Abstract
Mucosal immune responses against Pygidiopsis summa (Trematoda: Heterophyidae) infection were studied in ICR mice. Experimental groups consisted of group 1 (uninfected controls), group 2 (infection with 200 metacercariae), and group 3 (immunosuppression with Depo-Medrol and infection with 200 metacercariae). Worms were recovered in the small intestine at days 1, 3, 5, and 7 post-infection (PI). Intestinal intraepithelial lymphocytes (IEL), mast cells, and goblet cells were counted in intestinal tissue sections stained with Giemsa, astra-blue, and periodic acid-Schiff, respectively. Mucosal IgA levels were measured by ELISA. Expulsion of P. summa from the mouse intestine began to occur from days 3-5 PI which sustained until day 7 PI. The worm expulsion was positively correlated with proliferation of IEL, mast cells, goblet cells, and increase of IgA, although in the case of mast cells significant increase was seen only at day 7 PI. Immunosuppression suppressed all these immune effectors and inhibited worm reduction in the intestine until day 7 PI. The results suggested that various immune effectors which include IEL, goblet cells, mast cells, and IgA play roles in regulating the intestinal mucosal immunity of ICR mice against P. summa infection.
-
Key words: Pygidiopsis summa, mucosal immunity, intestinal fluke, intraepithelial lymphocyte, goblet cell, mast cell, IgA
INTRODUCTION
Pygidiopsis summa (Digenea: Heterophyidae), a minute intestinal trematode infecting avian and mammalian hosts, including humans [
1,
2], was originally described from dogs fed mullet harboring the metacercariae in Japan [
1]. This trematode is distributed in Japan and the Republic of Korea (=Korea), and human infections have been reported in both countries [
3-
6]. In Korea,
P. summa is distributed widely along coastal areas, including islands, and public health attention has been paid to this trematode infection [
5,
6].
In humans infected with
P. summa, gastrointestinal symptoms, such as abdominal pain and diarrhea, may occur, particularly in heavily infected cases [
1,
2]. Rats and mice experimentally infected with
P. summa display mucosal pathologies in the small intestine that include villous atrophy, crypt hyperplasia, and mucosal inflammation [
7]. Like other intestinal helminths (nematodes and cestodes),
P. summa worms are expelled spontaneously within 3 weeks after infection in mice [
7]. However, no information has been available regarding mucosal immune responses of the host in relation to expulsion of
P. summa from the host intestine.
The mechanisms of helminth expulsion from the gut of rodents were studied popularly in nematode infections, including
Strongyloides ratti,
Nippostrongylus brasiliensis,
Trichinella spiralis, and
Trichuris muris [
8-
11]. The mechanisms involved in worm expulsion are unique in each parasite species and even strains [
12]. For example, the major effector for expulsion of
S. ratti is mucosal mast cells, whereas it is goblet cells in
N. brasiliensis,
T. muris, and
T. spiralis [
10-
13]. In intestinal trematode infections, such as
Echinostoma spp. [
14-
16],
Neodiplostomum seoulense [
17-
19],
Metagonimus yokogawai [
20-
22], and
Gymnophalloides seoi [
23-
25], innate intestinal immune mechanisms operate, and goblet cells, mast cells, intestinal intraepithelial lymphocytes (IEL), and/or mucosal IgA increased remarkably. However, in heterophyid fluke infections other than
M. yokogawai, the role of these effectors has not been clarified.
The present study was performed to examine the intestinal mucosal immune responses of mice experimentally infected with P. summa in regulation to worm expulsion. Chronological changes in the number of IEL, mucosal mast cells, and goblet cells, were determined, and the levels of IgA were measured in immunocompetent (IC) and immunosuppressed (IS) P. summa-infected mice.
MATERIALS AND METHODS
Parasite
Naturally infected mullets, Mugil cephalus, with P. summa metacercariae were caught off the coast of Aphae-do (Island), Shinan-gun, Jeollanam-do, Korea. Their gills were separated and digested in artificial digestive juice (0.5% porcine pepsin in 0.6% HCl solution) (Sigma-Aldrich, St. Louis, Missouri, USA) at 37℃ for 1 hr. The digested mixture containing free metacercariae was successively filtered through mesh pore sizes of 600, 300, and 106 µm. Metacercariae were collected from the last mesh and counted using a stereomicroscope.
