Abstract
Ascidian soft tunic syndrome (AsSTS) caused by Azumiobodo hoyamushi (A. hoyamushi) is a serious aquaculture problem that results in mass mortality of ascidians. Accordingly, the early and accurate detection of A. hoyamushi would contribute substantially to disease management and prevention of transmission. Recently, the loop-mediated isothermal amplification (LAMP) method was adopted for clinical diagnosis of a range of infectious diseases. Here, the authors describe a rapid and efficient LAMP-based method targeting the 18S rDNA gene for detection of A. hoyamushi using ascidian DNA for the diagnosis of AsSTS. A. hoyamushi LAMP assay amplified the DNA of 0.01 parasites per reaction and detected A. hoyamushi in 10 ng of ascidian DNA. To validate A. hoyamushi 18S rDNA LAMP assays, AsSTS-suspected and non-diseased ascidians were examined by microscopy, PCR, and by using the LAMP assay. When PCR was used as a gold standard, the LAMP assay showed good agreement in terms of sensitivity, positive predictive value (PPV), and negative predictive value (NPV). In the present study, a LAMP assay based on directly heat-treated samples was found to be as efficient as DNA extraction using a commercial kit for detecting A. hoyamushi. Taken together, this study shows the devised A. hoyamushi LAMP assay could be used to diagnose AsSTS in a straightforward, sensitive, and specific manner, that it could be used for forecasting, surveillance, and quarantine of AsSTS.
-
Key words: Azumiobodo hoyamushi, LAMP, diagnostic method, soft tunic syndrome, ascidian aquaculture
As its name suggests, soft tunic syndrome (AsSTS) affects the tunics of affected ascidians, such as
Halocynthia roretzi (Drasche), which become thin and soft and are easily torn or ruptured and may result in mass mortality [
1]. The repeated occurrence of AsSTS presents serious problems to those involved in the aquaculture of ascidians with respect to the economic burden imposed by epizootic outbreaks and its incidence is gradually increasing in-line with massive increases in aquaculture and devastation of the marine environment. However, early diagnosis and management of AsSTS are difficult, and thus a rapid, sensitive, and easily performed diagnostic method that produces readily interpretable results is required to forecast outbreaks and to devise effective control programs [
2].
Azumiobodo hoyamush, a flagellated parasite belonging to the order Neobodonida, was recently identified as a causative agent of AsSTS. The diagnosis of AsSTS is established by histopathologic examination of the ascidian tunic by microscopy, but it has limited sensitivity in the case of low parasite density in early infection [
2]. Recently, a highly sensitive PCR method based on the 18S rRNA of
A. hoyamushi was developed for the diagnosis of AsSTS [
3], but it requires relatively expensive laboratory equipments, a trained specialist, and has a long turnaround time, which shows some difficulties of direct application of the massive surveillance in the field.
Recently, the loop-mediated isothermal amplification (LAMP) method, which is a relatively straightforward and sensitive technique based on rapid DNA amplification, was developed and adopted for the clinical diagnosis of a range of infectious diseases [
4]. In the LAMP assay, the target DNA is specifically amplified by
Bst DNA polymerase, which performs strand displacement DNA-synthesis under isothermal conditions and uses a set of 6 oligonucleotides that recognize independent regions of the target gene. The use of 6 oligonucleotides improves the specificity and speed of amplification and forms a loop-structured amplicon, which produces a typical ladder-pattern of multiple bands [
5]. A positive reaction is easily determined by eye as turbidity [
6] or by adding fluorescent dyes [
7,
8]. Until now, LAMP assays have been applied to detections of pathogens, such as, viruses, bacteria, fungi, and parasites [
5]. Accordingly, the present study was undertaken to develop a simple, rapid, and sensitive diagnostic assay for AsSTS based on LAMP that can be used in the field.
First, we designed a set of 6 LAMP primers targeting the kinetoplast gene for 18S ribosomal RNA (GenBank accession no. AB636162) [
9] using the Primer Explorer program (
http://primerexplorer.jp/elamp4.0.0/index.html; Eiken Chemical Co., Japan) [
4] (
Table 1).
