Abstract
Sparganosis is an infection with a parasitic tapeworm larva that occurs by eating infected foods or drinking contaminated water. The larvae can migrate to a tissue or muscle in the chest, abdominal wall, extremities, eyes, brain, urinary tract, pleura, pericardium, spinal canal, or scrotum. Herein, we report a 5-month old infant with scrotal sparganosis who was initially suspected to have a scrotal inflammatory mass with a history of applying raw frog meat into the umbilicus. Preoperative ultrasound examinations and computed tomography (CT) scanning misdiagnosed the mass as a scrotal teratoma. The scrotal mass was surgically removed, and the histopathology proved it to be scrotal sparganosis. This case displays the youngest patient ever reported with scrotal sparganosis, and the first description of CT characteristics of scrotal sparganosis. A detailed medical history is necessary for patients with scrotal masses suspected of sparganosis. In addition, ultrasound and CT examinations are helpful to rule out other causes of a scrotal mass.
-
Key words: Sparganum, sparganosis, scrotum, computed tomography, ultrasound
INTRODUCTION
Sparganosis mansoni is a parasitic infection caused by the plerocercoid larvae of diphyllobothroid tapeworms of the genus
Spirometra [
1]. Sparganosis has been reported sporadically around the world with a higher prevalence of the disease occurring in Eastern Asian countries that include South Korea, Japan, Thailand, and China [
2]. The reported age at onset is commonly in older children and adults [
3,
4]. Clinical symptoms also vary according to the location of the sparganum. The plerocercoid larvae can migrate to almost any part of the body after invasion, and the common destinations are subcutaneous tissues of the torso and extremities, the ocular, oral and maxillofacial region, and the viscera. Other sites, such as the scrotum are rare [
5].
Scrotal sparganosis is generally characterized by a painless subcutaneous scrotal lump, with no specific identifying characteristics and is difficult to diagnose preoperatively. We reviewed the literature related to scrotal sparganosis, and found that all cases were preoperatively misdiagnosed [
3,
6,
7,
8,
9,
10,
11,
12,
13,
14]. Although the histopathologic examination and serum detection of anti-sparganum antibody can be confirmed, they are often made after surgery.
Some authors feel that the serpiginous low attenuation structure discovered on ultrasonic images aids in the differential diagnosis of scrotal sparganosis [
9,
11]. However, up until now, there has been no report of CT characteristics of scrotal sparganosis. Here, we report CT features of a case of scrotal sparganosis in a 5-month old infant in China, which was preoperatively misdiagnosed as a scrotal teratoma. Our case illustrates that infants may also be infected with spargana, and a detailed medical history combined with a CT scan and ultrasound examination are helpful to arrive at a correct diagnosis of scrotal sparganosis.
CASE RECORD