Experimental animals and parasite infection
Specific pathogen-free ICR mice (4-week-old males) were purchased from the Samtaco Laboratory Animal Center (Osan-shi, Kyonggi-do, Korea). Experimental groups consisted of group 1 (uninfected controls), group 2 (IC and
P. summa-infected), and group 3 (IS and
P. summa-infected). Each group consisted of 5-6 mice. Immunosuppression was induced by intramuscular injection with 15 mg/kg Depo-Medrol (methylprednisolone; Upjohn Korea, Seoul, Korea) every other day from day 2 before infection until the end of the experiment. Glucocorticoids including prednisolone are known to inhibit T-cell, B-cell, and NK-cell functions [
26]. They were infected orally with 200 metacercariae of
P. summa, and worm recovery was observed at days 1, 3, 5, and 7 post-infection (PI). Animal experiments were carried out in accordance with the guidelines of Institutional Animal Care and User Committee, Seoul National University College of Medicine, Seoul, Korea.
Mice were killed by ether-induced hyperanesthesia. The abdomen of each mouse was opened, and the small intestine was resected, divided into several segments, and then longitudinally opened in Petri dishes containing physiological saline. The opened intestinal segments were put on the top of a Baermann's apparatus as described previously [
24]. The flukes were collected from the bottom of the tube in the apparatus. The intestinal segments were returned to Petri dishes to search for residual flukes using a stereomicroscope.
Small pieces (approximately 1-2 cm long) of the mouse jejunum were fixed in Carnoy's fixative for 24 hr. These fixed samples were sectioned at 5 µm thickness after dehydration and wax embedding. The sections were stained with 2% Giemsa solution (Diagnostica Merck, Darmstadt, Germany) for 20 min. IELs were located in the interspace or base of epithelial cells and were stained darker than nuclei of villous epithelial cells. The location of IELs was classified into apical, middle, and basal in comparison with location of the epithelial cell nuclei. The location of IELs in different experimental groups was compared. IEL numbers were counted per 10 villus-crypt units (VCU) from 5 mice for each group as previously described [
27].
For mast cell counting, sections of the mouse jejunum were prepared as described above for IEL counting. At a strong acidic condition of pH 0.3, the sections were stained with Astra blue and counterstained with weak eosin. Granulated mast cells were counted in the defined region of 10 VCU, and the mast cell counts were expressed as the mean number per VCU.
The intestinal segments, about 2 cm in length, were taken from the middle portion of each jejunum for goblet cell staining. The segments were washed twice with saline and fixed in Carnoy's fixative for 2-3 days. The fixed tissues were embedded in paraffin and sectioned at about 5 µm thickness. The tissue samples were stained with periodic acid Schiff (PAS). Briefly, the samples were oxidized with 1% periodic acid (Sigma-Aldrich) for 5 min and reacted with Schiff reagent to produce a colored end product. They were counterstained with hematoxylin (Sigma-Aldrich). Goblet cell numbers were counted per 10 VCU from 5 mice for each group.
Measurement of P. summa-specific IgA
The middle portion (about 5 cm) of the jejunum, which contained intestinal mucus, was collected for detection of IgA and stored at -20℃ until processing. Mucus was isolated based on a method described previously [
28]. Briefly, the tissues were thawed and mucus was scraped off with a glass slide. The scrapings were collected in a conical tube on ice. Three-milliliters of ice-cold PBS supplemented with protease inhibitors, 1 tablet for 10 ml (Complete TM, Roche, Indianapolis, Indiana, USA) was added to each sample. The samples were shaken for 1 hr at 4℃ and centrifuged at 3,000 g for 30 min at 4℃. Finally, the supernatant was spun down for 30 min at 4℃ and 15,000 g. The protein concentration was determined using a BCA assay kit (Pierce, Rockford, Illinois, USA). ELISA was performed for
P. summa antigen-specific IgA with samples adjusted to a final concentration of 5 mg protein per ml supernatant.
Experiments were repeated at least 3 times unless otherwise specified, with presentation of 1 representative set of results. The statistical significance was analyzed using the Student's t-test or χ2-test. P-values of <0.05 were considered significant.