A. hoyamushi LAMP was performed for 90 min at 64℃ in a 25 µl mixture containing 40 pmol each of FIP and BIP, 5 pmol each of F3 and B3, 20 pmol each of LF and LB, 1.4 mM of deoxynucleoside triphosphates, 0.8 M betaine, and 1 µl of
Bst DNA polymerase (NEB, Beverly, Massachusetts, USA) in 2.5 µl of buffer [20 mM Tris-HCl pH 8.8, 10 mM KCl, 10 mM (NH
4)
2SO
4, 8 mM MgSO
4, and 0.1% Tween 20], and 1 µl of template DNA in a Loopamp real-time turbidimeter (Realoop-30; Eiken Chemical Co., Tokyo, Japan). Reactions were inactivated by heating at 80℃ for 2 min. Optimal temperature and time for LAMP reaction were determined using cloned 18S rDNA (10
6 copies per reaction) under isothermal conditions at temperatures of 60-65℃ for 120 min, by monitoring turbidity. Although amplification targeting the 18S rDNA gene was detected at all temperatures tested, the LAMP assay reached a threshold value (0.1) quickest at 64℃ (data not shown). No non-specific amplification was detected in the negative control (plasmid containing no insert) after at least 120 min of incubation. Thus, subsequent LAMP reactions were conducted at 64℃ for 90 min.
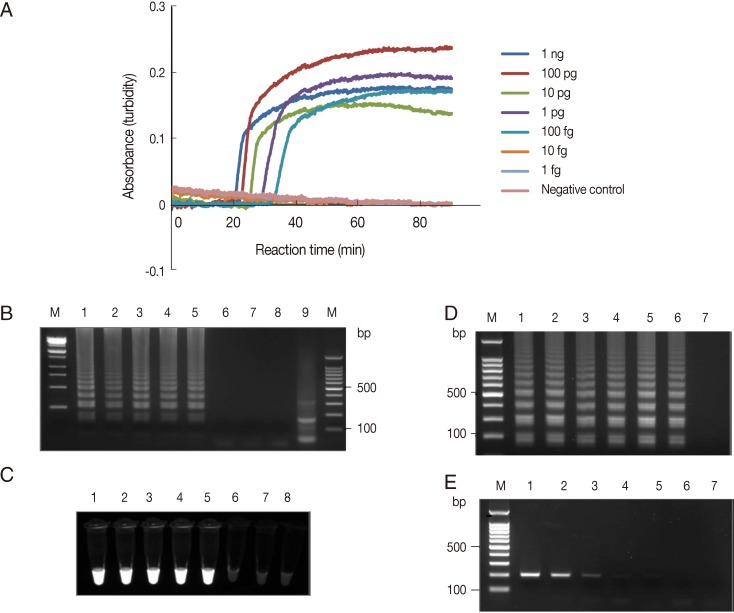
The sensitivity of the LAMP amplification was assessed in 2 ways. First, the LAMP assay was carried out using serially diluted
A. hoyamushi genomic DNA extracted from
in vitro cultured flagellates. The LAMP procedure amplified the targeted region at each dilution from the highest DNA concentration examined (1 ng per reaction) to as little as 1 fg per reaction, which reached the threshold value of absorbance (0.1) within 40 min (
Fig. 1A). As shown in
Fig. 1B, a typical ladder pattern of amplified LAMP products was observed by agarose gel (2.0%) electrophoresis in lanes containing 1 ng to 100 fg per reaction (
Fig. 1B, lanes 1-5). Amplified products of positive reactions were also visually detectable using the Loopamp fluorescent detection reagent (FD; Eiken Chemical Co.) under UV light (
Fig. 1C). Alternatively, DNA was extracted from ca 1×10
6 parasites, serially diluted to the equivalent of 1,000 to 0.01 parasites per reaction, and used as the template for the PCR and LAMP assays. F3 and B3 primers (
Table 1) were used for PCR and generated a product 205 bp long. The PCR conditions of 18S rDNA used were as follows: an initiation step of 94℃ for 10 min followed by 35 amplification cycles (94℃ for 30 sec, 64℃ for 30 sec, and 72℃ for 60 sec) and a final extension step at 72℃ for 5 min. The LAMP procedure amplified the DNA of parasites diluted to a concentration equivalent to 0.01 parasites per reaction (
Fig. 1D). In contrast, PCR amplified the DNA of parasites diluted to 0.1 parasite per reaction (
Fig. 1E). Thus, the detection limit of the LAMP assay was 10 times higher than that of PCR. To determine the specificity of the LAMP assay amplification, amplified LAMP products were digested with
MboI, of which cleavage site was represented in the target region of the 18S rDNA gene, and that generated the expected 144 bp fragment (
Fig. 1B, lane 9). No amplification was detected in LAMP assays conducted on the genomic DNAs of Pseudoalteromonas sp. or
Hasllibacter halocynthiae found in
H. roretzi [
10,
11] or in those conducted on the 2 pathogenic
H. roretzi unrelated flagellates (
Trichomonas vaginalis and
Trypanosoma brucei) (data not shown).