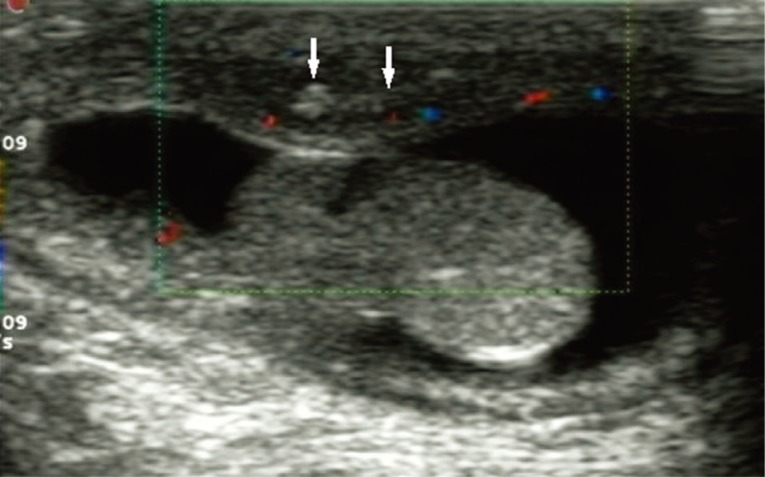
A 5-month-old boy was found to have an asymptomatic left scrotal mass for 3 months. The mass was soft, non-tender and without overlying scrotal skin abnormality. Ultrasound examination identified that the left scrotal wall was thickened with a mixed-echogenicity mass measuring 11×3 mm, which had an obscure boundary and increased blood supply on color Doppler (
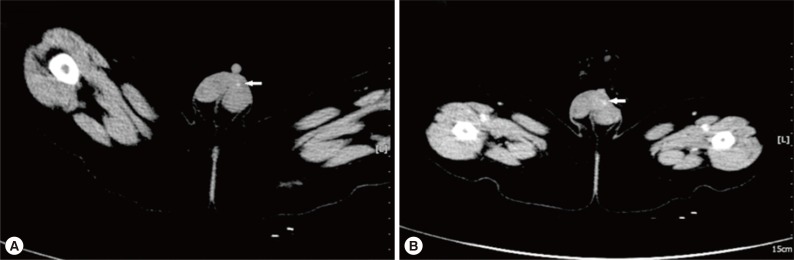
Fig. 1). The mass was thought to be inflammatory in origin, and observation was recommended. However, repeat ultrasound examinations demonstrated the mass to be increasing in size. The last ultrasound examination revealed a 19×9 mm hetero-echogenic mass with ill-defined margins. Physical examination demonstrated a flat mass of the left scrotum measuring 20×10×3 mm, with non-homogeneous consistency, ill-defined borders and no tenderness. CT showed a mass of 12×9 mm anterior to the left testicle, with heterogeneous density and punctate calcifications (
Fig. 2A) and obvious enhancement after administration of contrast agent (
Fig. 2B), which was diagnosed as a left testicular teratoma. The complete blood count (CBC), electrolytes, and liver function tests were all normal prior to surgery. The eosinophil differential count was 6% (normal range: 0.5 to 5%). No parasite eggs were found on routine stool testing. The α-fetoprotein (AFP), 19.5 µg/L (normal <20 µg/L), and human chorionic gonadotropin (HCG), 2.01 µg/L (normal <3.1 µg/L), were both normal.
The scrotal skin was edematous and thickened at surgery. A transverse incision was made at the base of the left scrotal mass. An oval, flat mass (15×17×3 mm) was located in the Dartos layer of the scrotum with non-homogeneous hardness and granular nodules within it. The mass was poorly circumscribed and external to the testicle and epididymis. The excised mass with surrounding edematous tissue was sent for pathological examinations. The left testis, epididymis, and vas deferens were normal based on direct examinations.
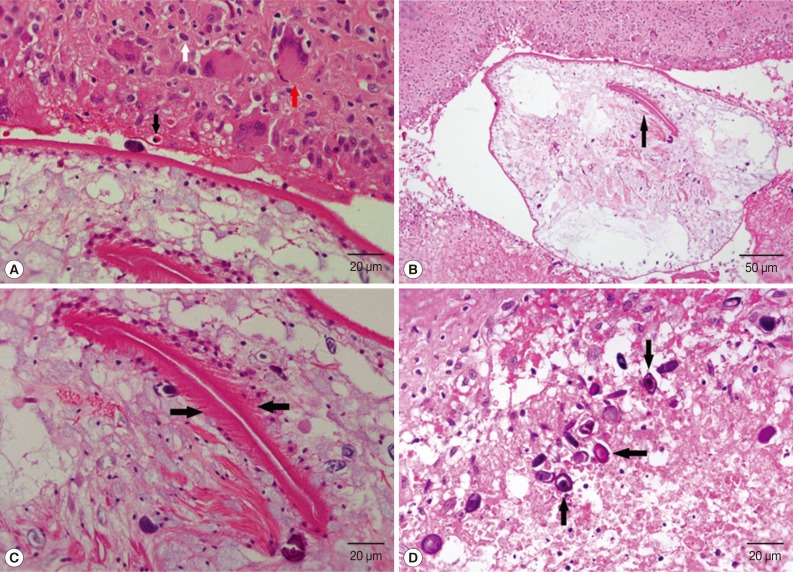
The pathological diagnosis was sparganosis (
Fig. 3). The postoperative (2 weeks after the surgery) ELISA for anti-sparganum antibody was positive (1:200 with the absorbance of 0.420 and the cut-off value of 0.197), while the serum antibodies of other parasites such as cysticercosis, lung trematode, filaria, and hydatid were all negative. After definitive diagnosis, re-questioning the family revealed that the infant was treated with topical raw frog meat to the umbilicus for 2 days at 20 days of life. No abnormalities of other organs were identified on postoperative brain CT, chest X-ray, and abdominal ultrasound. Postoperative oral praziquantel (25 mg/kg per day) was prescribed for 4 days to prevent residual infection. No new nodules were found in the left scrotum at the subsequent 4-month visit post surgery, and the serum test of anti-sparganum antibody 4-months post surgery was negative (<1:100), which indicated that the infant was successfully cured by the surgery.