RESULTS
Worm recovery
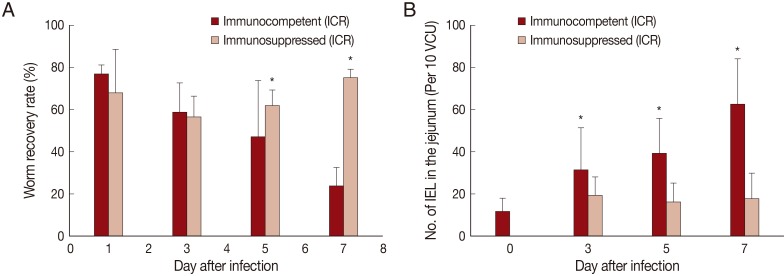
In IC mice,
P. summa worms were expelled slowly from day 1 PI (average WRR; 76.8%) until day 7 PI (23.8%) (
Fig. 1A). By comparison, in IS
P. summa-infected mice, the average WRR remained consistently high (72.3%) until day 7 PI. The WRR at days 5 and 7 PI were significantly (
P<0.05) different between IC and IS mice.
The number of IEL in the jejunum of IC mice infected with
P. summa increased significantly (
P<0.05) at days 3, 5, and 7 PI compared with uninfected controls (
Fig. 1B). However, in IS
P. summa-infected mice, the IEL counts did not increase significantly (
P>0.05) throughout days 3 to 7 PI (
Fig. 1B).
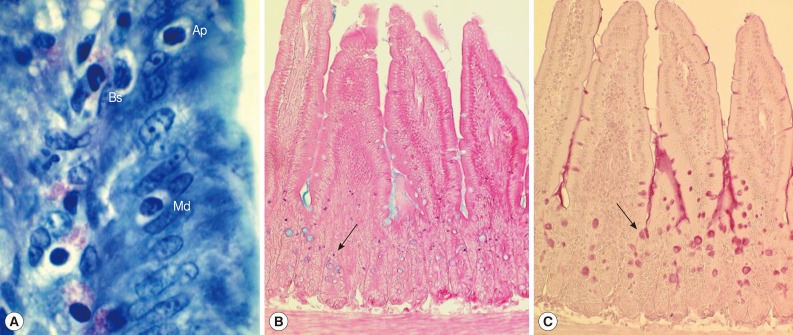
In uninfected control mice, the IEL position (
Fig. 2A) in relation to the level of epithelial cell nuclei was predominantly basal (96.4%) rather than apical (1.2%) or middle (2.4%) (
Table 1). However, the position changed significantly (
P<0.05) in IC
P. summa-infected mice at days 5 and 7 PI compared with those in uninfected controls and IS
P. summa-infected mice (
Table 1). For example, at day 5 PI, significantly more IEL (
P<0.05) were located at the apical (3.7%) or middle (10.2%) portion in IC mice than in uninfected controls (1.2% and 2.4%, respectively), whereas such IEL position changes were not recognizable in IS mice. Similar position changes were observed at day 7 PI (
Table 1).
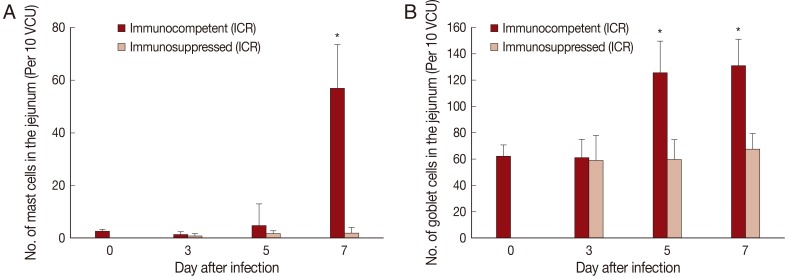
The number of mast cells did not increase until day 5 PI in in the jejunum of
P. summa-infected mice. However, the count increased significantly (
P<0.05) at day 7 PI compared with uninfected controls (23-fold) and IS
P. summa-infected mice (32-fold) (
Figs. 2B,
3A).
Goblet cell counts in IC mice infected with
P. summa were similar to those of uninfected mice at day 3 PI. However, remarkable increases were observed at days 5 and 7 PI (