Then, template DNAs from non-diseased and AsSTS-positive ascidians (determined the presence of
A. hoyamushi flagellates by microscopic examinations) were tested for the detection of
A. hoyamishi DNA using the PCR and LAMP assays. Ascidians with or without AsSTS were collected from aquaculture sites in Tongyeong-gun (County) on the southeastern coast of the Republic of Korea in March and April 2013. Animals were classified as Grade 0 to Grade 4, as previously described (Grade 0=healthy ascidian in a non-infected population; Grade 1=apparently non-diseased ascidian in a diseased population; Grade 2=slightly soft tunicate; Grade 3=considerably soft tunicate; Grade 4=severe soft tunicate) [
12]. Grade 0 specimens were not available in Korea and thus Grade 1 specimens (non-diseased) were used as a control following the previous criteria. The collected ascidians were washed several times with 0.2 µm-filtered artificial seawater, tunics were dissected adjacent to the 2 siphons, chopped into small pieces (~0.2×0.2 cm) and placed in tubes containing 20 ml of sterilized artificial seawater, incubated at room temperature overnight, and directly examined under microscope the presence of flagellates as previously described [
13]. To prepare ascidians genomic DNA for PCR and LAMP, the dissected ascidians prepared above were incubated with 10 ml of PBS at room temperature for 30 min. After brief centrifugation, 1 ml of each supernatant was transferred to a new tube, centrifuged, and total DNA was extracted using a DNeasy tissue kit (Qiagen, Valencia, California, USA). One microliter of extracted DNA dissolved in 20 µl of doubly distilled water was used as the template for the PCR and LAMP assays.
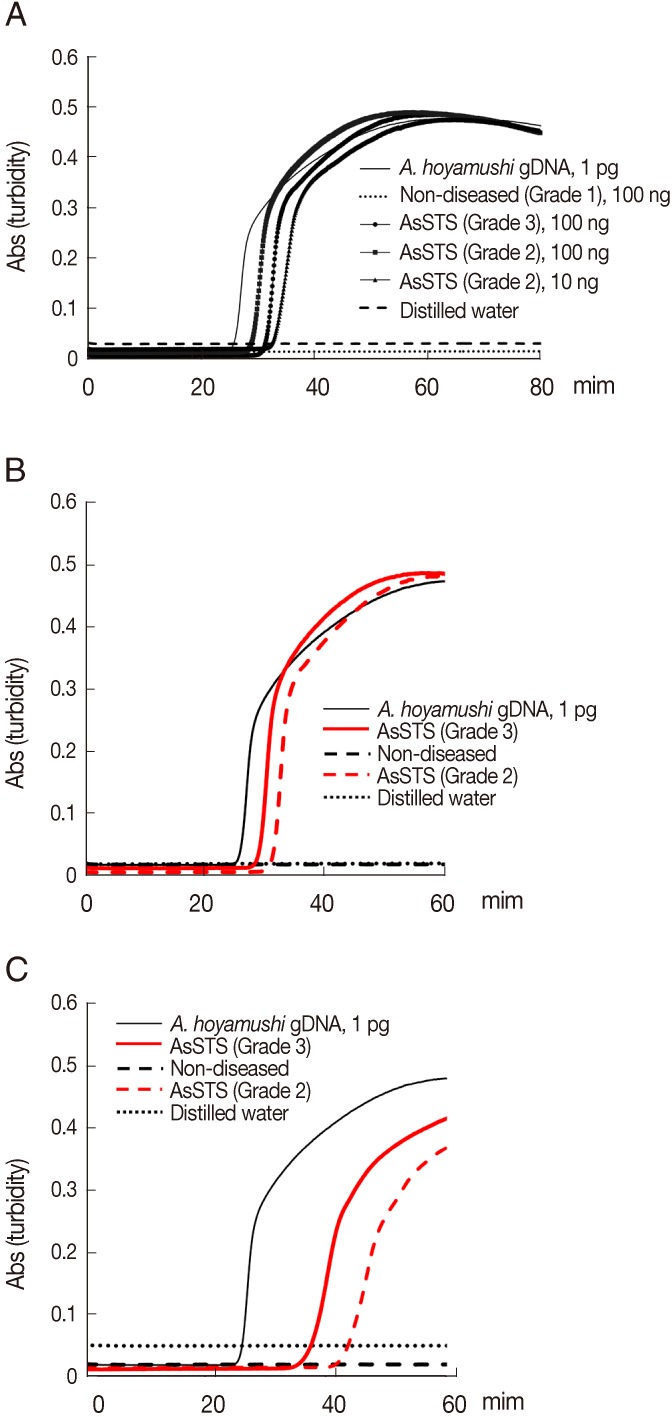
Results of the amplification of
A. hoyamishi DNA by PCR or LAMP were positive only for AsSTS-positive ascidians (Grade 2 to Grade 3) by microscopy. No amplification was detected in non-diseased (Grade 1) ascidians. PCR using F3 and B3 primers amplified the DNA from 100 ng of template DNA of AsSTS-positive ascidians (Grade 2 to Grade 3) (data not shown), whereas the
A. hoyamishi LAMP assay amplified DNA from 10 ng of template DNA (Grade 2) within 40 min from reaction initiation (
Fig. 2A). Thus, the detection limit of the LAMP assay using the DNA extracted from ascidians was 10 times higher than that of PCR, which was consistent with the results above using the pure DNA from in vitro cultured flagellates (
Fig. 1D, E).