DISCUSSION
Sparganosis is a rare parasitic infection in humans caused by a larval cestode of the genus
Spirometra. The disease has been reported in 39 countries, mostly in Southeast Asia and occurs mainly in people who commonly eat raw snakes or frogs [
8]. In China, the main route of infection is using unboiled frog meat as local poultice, which relates to Chinese folklore that raw frog skin or frog meat has a healing effect on wounds [
5]. The infected infant in our report was treated with raw frog meat applied to his umbilicus for abdominal distension 20 days after birth.
After gaining entrance, spargana can reside in almost any part of the body; the destination is commonly a muscle or subcutaneous tissue in the lower abdomen, inguinal, and femoral regions, as well as the eye, oral and maxillofacial region, abdominal viscera, and brain, while the scrotum is rare [
9,
11,
13]. There have been only 10 reported cases of scrotal sparganosis in the literature dating back to 1964 (
Table 1).
Human sparganosis occurs in both children and adults, but very rarely in children less than 1 year of age [
5,
10]. The 5-month old patient in this case is the youngest reported patient with scrotal sparganosis. The most common clinical manifestations of scrotal sparganosis is a painless lump of the scrotal wall that was initially misdiagnosed in all patients. In the reviewed literature, the time interval from initial presentation of a scrotal mass to resection was 3 to 24 months [
8,
14]. This indicates that scrotal sparganosis is difficult to diagnosis due to its rarity and non-specific clinical manifestations.
Serum immunology testing with ELISA is a very reliable method for preoperative diagnosis of sparganosis [
14,
15]. However, given the extremely low incidence of scrotal sparganosis, serum immunology testing is not routinely used for patients presenting with a scrotal mass. Therefore, the key to correct preoperative diagnosis of scrotal sparganosis is radiologic examinations.
Ultrasound is often used for evaluation of superficial subcutaneous masses, including the scrotum. The ultrasound characteristic of internal serpiginous tubular structures suggests sparganosis, but this can also be seen in radiation edema and superficial phlebitis [
9,
11,
14]. In this case, multiple ultrasound examinations demonstrated a heterogeneous echogenic mass of the scrotal wall without internal anechoic serpiginous tubular structures. Therefore, ultrasound has limitations in the differential diagnosis of scrotal sparganosis [
11]. The CT findings of scrotal sparganosis are similar to the reported triad manifestations of cerebral sparganosis: low density mass, punctate calcifications, and nodular or irregular enhancement [
16,
17]. The CT scan revealed a heterogeneous density mass of the scrotal wall with punctate calcifications on plain CT in our patient (
Fig. 2A) and obvious enhancement on contrast-enhanced CT (
Fig. 2B), which was misdiagnosed as a scrotal teratoma.
The differential diagnosis of a scrotal wall mass includes trauma, inflammation, and tumor [
18]. If there is no history of trauma and no evidence of inflammation, then a CT scan should be performed to rule out a tumor. Scrotal tumors generally do not display punctate calcifications on CT imaging [
18]. Therefore, if calcifications are identified, the diagnosis of scrotal sparganosis should be suspected. A detailed medical history is necessary, with attention to whether the patient has a history of eating unboiled reptile meat. If so, immunologic testing should be performed.
Surgical excision is the treatment of choice for sparganosis regardless of the affected location. Excision of the entire worm, in particular the scolex, is critical to achieve a radical cure for the patient. The 10 patients reported in the literature review were all cured by surgical removal of the tumors and the spargana larvae [
3,
6,
7,
8,
9,
10,
12,
13,
14]. It is generally considered that there is no effective drug treatment for sparganosis. However, praziquantel is recommended for multiple-site sparganosis of the viscera that cannot be effectively cured by surgery. Praziquantel is a broad-spectrum drug used for resistant trematodes and tapeworms, but its efficacy for treating sparganosis remains uncertain [
19,
20,
21,
22,
23]. Since it is unknown whether live sparganum larvae remain in this patient after complete resection of the scrotal mass, the postoperative use of oral praziquantel is controversial. Our patient was treated with oral praziquantel for 1 cycle after surgery.