Figs. 2C,
3B). In IS
P. summa-infected mice, no significant changes in goblet cell counts were recognized through day 3 to day 7 PI.
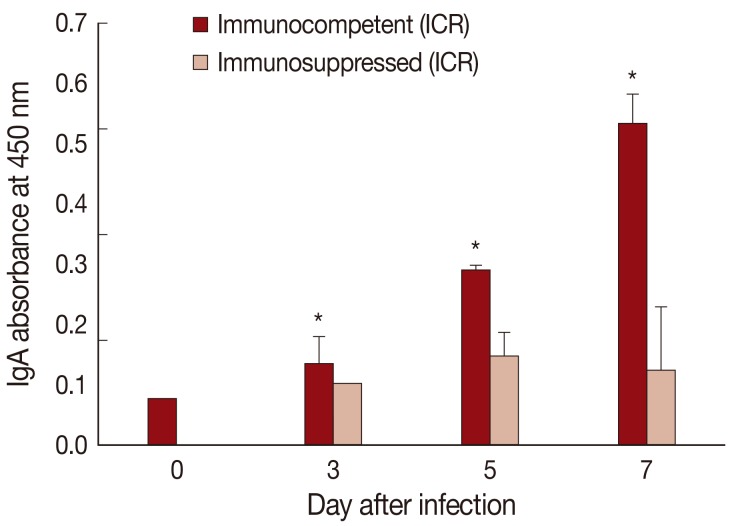
The absorbance for mucosal IgA was significantly (
P<0.05) increased in IC
P. summa-infected mice compared with uninfected controls through days 3, 5, and 7 PI (
Fig. 4). By contrast, in IS
P. summa-infected mice, the absorbance for mucosal IgA did not increase significantly (
P>0.05) through days 3 to 7 PI.
DISCUSSION
Against intestinal helminth infections, IEL, lamina propria lymphocytes, mast cells, goblet cells, paneth cells, mucus, and IgA as well as various cytokines play crucial roles in the host defense and worm expulsion [
9,
11,
12,
29]. In our study, mucosal immune responses against
P. summa in ICR mice were characterized by significant increase of IEL count, change of IEL position, increase in number of mast cells and goblet cells, and increased level of mucosal IgA.
However, some debates have been raised on the role of mucosal mast cells [
9]. For example, in infection with nematodes such as
Strongyloides venezuelensis, worm expulsion was strongly mediated by mucosal mast cells [
30]. However, in
N. brasiliensis, the role of mast cells in worm expulsion was negligible [
12]. In
Heligmosomoides polygyrus bakeri and
T. muris infections in mice, mast cells were highly active in orchestrating T helper 2 (Th2) type immunity through regulation of various cytokines [
9]. In trematode models, such as
Echinostoma hortense and
M. yokogawai, mucosal mast cells appeared to play important roles in worm expulsion [
20,
31]. Contrarily, no significant role for mast cells was reported in
N. seoulense-infected mice [
17].
In our study with P. summa, significant increases of mast cells were observed only at day 7 PI but not at days 3 and 5 PI when worm expulsion occurred most actively, suggesting no direct relationship between mastocytosis and worm expulsion. This discrepancy may be due to a more rapid expulsion of P. summa (days 7-14 PI) compared with E. hortense (days 40-50 PI) and intestinal nematodes (days 14-30 PI). Therefore, the role of mast cells in protective immunity against P. summa infection needs further elucidation.
The role of goblet cells has been documented in various nematode and cestode infections [
8,
10,
11,
32,
33]. In
N. brasiliensis and
T. spiralis, there was a chronologic association of worm expulsion and goblet cell hyperplasia, and the goblet cell response was regulated by CD4
+ T-cells, in particular, Th2 cells [
12,
13,
32]. In infection with an intestinal trematode,
Echinostoma trivolvis, goblet cells were important for worm expulsion in primary infections and also challenge infections [
14]. In our previous studies, the importance of goblet cells in expulsion of a human-infecting gymnophallid,
G. seoi, has been elucidated [
23] and the role of CD4
+ T-cells on goblet cell proliferation and worm expulsion has been demonstrated [
24]. We also have shown that
MUC2 gene and Toll-like receptor 2 were highly expressed on intestinal epithelial cells upon stimulation with
G. seoi antigen, together with goblet cell hyperplasia and mucin hypersecretion [
25]. In the present study, goblet cell hyperplasia was evident at days 5 and 7 PI in IC
P. summa-infected mice, when worm expulsion occurred actively. Immunosuppression abrogated these goblet cell responses.
IEL are in close contact with foreign substances derived from the gut lumen and are thought to play a key role in immune responses to these antigens [
34]. Many IELs in the small intestine are CD8
+ T-cells and develop independent of the thymus [
35]. In C57BL/6 mice infected with
T. spiralis, IEL released mediators that influence goblet cell differentiation and enhance worm expulsion [
36].