Because the straightforward sample preparation for molecular diagnosis reduces the risk of cross-contamination and the turnaround time for diagnosis, we prepared the crude cell lysates of ascidians by direct heat-treatment. Briefly, 1 ml of the PBS supernatants above were collected, heated at 100℃ for 5 min to disrupt
A. hoyamushi flagellates, and centrifuged at 12,000 g for 2 min at room temperature. One microliter aliquots of supernatants were collected and used as templates for PCR and LAMP. LAMP assays conducted using heat-treated lysates (
Fig. 2C) produced the same results as those obtained using DNA extracted using a DNeasy tissue kit (
Fig. 2B), although the incubation time required for LAMP assay to reach a threshold absorbance value of 0.1 was slightly delayed (
Fig. 2C).
Next, AsSTS-suspected ascidians (n=36) (Grades 2 or 3) were then subjected to microscopic analysis, PCR, and to the LAMP assay (
Table 2). Of the 36 AsSTS-suspected ascidians, positive results by microscopy, PCR, and LAMP were 27 (75%), 32 (89%), and 33 (92%), respectively. All the 27 positive samples by microscopy were positive by PCR and LAMP. Of the 9 negatives by microscopy, 5 samples (56%) were positive by PCR and LAMP and thus considered false negatives. We attribute these 5 false negatives by microscopy to have been caused by a low parasite density in early infection. Of the 4 negatives by microscopy and PCR, 1 was positive by LAMP. Of the 50 non-diseased ascidians (Grade 1) subjected to the same 3 tests, all were negative by microscopy, and of these 50 negatives by microscopy, 1 was positive by PCR and LAMP and 2 were only positive by LAMP.
To determine the accuracy of the 3 tests for diagnosis of AsSTS, PCR was used as a gold standard and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated (
Table 2). The sensitivity and specificity of microscopic analysis were 79.4% with 95% confidence interval (CI) from 61.6 to 90.7% and 100% (95% CI, 91.6-100%), respectively, whereas those of the LAMP assay were 100% (95% CI, 87.4-100%) and 94.3% (95% CI, 83.4-98.5%), respectively. These results indicate that PCR and LAMP had higher sensitivity compared to that of optical microscopy and thus can be used in the diagnosis of early infections of AsSTS. When PCR was used as the gold standard, LAMP showed excellent PPV (91.9%, 95% CI: 77.0-97.9%) and NPV (100%, 95% CI: 91.1-100%) and better agreement with PCR (Kappa=0.929) (95% CI, 0.850-1.000) (
P<0.001) than microscopy (Kappa=0.825) (95% CI, 0.702-0.947) (
P<0.001).
Since AsSTS was first reported, efforts have been made to identify the causative agent, but recently,
A. hoyamushi was found to be responsible [
9,
13]. In agreement with previous studies, the LAMP assay detected
A. hoyamushi only in ascidians with AsSTS (
Fig. 2A). Furthermore, the
A. hoyamushi LAMP assay showed no cross-reactivity with the ascidian tunic, with other organisms previously suggested to be associated with AsSTS, or with AsSTS unrelated flagellates (data not shown). In addition, the devised LAMP assay showed good agreement with PCR and high PPV and NPV values.