In conclusion, a detailed medical history followed by scrotal ultrasound and CT scan is necessary to establish the diagnosis of scrotal sparganosis. Serum immunology testing can then confirm the diagnosis prior to surgical excision.
Notes
-
We have no conflict of interest related to this study.
References
- 1. John DT, Petri WA. Markell and Voge's Medical Parasitology. 9th ed. St. Louis, Missouri, USA. Saunders Elsevier; 2006.
- 2. Li MW, Song HQ, Li C, Lin HY, Xie WT, Lin RQ, Zhu XQ. Sparganosis in mainland China. Int J Infect Dis 2011;15:e154-e156.
- 3. Yeo JY, Han JY, Lee JH, Park YH, Lim JH, Lee MH, Kim CS, Yi HG. A case of inguinal sparganosis mimicking myeloid sarcoma. Korean J Parasitol 2012;50:353-355.
- 4. Wiwanitkit V. A review of human sparganosis in Thailand. Int J Infect Dis 2005;9:312-316.
- 5. Lin X, Wang Z. Overview of clinical features of sparganosis mansoni in China. J Pathog Biol 2011;6:467-468.
- 6. Seo BS, Rim HJ, Yoon JJ, Lee DJ. A case report of sparganosis. Korean J Parasitol 1964;2:179-182.
- 7. Ishida Y, Naitoh Y, Nishida M, Soh J, Ito Y, Uehara H, Uchida M, Watanabe H. A case of sparganosis mansoni with a painless mass in the inguinal region and the scrotum. Acta Urol Jap 1996;42:983-985.
- 8. Jeong HJ. Fournier's gangrene associated with sparganosis in the scrotum. Urology 2004;63:176-177.
- 9. Kim YJ, Lee MW, Jeon HJ, Yi JG, Paick SH, Kim HG, Lim SD, Hwang TS. Sparganosis in the scrotum sonographic findings. J Ultrasound Med 2007;26:129-131.
- 10. Lim DH, Kim CS, Kim SI. Sparganosis presenting as spermatic cord hydrocele in six-year-old boy. Urology 2007;70:1223.e1-1223.e2.
- 11. Kim SH, Park S, Paik JH. Scrotal sparganosis: with an emphasis on ultrasonographic findings. Urology 2008;71:351.e11-351.e12.
- 12. Huang AM, Li X, Su SL. A case of sparganosis mansoni in scrotum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2009;27:79.
- 13. Yun SJ, Park MS, Jeon HK, Kim YJ, Kim WJ, Lee SC. A case of vesical and scrotal sparganosis presenting as a scrotal mass. Korean J Parasitol 2010;48:57-59.
- 14. Hong SJ, Kim YM, Seo M, Kim KS. Breast and scrotal sparganosis sonographic findings and pathologic correlation. J Ultrasound Med 2010;29:1627-1633.
- 15. Yeo IS, Yong TS, Im KI. Serodiagnosis of human sparganosis by a monoclonal antibody-based competition ELISA. Yonsei Med J 1994;35:43-48.
- 16. Chang KH, Chi JG, Cho SY, Han MH, Han DH, Han MC. Cerebral sparganosis: analysis of 34 cases with emphasis on CT features. Neuroradiology 1992;34:1-8.
- 17. Qian SK, Meng W, Liu LY, Deng L, Hu SW, Yang YF. Cerebral sparganosis: a report of 4 cases and review of literatures. Chin J Neuromed 2008;7:88-90.
- 18. Li YZ, Cai DM, Qiu L, Wen XR, Zhuang H. Characteristics of scrotal wall diseases in high frequency ultrasonography and pathological findings. Chin J Med Imaging Technol 2007;23:1202-1204.
- 19. Wu W, Huang YX. Sparganosis mansoni treated with praziquantel: one case report. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2009;21:472.
- 20. Kim DG, Paek SH, Chang KH, Wang KC, Jung HW, Kim HJ, Chi JG, Choi KS, Han DH. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg 1996;85:1066-1071.
- 21. Bai J, He ZY, Liu GN, Zhang JQ, Deng JM, Li MH, Zhong XN. Clinical solutions. Bronchial sparganosis mansoni accompanied by abnormal hyperplasia diagnosed by bronchoscopy. Chin Med J (Engl) 2012;125:3183-3187.