M. yokogawai-infected Sprague-Dawley rats also displayed increased numbers of IEL, peaking at day 5 PI, although the number decreased subsequently to stay at lower levels during days 10-24 PI [
21]. In the present study, IEL counts increased remarkably in ICR mice infected with
P. summa from day 3 PI and continued to increase until day 7 PI. It is strongly suggested that IEL actively take part in expulsion of
P. summa within the first 1 week of infection.
The position of IEL within the mucosal epithelial layer has been suggested to play a role in protective responses of the host against helminth parasites [
21]. In a previous study, during
M. yokogawai infection, some IEL changed their position from basal to intermediate or apical portions of the epithelial cell at day 5 PI and this position change was sustained until day 15 PI [
21]. In the present study, a similar phenomenon was observed during the early stage (until day 7 PI) of infection with
P. summa. It is strongly suggested that the position change of IEL is to enhance their functions as cytotoxic T-cells which act directly against the parasites.
It was also shown in this study that mucosal IgA levels were significantly increased at days 3, 5, and 7 PI in
P. summa-infected mice, whereas no increases were seen in IS
P. summa-infected mice from which higher worm recoveries were obtained. This strongly suggests an involvement of IgA in the worm expulsion of
P. summa. A similar suggestion was made in
Ascaris suum infection in pigs [
37] and
T. spiralis infection in mice [
38]. By contrast, in
E. caproni infection, IgA was considered not sufficient for worm expulsion because a resistant rat strain did not exhibit any IgA response, whereas a susceptible mouse strain exhibited a high IgA response [
39]. The induction of IgA response in mice was suggested to be a consequence of IL-6 (a Th2 cytokine) production in response to
E. caproni antigens [
39]. Similarly, in
T. muris infection in mice, the role of B-cells and antibodies in worm expulsion remained controversial and required further investigation [
30].
In intestinal trematode infections, the role of T-cells has been reported to be variable depending on different parasite species and also on different host species or strain. For example, in
E. trivolvis infection, athymic nude mice could expel worms, prompting the suggestion that T-cells are not an essential element for worm expulsion [
40]. However, in
G. seoi infection, T-cells were found to be important in goblet cell hyperplasia and worm expulsion [
24]. In
P. summa infection, it is presumed that T-cells may be important in inducing intestinal IEL, mast cells, goblet cells, IgA, and also worm expulsion of
P. summa from ICR mice. However, the role of T-cells remains to be further studied.
Notes
-
There is no conflict of interest related with this study.
ACKNOWLEDGMENT
We are grateful to Jae-Lip Kim for his excellent laboratory works including collection of P. summa metacercariae and experimental infection of animals.
References
- 1. Chai JY, Shin EH, Lee SH, Rim HJ. Foodborne intestinal flukes in southeast Asia. Korean J Parasitol 2009;47(suppl):S69-S102.
- 2. Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int 2002;51:129-154.
- 3. Yokogawa M, Sano M, Itabashi T, Kachi S. Studies on the intestinal flukes. II. Epidemiological studies on heterophyid trematodes of man in Chiba Prefecture. Jpn J Parasitol 1965;14:577-585.
- 4. Seo BS, Hong ST, Chai JY. Studies on intestinal trematodes in Korea. III. Natural human infections of Pygidiopsis summa and Heterophyes nocens. Seoul J Med 1981;22:228-235.
- 5. Chai JY, Kim IM, Seo M, Guk SM, Kim JL, Sohn WM, Lee SH. A new endemic focus of Heterophyes nocens, Pygidiopsis summa, and other intestinal flukes in a coastal area of Muan-gun, Chollanam-do. Korean J Parasitol 1997;35:233-238.
- 6. Chai JY, Park JH, Han ET, Shin EH, Kim JL, Guk SM, Hong KS, Lee SH, Rim HJ. Prevalence of Heterophyes nocens and Pygydiopsis summa infections among residents of the western and southern coastal islands of the Republic of Korea. Am J Trop Med Hyg 2004;71:617-622.
- 7. Seo BS, Cheong SK, Chai JY, Lee SH, Lee JB. Histopathology of small intestines of rats and mice experimentally infected with Pygidiopsis summa. Seoul J Med 1986;27:125-134.
- 8. Tsubokawa D, Goso Y, Nakamura T, Maruyama H, Yatabe F, Kirihara M, Ichikawa T, Ishihara K. Rapid and specific alteration of goblet cell mucin in rat airway and small intestine associated with resistance against Nippostrongylus brasiliensis reinfection. Exp Parasitol 2012;130:209-217.
- 9. Hepworth MR, Danilowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, Hartmann S. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A 2012;109:6644-6649.
- 10. Forman RA, deSchoolmeester ML, Hurst RJM, Wright SH, Pemberton AD, Else KJ. The goblet cell is the cellular source of the anti-microbial angiotensin 4 in the large intestine post Trichuris muris infection. PLoS One 2012;7:e42248.