Previous authors have reported that the clinical signs of AsSTS are evident at siphons during the early stage of infection and subsequently extend into adjacent regions [
2]. In the present study, to enhance the sensitivity of diagnostic methods, tunics adjacent to the siphons were chopped, incubated with PBS for only 30 min, heat-treated, and directly used as the template for the LAMP assay instead of using the extracted DNA by conventional methods from the overnight enrichment of the sliced tunics with seawater [
13]. The LAMP assay conducted on heat-treated samples was found to be as efficient at detecting
A. hoyamushi as DNA extracted using a commercial kit probably due to the ability of LAMP to better tolerate biological contaminants than PCR [
14,
15]. In this study, the LAMP assay conducted using heat-treated lysates was completed within 50 min (
Fig. 2C), which is substantially faster than PCR using DNA prepared by conventional methods or commercially available kits. Thus, the devised
A. hoyamushi LAMP assay could eliminate the need for DNA extraction without compromising sensitivity and reduce the time required to reach a diagnosis. Furthermore, it could be applied for AsSTS surveillance and quarantine for importation and exportation purposes. In addition, simplicity, rapid sample preparation, and straightforward detection suggest that the devised assay has potential forecasting applications in seawater regions where AsSTS is prevalent [
16,
17].
RP-2012-AQ-32
Notes
-
We have no conflict of interest related to this study.
ACKNOWLEDGMENT
This study was supported by the Aquaculture Management Division, National Fisheries Research & Development Institute (Grant no. RP-2012-AQ-32).
References
- 1. Hirose E, Ohtake SI, Azumi K. Morphological characterization of the tunic in the edible ascidian, Halocynthia roretzi (Drasche), with remarks on 'soft tunic syndrome' in aquaculture. J Fish Dis 2009;32:433-445.
- 2. Kumagai A, Suto A, Ito H, Tanabe T, Takahashi K, Kamaishi T, Miwa S. Mass mortality of cultured ascidians Halocynthia roretzi associated with softening of the tunic and flagellate-like cells. Dis Aquat Organ 2010;90:223-234.
- 3. Kumagai A, Kamaishi T. Development of polymerase chain reaction assays for detection of the kinetoplastid Azumiobodo hoyamushi, the causative agent for soft tunic syndrome in the ascidian Halocynthia roretzi. Fish Pathol 2013;48:42-47.
- 4. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucl Acids Res 2000;28:E63.
- 5. Mori Y, Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 2009;15:62-69.
- 6. Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 2001;289:150-154.
- 7. Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques 2009;46:167-172.
- 8. Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 2008;3:877-882.
- 9. Hirose E, Nozawa A, Kumagai A, Kitamura S. Azumiobodo hoyamushi gen. nov. et sp. nov. (Euglenozoa, Kinetoplastea, Neobodonida): a pathogenic kinetoplastid causing the soft tunic syndrome in ascidian aquaculture. Dis Aquat Organ 2012;97:227-235.
- 10. Kim D, Baik KS, Park SC, Kim SJ, Shin TS, Jung SJ, Oh MJ, Seong CN. Cellulase production from Pseudoalteromonas sp. NO3 isolated from the sea squirt Halocynthia rorentzi. J Ind Microbiol Biotechnol 2009;36:1375-1382.
- 11. Kim SH, Yang HO, Shin YK, Kwon HC. Hasllibacter halocynthiae gen. nov., sp. nov., a nutriacholic acid-producing bacterium isolated from the marine ascidian Halocynthia roretzi. Int J Syst Evol Microbiol 2012;62:624-631.
- 12. Kitamura SI, Ohtake SI, Song JY, Jung SJ, Oh MJ, Choi BD, Azumi K, Hirose E. Tunic morphology and viral surveillance in diseased Korean ascidians: soft tunic syndrome in the edible ascidian, Halocynthia roretzi (Drasche), in aquaculture. J Fish Dis 2010;33:153-160.
- 13. Kumagai A, Ito H, Sasaki R. Detection of the kinetoplastid Azumiobodo hoyamushi, the causative agent of soft tunic syndrome, in wild ascidians Halocynthia roretzi. Dis Aquat Organ 2013;106:267-271.
- 14. Enomoto Y, Yoshikawa T, Ihira M, Akimoto S, Miyake F, Usui C, Suga S, Suzuki K, Kawana T, Nishiyama Y, Asano Y. Rapid diagnosis of herpes simplex virus infection by a loop-mediated isothermal amplification method. J Clin Microbiol 2005;43:951-955.
- 15. Kaneko H, Kawana T, Fukushima E, Suzutani T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J Biochem Biophys Methods 2007;70:499-501.