- 22. Gonzenbach RR, Kong Y, Beck B, Buck A, Weller M, Semmler A. High-dose praziquantel therapy for cerebral sparganosis. J Neurol 2013;260:1423-1425.
- 23. Hong D, Xie H, Zhu M, Wan H, Xu R, Wu Y. Cerebral sparganosis in mainland Chinese patients. J Clin Neurosci 2013;20:1514-1519.
Fig. 1Scrotal ultrasound showing that the left scrotal wall is thickened and there is a hetero-echogenic mass (arrows) measuring 11×3 mm with ill-defined margins.
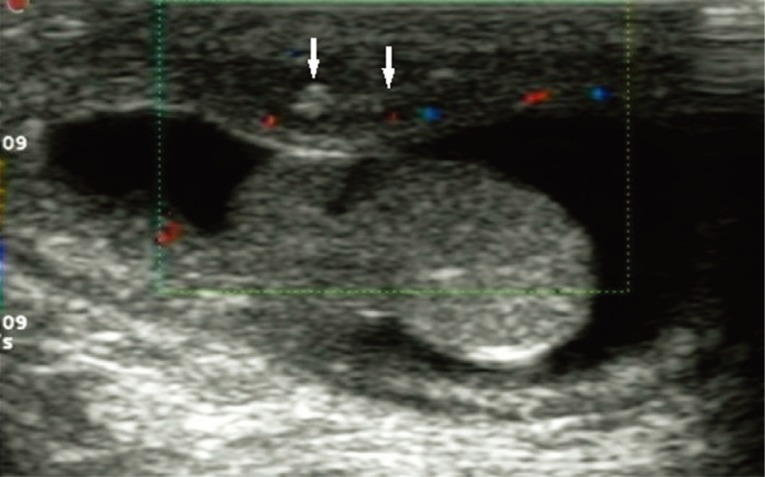
Fig. 2The CT appearance of scrotal sparganosis. (A) Focal nodule of 12×9 mm in size (arrow) was found anterior to the left testis with heterogeneous density and punctate calcifications on non-contrast CT scan. (B) The lesion (arrow) anterior to the left testis was very prominent on contrast-enhanced CT.
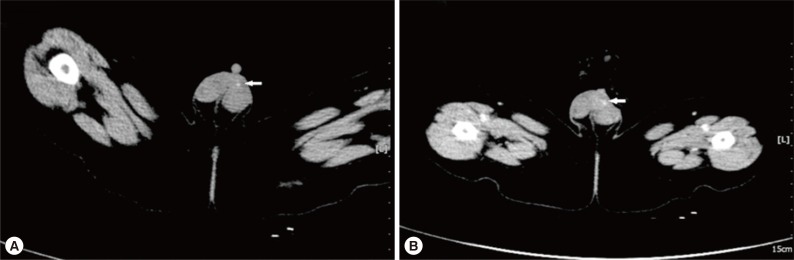
Fig. 3The histopathologic images of scrotal sparganosis. (A) Chronic granulomas with eosinophils (black arrow), lymphocytes (white arrow), and multinucleated giant cells (red arrow) infiltration surrounding the worm's body. H&E, ×400. (B) The central portion of the worm head is embolic, which appears as a lip-like structure (arrow) on cross sectional image of the scolex with many small spines around it. H&E, ×200. (C) There are many small spines around the lip-like structure (arrows). H&E, ×400. (D) Many calcareous bodies (arrows) are displayed on the cross section of the sparganum larvae, which are round or oval neutrophilic small bodies arranged in concentric circles. H&E, ×400.
