic diversity of malaria parasites is essential for developing effective drugs and vaccines. The genetic diversity of the merozoite surface protein-1 (PfMSP-1) and merozoite surface protein-2 (PfMSP-2) genes was investigated in a total of 145 P. falciparum isolates collected from Mae Sot District, Tak Province, Thailand during 3 different periods (1997-1999, 2005-2007, and 2009-2010). Analysis of genetic polymorphisms was performed to track the evolution of genetic change of P. falciparum using PCR. Both individual genes and their combination patterns showed marked genetic diversity during the 3 study periods. The results strongly support that P. falciparum isolates in Thailand are markedly diverse and patterns changed with time. These 2 polymorphic genes could be used as molecular markers to detect multiple clone infections and differentiate recrudescence from reinfection in P. falciparum isolates in Thailand.
-
Key words: Plasmodium falciparum, merozoite surface protien, genetic polymorphism, Thailand
Malaria remains one of the most important public health problems in several tropical countries.
Plasmodium falciparum infection causes clinical symptoms ranging from asymptomatic to the rarer complications of severe manifestations. Cerebral malaria (CM) is one of the major pathological complications of
P. falciparum infection in humans manifesting as coma that can lead to death. The emergence and spread of resistance of
P. falciparum to antimalarial drugs is an important factor for malaria control in endemic areas [
1]. The resistance of
P. falciparum has occurred to all classes of antimalarial drugs except artemisinin and its derivatives. The understanding of genetic diversity of malaria parasites is essential for developing effective drugs and vaccines. The merozoite surface protein-1 (MSP-1) of
P. falciparum is a major surface protein with an approximate molecular size of 190 kDa. MSP-1 exerts a key role in erythrocyte invasion by the merozoite [
2]. It is a target of human immune responses [
3] and a promising candidate for a blood stage subunit vaccine [
4]. MSP-2 of
P. falciparum is another candidate antigen for a subunit malaria vaccine [
5]. The objective of this study was to investigate genetic diversity of
PfMSP-1 and
PfMSP-2 genes in blood samples collected from 145 patients with uncomplicated
P. falciparum malaria in Mae Sot District of Thailand during the 3 different study periods.
A total of 145 blood samples were collected from patients attending the malaria clinic in Mae Sot District, Tak Province during 3 different periods, i.e., 1997-1999 (n=49), 2005-2007 (n=50), and 2009-2010 (n=46). Approval of the study protocol was obtained from the Ethics Committees of Ministry of Public Health, Thailand. Tak Province has been reported as the province with highest malaria incidence with approximately equal ratio of P. falciparum and P. vivax. Two milliliters of blood samples were collected by venipuncture prior to treatment with standard regimens for P. falciparum (a 3-day artesunate-mefloquine combination) and collected into EDTA collecting tubes. Giemsa-stained thin and thick blood smears were prepared and examined microscopically for P. falciparum. Parasite genomic DNA was extracted from whole blood using Chelex extraction method and used as the template for PCR amplification.
The amplification of
PfMSP-1 and
PfMSP-2 was carried out using PCR technique [
6]. In the reaction, primer pairs corresponding to the conserved sequences spanning the polymorphic regions consisted of forward-5'GAAGATGCAGTATTGACAGG3' and reverse-5'GAGTTCTTTAATAGTGAACAAG3' for MSP-1 and forward-5'GAGTTCTTTAATAGTGAACAAG3' and reverse-5'CCTGTACCTTTATTCTCTGG3' for MSP-2 [
6]. The reaction volume was 20 µl containing 1 µM of each of primer, 0.5 U of Taq polymerase, 1x of buffer with KCl (Fermentas, Burlington, Canada), 2.5 mM of MgCl
2 (Fermentas), 0.5 mM of dNTP and DNA template. PCR was performed under 1 cycle of 5 min at 94℃, then 30 cycles of 1 min at 94℃, 1 min at 50℃, 1 min at 72℃, and final extension at 72℃ for 5 min of amplification condition. PCR products were analyzed on a 2% agarose gel containing ethidium bromide. The variation in size of the amplified products was observed.