- 16. Sharma S, Srinivasan M, George C. Acanthamoeba keratitis in non-contact lens wearers. Arch Ophthalmol 1990;108:676-678.
- 17. Srinivasan M, Burman S, George C, Nirmalan PK. Non-contact lens related Acanthamoeba keratitis at a tertiary eye care center in south India: implications for eye care programs in the region. Med Sci Monit 2003;9:CR125-CR129.
Fig. 1Detection limit of
Azumiobodo hoyamushi 18S rDNA LAMP assays (A). LAMP assays were performed using serial dilutions of
A. hoyamushi genomic DNA (1 ng to 1 fg per reaction). Distilled water was used as a negative control. LAMP products were visualized by gel electrophoresis (B) and using Loopamp® fluorescent detection reagent (FD) (C). (B, C) Lane M, 100-bp DNA marker; lane 1, 1 ng; lane 2, 100 pg; lane 3, 10 pg; lane 4, 1 pg; lane 5, 100 fg; lane 6, 10 fg; lane 7, 1 fg of
A. hoyamushi genomic DNA; lane 8, distilled water; and lane 9, LAMP product after
MboI digestion. (D-E)
A. hoyamushi at a density of 1×10
3 parasites/µl was serially diluted and tested (D) using the LAMP assay (D) and by PCR (E) using F3 and B3 primers. Lane M, 100-bp DNA marker; lane 1, 1,000; lane 2, 100; lane 3, 10; lane 4, 1; lane 5, 0.1; lane 6, 0.01 of parasites per reaction; lane 7, distilled water.
A. hoyamushi genomic DNA was prepared using DNeasy tissue kits (Qiagen) from in vitro cultured
A. hoyamushi species [
9] which were kindly provided by Dr. Kyung Il Park (Kunsan National University, Gunsan, Korea).

Fig. 2Results of the LAMP assay for detection of Azumiobodo hoyamushi from AsSTS (Grades 2, 3) and non-diseased (Grade 1) ascidians. (A) The amplified products of LAMP reactions were examined by real-time turbidity: A. hoyamushi genomic DNA (1 pg), non-diseased ascidian DNA (100 ng), AsSTS ascidian DNA (Grade 3, 100 ng; Grade 2, 100 ng; [please confirm here] Grade 2, 10 ng), and distilled water. (B-C) Detection of A. hoyamushi in non-diseased (Grade 1) and in AsSTS infected ascidians (Grades 2, 3) using DNAs (B) prepared by a commercial kit (DNeasy tissue kit, Qiagen) or heat-treated lysates (C).

Table 1.Names and sequences of the primers used for A. hoyamushi 18S rDNA LAMP reaction
Table 1.
|
Primer |
Sequence (5´-3´) |
|
F3 |
GAATGGTGGTGCATGGCC |
|
B3 |
GCCCAAAATCTCACCTCGTT |
|
FIP (F1c-F2) |
CTACTGGGCGGCTTGGATCTCGCTTTTGGTCGGTGGAGT |
|
BIP (B1c-B2) |
AGCAATCCTCTTGCTCGGCTTAGGAATCCCGCAGAGAAGG |
|
LF |
GTTGACGGAATCAACCAAACAAATC |
|
LB |
CGGCTGACTGAGGCAACCT |
Table 2.Sensitivity, specificity, PPV, NPV, and agreements of microscopy and LAMP versus PCR (the gold standard)
Table 2.
|
Assay |
Microscopy |
PCR |
LAMP |
|
AsSTS-suspected ascidians (n = 36) |
Positive: 27 |
Positive: 27 |
Positive: 27 |
|
Negative: 9 |
Positive: 5 |
Positive: 5 |
|
Negative: 4 |
Positive: 1 |
|
|
Negative: 3 |
|
Non-diseased ascidians (n = 50) |
Positive: 0 |
Positive: 1 |
Positive: 3 |
|
Negative: 50 |
Negative: 49 |
Negative: 47 |
|
Sensitivity (%) (95% CI) |
79.4 (61.6-90.7) |
|
100 (87.4-100) |
|
Specificity (%) (95% CI) |
100 (91.6-100) |
|
94.3 (83.4-98.5) |
|
PPV (%) (95% CI) |
100 (84.5-100) |
|
91.9 (77.0-97.9) |
|
NPV (%) (95% CI) |
88.3 (76.8-94.8) |
|
100 (91.1-100) |
|
Kappaa (95% CI) |
0.825 (0.702-0.947) |
|
0.926 (0.850-1.000) |