- 11. Hasnain SZ, Gallagher AL, Grencis RK, Thornton DJ. A new role for mucins in immunity: insights from gastrointestinal nematodes. Int J Biochem Cell Biol 2013;45:364-374.
- 12. Nawa Y, Ishikawa N, Tsuchiya K, Horii Y, Abe T, Khan AI, Bing-Shi , Itoh H, Ide H, Uchiyama F. Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol 1994;16:333-338.
- 13. Ishikawa N, Wakelin D, Mahida YR. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 1997;113:542-549.
- 14. Fujino T, Fried B, Ichikawa H, Tada I. Rapid expulsion of the intestinal trematodes Echinostoma trivolvis and E. caproni from C3H mice by trapping with increased goblet cell mucins. Int J Parasitol 1996;26:319-324.
- 15. Toledo R, Monteagudo C, Espert A, Fried B, Esteban JG, Marcilla A. Echinostoma caproni: intestinal pathology in the golden hamster, a highly compatible host, and the Wistar rat, a less compatible host. Exp Parasitol 2006;112:164-171.
- 16. Ryang YS, Yang EJ, Kim JL, Lee KJ, Sung HJ, Kim JB, Kim IS. Immune response and inhibitory effect of ketotifen on the BALB/c and C3H/HeN mice infected with Echinostoma hortense. Parasitol Res 2007;101:1103-1110.
- 17. Chai JY, Kim TK, Cho WH, Seo M, Kook J, Guk SM, Lee SH. Intestinal mastocytosis and goblet cell hyperplasia in BALB/c and C3H mice infected with Neodiplostomum seoulense. Korean J Parasitol 1998;36:109-119.
- 18. Kook J, Nawa Y, Lee SH, Chai JY. Pathogenicity and lethality of a minute intestinal fluke, Neodiplostomum seoulense, to various strains of mice. J Parasitol 1998;84:1178-1183.
- 19. Chai JY, Shin EH, Han ET, Guk SM, Choi MH, Lee SH. Genetic difference in susceptibility and fatality of three strains of mice experimentally infected with Neodiplostomum seoulense. J Parasitol 2000;86:1140-1144.
- 20. Chai JY, Kim TH, Kho WG, Chung SW, Hong ST, Lee SH. Mucosal mast cell responses to experimental Metagonimus yokogawai infection in rats. Korean J Parasitol 1993;31:129-134.
- 21. Chai JY, Yun TY, Kim J, Huh S, Choi MH, Lee SH. Chronological observation on intestinal histopathology and intraepithelial lymphocytes in the intestine of rats infected with Metagonimus yokogawai. Korean J Parasitol 1994;32:215-221. (in Korean).
- 22. Guk SM, Park JY, Seo M, Han ET, Kim JL, Chai JY. Susceptibility of inbred mouse strains to infection with three species of Metagonimus prevalent in the Republic of Korea. J Parasitol 2005;91:12-16.
- 23. Seo M, Guk SM, Han ET, Chai JY. Role of intestinal goblet cells in the expulsion of Gymnophalloides seoi from mice. J Parasitol 2003;89:1080-1082.
- 24. Guk SM, Lee JH, Kim HJ, Kim WH, Shin EH, Chai JY. CD4+ T-cell-dependent goblet cell proliferation and expulsion of Gymnophalloides seoi from the intestine of C57BL/6 mice. J Parasitol 2009;95:581-590.
- 25. Lee KD, Guk SM, Chai JY. Toll-like receptor 2 and MUC2 expression on human intestinal epithelial cells by Gymnophalloides seoi adult antigen. J Parasitol 2010;96:58-66.
- 26. Cohen JJ. Glucocorticoids. In Roill IM, Delves eds, Encyclopedia of Immunology. London, UK. Academic Press; 1992, pp 616-617.
- 27. Miller HR, Jarrett WF. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology 1971;20:277-288.
- 28. Kanobana K, Ploeger HW, Vervelde L. Immune expulsion of the trichostrongylid Cooperia oncophora is associated with increased eosinophilia and mucosal IgA. Int J Parasitol 2002;32:1389-1398.
- 29. Klementowicz JE, Travis MA, Grencis RK. Trichuris muris: a model of gastrointestinal parasite infection. Semin Immunopathol 2012;34:815-828.
- 30. Maruyama H, Osada Y, Yoshida A, Futakuchi M, Kawaguchi H, Zhang R, Fu J, Shirai T, Kojima S, Ohta N. Protective mechanisms against the intestinal nematode Strongyloides venezuelensis in Schistosoma japonicum-infected mice. Parasite Immunol 2000;22:279-286.