The genetic diversity pattern of PfMSP-1 and PfMSP-2 were analyzed using GeneTools software (SYNGENE™, Cambridge, UK). This software automatically compensates for smiling or distorted bands and tracks. Molecular weight or base pair values can be calculated using 2 standards for comfirmation. Comparison of difference in gene patterns during 3 different periods of sample collection was performed using the chi-square test (SPSS version 12.0 software, SPSS Inc., Chicago, Illinois, USA). Statistical significance level was set at P=0.05.
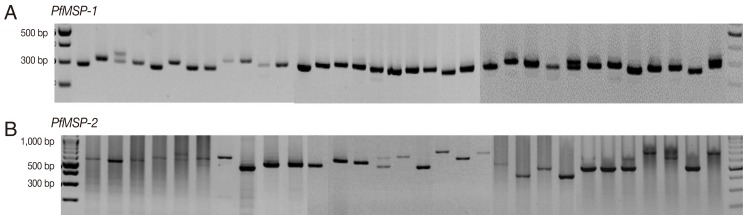
The amplification results of 145 samples during the 3 study periods (1997-1999, 2005-2007, and 2009-2010) were successful in 46 (94%), 50 (100%), and 46 (100%) for
PfMSP-1 and 33 (67%), 29 (58%), and 39 (85%) for
PfMSP-2, respectively. Both
P. falciparum genes were highly polymorphic (
Fig. 1) with different gene patterns in samples collected during the 3 periods (
Tables 1-
2). The dominant polymorphic sizes of
PfMSP-1 and
PfMSP-2 detected during 1997-1999, 2005-2007, and 2009-2010 were 300 and 500 bp, 300 and 480 bp, and 310 and 650 bp, respectively. The multiple clone infections were detected by 2 or more PCR fragments. A significant difference in the pattern of
PfMSP-1 was observed between isolates collected during the period 1997-1999 vs 2005-2007 (
P=0.002), 2005-2007 vs 2009-2010 (
P<0.001), and between 1997-1999 vs 2009-2010 (
P=0.028). For the pattern of
PfMSP-2, significant difference was found between isolates collected during the period 1997-1999 vs 2005-2007 (
P=0.050).
The polymorphic sizes of the combined
PfMSP-1/
PfMSP-2 were more diversed than each individual gene alone; 25, 23, and 32 patterns of
PfMSP-1/
PfMSP-2 polymorphisms were observed (
Table 3). The dominant combination pattern found in samples collected during the period 1997-1999, 2005-2007, and 2009-2010 were 300/500, 300/610, and 310/650, respectively. A total of 5 (15.5%), 3 (17.8%), and 7 (18.4%) samples collected during the 3 periods showed multiple clone infections, respectively.
Several malarial proteins have been proposed as vaccine candidate antigens but MSP-1 is the most promising candidate [
7,
8]. Results of the phase 1-2b clinical trial of a MSP-2 based vaccine showed that 1 allelic type included in the vaccine may be more effective against malaria parasite [
9].
P. falciparum MSP-2, apical membrane antigen-1 (AMA-1), and circumsporozoite protein (CSP) are also under investigation as candidate antigens for the development of malaria vaccine [
10,
11]. The polymorphisms of
PfMSP-1 and
PfMSP-2 have been investigated in isolates collected from several malaria endemic areas [
12-
16]. All showed highly polymorphic patterns of these 2 genes. High levels of
PfMSP-1 and
PfMSP-2 polymorphisms and multiple clonal infections were reported in 3 malaria endemic regions of Lao PDR [
13]. Similarly, sequence analysis of
PfMSP-1 block 2 in
P. falciparum isolates collected from Myanmar demonstrated 14 different genotypes (5 for K1 type and 9 for MAD20 type), whereas 22 genotypes (7 for FC27 type and 15 for 3D7 type) were found with
PfMSP-2 block 3 [
12]. A recent report from Republic of Congo revealed high polymorphisms and multiple clones of
P. falciparum isolates [
15]. Moreover, isolates collected from Malawi, Tanzania, Uganda, Burkina Faso, and São Tomé exhibited highly polymorphic and low allele frequencies of
PfMSP-1,
PfMSP-2, and
glurp, with a total of 17
PfMSP-1, 116
PfMSP-2, and 14
glurp genotypes [
16]. In contrast, relatively low levels of genetic diversity were found in isolates collected from Haiti (9
PfMSP-1 genotypes) [
14].