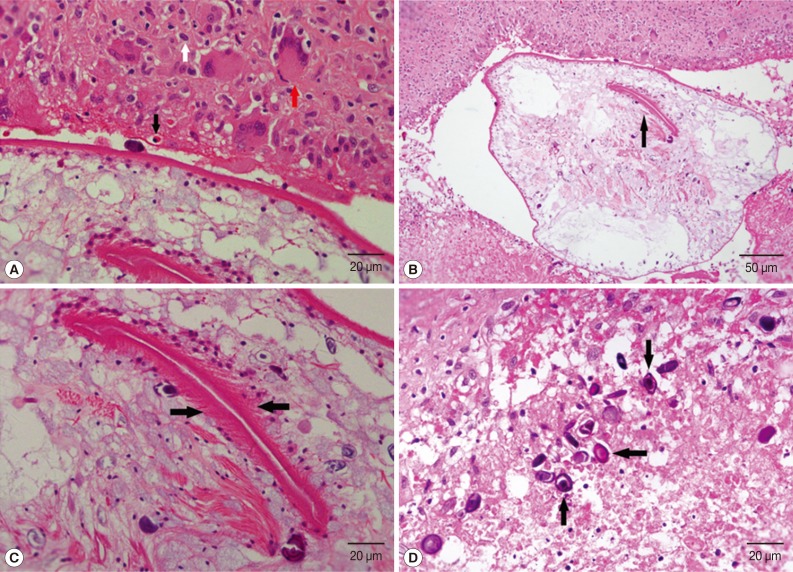
Table 1.Review of scrotal sparganosis cases reported in the literature
Table 1.
|
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
Case 6 |
Case 7 |
Case 8 |
Case 9 |
Case 10 |
|
Reported year |
1964 |
1996 |
2004 |
2007 |
2007 |
2008 |
2009 |
2010 |
2010 |
2012 |
|
Country |
Korean |
Japanese |
Korean |
Korean |
Korean |
Korean |
Chinese |
Korean |
Korean |
Korean |
|
Age |
43 |
67 |
- |
62 |
6 |
44 |
26 |
59 |
47 |
56 |
|
Gender |
Male |
Male |
- |
Male |
Male |
Male |
Male |
Male |
Male |
Male |
|
History of eating raw food |
Yes |
- |
- |
No |
No |
No |
No |
Yes |
No |
Yes |
|
External use of frog or snake meat |
- |
- |
- |
- |
No |
Yes |
No |
- |
No |
No |
|
Course of disease (months) |
24 |
- |
- |
3 |
6 |
12 |
- |
12 |
- |
- |
|
Painless mass |
Yes |
Yes |
|
Yes |
Hydrocele |
Yes |
Yes |
Yes |
Yes |
|
|
Locations |
Scrotum |
Inguinal region and scrotum |
Scrotum |
Scrotum |
Scrotum and spermatic cord |
Scrotum |
Scrotum |
Scrotum |
Scrotum and bladder |
|
|
Migration |
No |
Yes |
- |
No |
- |
Yes |
No |
No |
No |
- |
|
Gangrene |
No |
No |
Yes |
No |
- |
No |
No |
No |
No |
- |
|
Fever |
No |
- |
- |
No |
- |
No |
No |
No |
No |
- |
|
Acidophilic cells |
- |
- |
- |
Normal |
Increased |
Increased |
- |
- |
- |
- |
|
Ultrasound |
- |
- |
- |
Yes |
- |
Yes |
Yes |
Yes |
Yes |
- |
|
CT |
- |
- |
- |
No |
- |
No |
No |
No |
No |
- |
|
Anti-sparganum antibody test |
- |
- |
- |
- |
Positive |
Negative |
- |
Positive |
Negative |
- |
|
Histology |
Yes |
Yes |
- |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
- |
|
Treatment |
Surgery |
Surgery |
Surgery |
- |
Surgery |
Surgery |
Surgery |
Surgery |
Surgery |
- |
|
Prognosis |
- |
- |
- |
Cured |
- |
Cured |
- |
Cured |
- |
- |
Citations
Citations to this article as recorded by

- Low prevalence of spargana infection in farmed frogs in the Yangtze River Delta of China
Xiaoli Zhang, Rongsheng Mi, Yehua Zhang, Shijie Zhang, Tao Sun, Haiyan Jia, Yan Huang, Haiyan Gong, Xiangan Han, Zhaoguo Chen
Infection, Genetics and Evolution.2020; 85: 104466. CrossRef - Sparganosis as an accidental human parasitic disease
Jolanta Czyżewska, Joanna Matowicka-Karna
Diagnostyka Laboratoryjna.2018; 54(3): 167. CrossRef - Seroprevalence of Sparganosis in Rural Communities of Northern Tanzania
Nicholas Kavana, Parthasarathy Sonaimuthu, Christopher Kasanga, Ayub Kassuku, Hesham M. Al-Mekhlafi, Mun Yik Fong, Mohammad Behram Khan, Rohela Mahmud, Yee Ling Lau
The American Journal of Tropical Medicine and Hygiene.2016; 95(4): 874. CrossRef