- 31. Kim I, Im JA, Lee KJ, Ryang YS. Mucosal mast cell responses in the small intestine of rats infected with Echinostoma hortense. Korean J Parasitol 2000;38:139-143.
- 32. Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis infection in mice. Parasite Immunol 1995;17:485-491.
- 33. Yamauchi J, Kawai Y, Yamada M, Uchikawa R, Tegoshi T, Arizono N. Altered expression of goblet cell- and mucin glycosylation-related genes in the intestinal epithelium during infection the with nematode Nippostrongylus brasiliensis. APMIS 2006;114:270-278.
- 34. Lillehoj HS, Chai JY. Comparative natural killer cell activities of thymic, bursal, splenic and intestinal intraepithelial lymphocytes of chickens. Dev Comp Immunol 1988;12:629-643.
- 35. Little MC, Bell LV, Cliffe LJ, Else KJ. The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. J Immunol 2005;175:6713-6722.
- 36. Bozić F, Marinculić A, Duraković E. Analysis of intestinal intraepithelial lymphocyte populations in experimental Trichinella spiralis infection of mice. Folia Parasitol (Praha) 2000;47:55-59.
- 37. Miquel N, Roepstorff A, Bailey M, Eriksen L. Host immune responses and worm kinetics during the expulsion of Ascaris suum in pigs. Parasite Immunol 2005;27:79-88.
- 38. Inaba T, Sato H, Kamiya H. Monoclonal IgA antibody-mediated expulsion of Trichinella from the intestine of mice. Parasitology 2003;126:591-598.
- 39. Sotillo J, Muňoz-Antoli C, Marcilla A, Fried B, Guillermo Esteban J, Toledo R. Echinostoma caproni: kinetics of IgM, IgA and IgG subclasses in the seum and intestine of experimentally infected rats and mice. Exp Parasitol 2007;116:390-398.
- 40. Fujino T, Fried B, Tada I. The expulsion of Echinostoma trivolvis: worm kinetics and intestinal cytopathology in conventional and congenitally athymic BALB/c mice. Parasitology 1993;106:297-304.
Fig. 1Worm recovery and chronologic changing patterns of intestinal IEL in ICR mice infected with 200 metacercariae of Pygidiopsis summa. (A) Worm recovery in immunocompetent (IC) and immunosuppressed (IS) mice at days 1, 3, 5, and 7 PI. Note significantly higher WRR (P<0.05) in IS mice at days 5 and 7 PI (*). (B) IEL counts in the jejunum showing a significant increase (P<0.05) in IC mice at days 3, 5, and 7 PI (*), but not in IS mice, compared with uninfected controls (day 0).
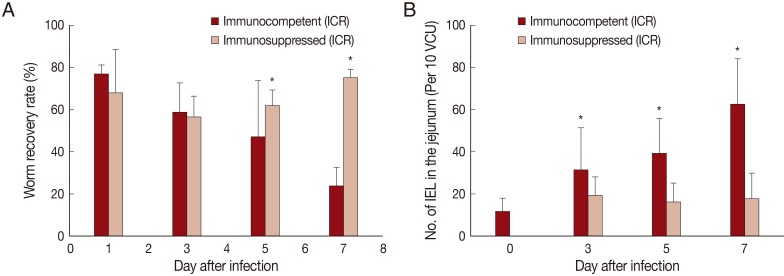
Fig. 2Histological sections of the jejunum showing IEL, mast cells, and goblet cells in ICR mice infected with Pygidiopsis summa. (A) A jejunal section showing the location of IEL (Ap, apical; Md, middle; Bs, basal) in the epithelial layer. 'Apical' was desginated when the IEL was located anteriorly in comparison with the nucleus of the epithelial cell. 'Middle' was when the IEL was located nearby the nucleus of the epithelial cell. 'Basal' was when the IEL was located posterior to the nucleus of the epithelial cell. Giemsa stain. ×1,000. (B) Mast cell (arrow) hyperplasia in P. summa-infected mice at day 7 PI. Astra blue and eosin stained. ×100. (C) Goblet cell (arrow) hyperplasia in P. summa-infected mice at day 5 PI. Periodic acid-Schiff (PAS) stained. ×100.