The results of the present study confirmed the genetic variations of PfMSP-1 and PfMSP-2 in isolates collected from Mae Sot District, the endemic area of Thailand with highest malaria incidence. Moreover, the combination of PfMSP-1/PfMSP-2 was relatively more polymorphic, and thus appropriate for application to detect multiple clone infections and differentiate recrudescence from reinfection in P. falciparum isolates in Thailand. The low efficacy of vaccine candidate antigens observed in various clinical trials would be due to the highly variable genetic polymorphisms of PfMSP-1 and PfMSP-2 in P. falciparum isolates.
Commission on Higher Education, Ministry of Education, ThailandNational Research University Project of Thailand Office of Higher Education Commission of Thailand
Notes
-
We have no conflict of interest related with this study.
ACKNOWLEDGMENTS
This work was supported by the Commission on Higher Education, Ministry of Education, Thailand, and the National Research University Project of Thailand Office of Higher Education Commission of Thailand.
References
- 1. Na-Bangchang K, Congpuong K. Current malaria status and distribution of drug resistance in East and Southeast Asia with special focus to Thailand. Tohoku J Exp Med 2007;211:99-113.
- 2. Holder AA, Blackman MJ. What is the function of MSP-1 on the malaria merozoite. Parasitol Today 1994;10:182-184.
- 3. Woehlbier U, Epp C, Kauth CW, Lutz R, Long CA, Coulibaly B, Kouyaté B, Arevalo-Herrera M, Herrera S, Bujard H. Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect Immun 2006;74:1313-1322.
- 4. Holder AA, Guevara Patiño JA, Uthaipibull C, Syed SE, Ling IT, Scott-Finnigan T, Blackman MJ. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 1999;41:409-414.
- 5. Lawrence G, Cheng QQ, Reed C, Taylor D, Stowers A, Cloonan N, Rzepczyk C, Smillie A, Anderson K, Pombo D, Allworth A, Eisen D, Anders R, Saul A. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in nonimmune volunteers. Vaccine 2000;18:1925-1931.
- 6. Wooden J, Kyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today 1993;9:303-305.
- 7. Genton B, Al-Yaman F, Betuela I, Anders RF, Saul A, Baea K, Mellombo M, Taraika J, Brown GV, Pye D, Irving DO, Felger I, Beck HP, Smith TA, Alpers MP. Safety and immunogenicity of a 3-component blood-stage malaria vaccine (MSP1, MSP2, RESA) against Plasmodium falciparum in Papua New Guinean children. Vaccine 2003;22:30-41.
- 8. Reed ZH, Kieny MP, Engers H, Friede M, Chang S, Longacre S, Malhotra P, Pan W, Long C. Comparison of immunogenicity of five MSP1-based malaria vaccine candidate antigens in rabbits. Vaccine 2009;27:1651-1660.
- 9. Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, Lorry K, Brown GV, Pye D, Irving DO, Smith TA, Beck HP, Alpers MP. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis 2002;185:820-827.
- 10. Collins WE, Pye D, Crewther PE, Vandenberg KL, Galland GG, Sulzer AJ, Kemp DJ, Edwards SJ, Coppel RL, Sullivan JS, Morris CL, Anders RF. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg 1994;51:711-719.
- 11. Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garçon N, Krzych U, Marchand M. RTS,S Malaria Vaccine Evaluation Group. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med 1997;336:86-91.
- 12. Kang JM, Moon SU, Kim JY, Cho SH, Lin K, Sohn WM, Kim TS, Na BK. Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum field isolates from Myanmar. Malar J 2010;9:131.
- 13. Khaminsou N, Kritpetcharat O, Daduang J, Charerntanyarak L, Kritpetcharat P. Genetic analysis of the merozoite surface protein-1 block 2 allelic types in Plasmodium falciparum clinical isolates from Lao PDR. Malar J 2011;10:371.
- 14. Londono-Renteria B, Eisele TP, Keating J, Bennett A, Krogstad DJ. Genetic diversity in the merozoite surface protein 1 and 2 genes of Plasmodium falciparum from the Artibonite Valley of Haiti. Acta Trop 2012;121:6-12.
- 15. Mayengue PI, Ndounga M, Malonga FV, Bitemo M, Ntoumi F. Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum isolates from Brazzaville, Republic of Congo. Malar J 2011;10:276.
- 16. Mwingira F, Nkwengulila G, Schoepflin S, Sumari D, Beck HP, Snounou G, Felger I, Olliaro P, Mugittu K. Plasmodium falciparum msp1, msp2 and glurp allele frequency and diversity in sub-Saharan Africa. Malar J 2011;10:79.
Fig. 1Genetic polymorphisms of PfMSP-1 and PfMSP-2.
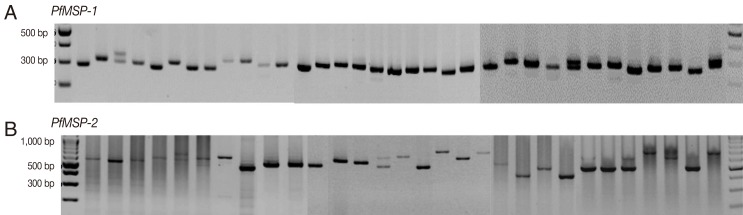
Table 1.Summary of polymorphic sizes of PfMSP-1 in Plasmodium falciparum isolates collected during the 3 different study periods (1997-1999, 2005-2007, and 2009-2010)
Table 1.
|
Study period |
Polymorphic size (bp)
|
|
1997-1999 |
2005-2007 |
2009-2010 |
|
260 |
280 |
280 |
|
290 |
290 |
285 |
|
300 |
295 |
290 |
|
310 |
300 |
300 |
|
320 |
320 |
310 |
|
300/320 |
290/300/320 |
315 |
|
300/330 |
300/310 |
320 |
|
|
300/320 |
325 |
|
|
300/320/340 |
330 |
|
|
310/320 |
300/400 |
|
|
|
310/360 |
|
|
|
315/370 |
|
|
|
320/360 |
|
|
|
325/360 |
|
|
|
330/380 |
|
Total no. of polymorphisms |
7 |
10 |
15 |
Table 2.Summary of polymorphic sizes of PfMSP-2 in Plasmodium falciparum isolates collected during the 3 different study periods (1997-1999, 2005-2007, and 2009-2010)
Table 2.
|
Study period |
Polymorphic size (bp)
|
|
1997-1999 |
2005-2007 |
2009-2010 |
|
495 |
450 |
450 |
|
500 |
480 |
480 |
|
550 |
490 |
490 |
|
570 |
500 |
500 |
|
580 |
510 |
510 |
|
590 |
520 |
520 |
|
600 |
530 |
590 |
|
620 |
550 |
600 |
|
630 |
570 |
610 |
|
650 |
580 |
630 |
|
690 |
590 |
650 |
|
700 |
595 |
660 |
|
900 |
600 |
690 |
|
550/620 |
610 |
700 |
|
590/610 |
690 |
500/520 |
|
590/690 |
480/560 |
520/530 |
|
500/600 |
500/690 |
|
|
500/650 |
|
|
|
Total no. of polymorphisms |
18 |
17 |
16 |
Table 3.Polymorphic sizes of various combination patterns of PfMSP-1/PfMSP-2
Table 3.