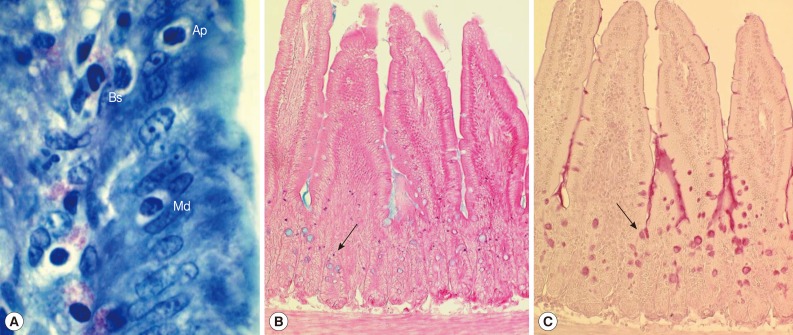
Fig. 3Mucosal mast cell and goblet cell responses in the intestine of ICR mice infected with 200 metacercariae of Pygidiopsis summa. (A) Mast cell responses in the jejunum that show a significant increase (P<0.05) in IC mice at day 7 PI (*) compared with uninfected controls (day 0) and IS mice. (B) Goblet cell responses in the jejunum showing a significant increase (P<0.05) in IC mice at days 5 and 7 PI (*) compared with uninfected controls (day 0) and IS mice.
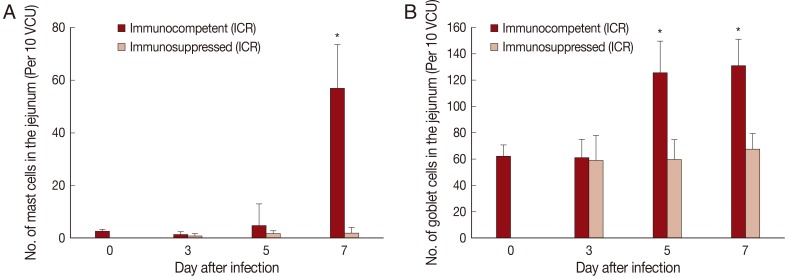
Fig. 4IgA absorbance in the jejunum showing a significant increase (P<0.05) in IC mice at days 3, 5, and 7 PI (*), but not in IS mice, compared with uninfected controls (day 0).
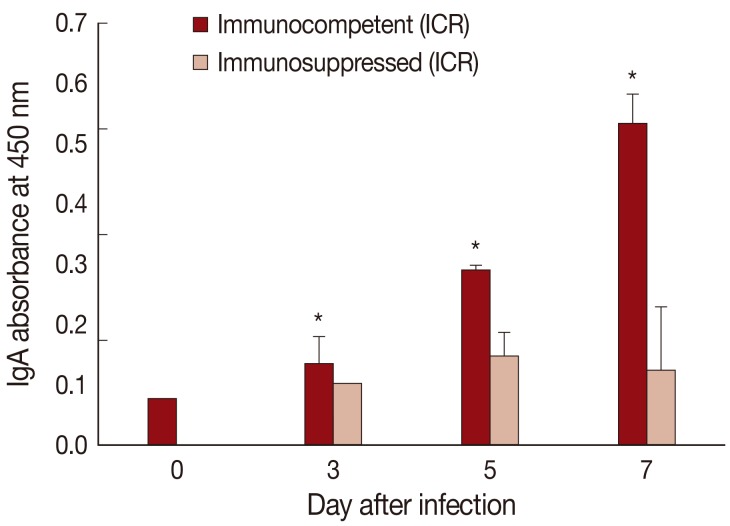
Table 1.Changes in the position of intraepithelial lymphocytes (IEL) in comparison with the level of epithelial cell nuclei in the jejunum of ICR mice at days 3, 5, and 7 post-infection with Pygidiopsis summa
Table 1.
|
Positiona of IEL (%) after P. summa infection
|
|
Uninfected (Day 0)
|
Day 3 PI
|
Day 5 PI
|
Day 7 PI
|
|
Apical |
Middle |
Basal |
Apical |
Middle |
Basal |
Apical |
Middle |
Basal |
Apical |
Middle |
Basal |
|
Controls |
1.2 |
2.4 |
96.4 |
|
|
|
|
|
|
|
|
|
|
Immunocompetent mice |
|
|
|
1.0 |
3.7 |
96.3 |
3.7b
|
10.2b
|
86.1b
|
2.5b
|
6.9b
|
90.7b
|
|
Immunosuppressed mice |
|
|
|
0.4 |
3.1 |
96.5 |
1.5 |
1.2 |
97.2 |
0.2 |
3.4 |
96.4 |