|
Pattern |
PfMSP-1/PfMSP-2 (bp) |
1997-1999 |
2005-2007 |
2009-2010 |
|
1 |
320 |
495 |
290 |
450 |
300 |
450 |
|
2 |
290 |
500 |
290 |
480 |
310 |
450 |
|
3 |
300 |
500 |
300 |
480 |
300 |
480 |
|
4 |
310 |
500 |
320 |
480 |
310 |
480 |
|
5 |
320 |
500 |
290/300/320 |
480/560 |
300 |
490 |
|
6 |
310 |
500/600 |
320 |
490 |
300 |
500 |
|
7 |
300 |
500/650 |
300/320 |
500 |
310/360 |
500 |
|
8 |
300 |
550 |
300/310 |
500/690 |
315 |
500 |
|
9 |
310 |
550 |
310/320 |
500/690 |
320/360 |
500 |
|
10 |
290 |
550/620 |
290 |
510 |
320/360 |
500/520 |
|
11 |
300 |
570 |
300/320/340 |
520 |
325/360 |
500/520 |
|
12 |
310 |
580 |
290 |
530 |
290 |
510 |
|
13 |
320 |
580 |
290 |
550 |
300 |
510 |
|
14 |
310 |
590 |
300 |
570 |
310 |
510 |
|
15 |
300/320 |
590/610 |
290 |
580 |
320/360 |
510 |
|
16 |
300 |
590/690 |
300 |
580 |
330/380 |
520 |
|
17 |
310 |
600 |
300 |
590 |
310/360 |
520/530 |
|
18 |
320 |
600 |
300 |
595 |
290 |
530 |
|
19 |
290 |
620 |
310/320 |
595 |
290 |
590 |
|
20 |
310 |
620 |
300 |
600 |
300 |
590 |
|
21 |
290 |
630 |
300 |
610a
|
310 |
590 |
|
22 |
300 |
650a
|
300 |
690 |
300 |
600 |
|
23 |
310 |
690 |
320 |
690 |
310 |
600 |
|
24 |
290 |
700 |
|
|
310 |
610a
|
|
25 |
260 |
900 |
|
|
290 |
650a
|
|
26 |
|
|
|
|
310 |
650 |
|
27 |
|
|
|
|
315 |
650 |
|
28 |
|
|
|
|
310 |
660 |
|
29 |
|
|
|
|
300 |
690 |
|
30 |
|
|
|
|
310 |
690 |
|
31 |
|
|
|
|
280 |
700 |
|
32 |
|
|
|
|
300 |
700 |
Citations
Citations to this article as recorded by

- Immunization with PfGBP130 generates antibodies that inhibit RBC invasion by P. falciparum parasites
Yannick Johnson, Ahmad Rushdi Shakri, Sunthorn Pond-Tor, Anup Jnawali, Tanbir Najrana, Haiwei Wu, Jhasketan Badhai, Mohamad-Gabriel Alameh, Drew Weissman, Edward Kabyemela, Patrick Duffy, Michal Fried, Jonathan Kurtis, Dipak Kumar Raj
Frontiers in Immunology.2024;[Epub] CrossRef - Allelic diversity of MSP1 and MSP2 repeat loci correlate with levels of malaria endemicity in Senegal and Nigerian populations
Mary A. Oboh, Tolla Ndiaye, Khadim Diongue, Yaye D. Ndiaye, Mouhamad Sy, Awa B. Deme, Mamadou A. Diallo, Mamadou S. Yade, Sarah K. Volkman, Aida S. Badiane, Alfred Amambua-Ngwa, Daouda Ndiaye
Malaria Journal.2021;[Epub] CrossRef - Genetic polymorphism of merozoite surface proteins 1 and 2 of Plasmodium falciparum in the China–Myanmar border region
Cang-Lin Zhang, Hong-Ning Zhou, Quan Liu, Ya-Ming Yang
Malaria Journal.2019;[Epub] CrossRef