Abstract
While imported falciparum malaria has been increasingly reported in recent years in Korea, clinicians have difficulties in making a clinical diagnosis as well as in having accessibility to effective anti-malarial agents. Here we describe an unusual case of imported falciparum malaria with severe hemolytic anemia lasting over 2 weeks, clinically mimicking a coinfection with babesiosis. A 48-year old Korean man was diagnosed with severe falciparum malaria in France after traveling to the Republic of Benin, West Africa. He received a 1-day course of intravenous artesunate and a 7-day course of Malarone (atovaquone/proguanil) with supportive hemodialysis. Coming back to Korea 5 days after discharge, he was readmitted due to recurrent fever, and further treated with Malarone for 3 days. Both the peripheral blood smears and PCR test were positive for Plasmodium falciparum. However, he had prolonged severe hemolytic anemia (Hb 5.6 g/dl). Therefore, 10 days after the hospitalization, Babesia was considered to be potentially coinfected. A 7-day course of Malarone and azithromycin was empirically started. He became afebrile within 3 days of this babesiosis treatment, and hemolytic anemia profiles began to improve at the completion of the treatment. He has remained stable since his discharge. Unexpectedly, the PCR assays failed to detect DNA of Babesia spp. from blood. In addition, during the retrospective review of the case, the artesunate-induced delayed hemolytic anemia was considered as an alternative cause of the unexplained hemolytic anemia.
-
Key words: Plasmodium falciparum, imported malaria, falciparum malaria, babesiosis, hemolytic anemia, artesunate
INTRODUCTION
In Korea, the number of cases with resurgent indigenous vivax malaria has decreased to less than 500 cases per year, but imported malaria has been increasingly reported as of late, up to 46 cases per year [
1]. The majority of the imported cases occurred after travel to malaria-endemic countries.
Plasmodium falciparum and
Plasmodium vivax are the 2 more common etiological agents, accounting for 43% and 39% of the cases, respectively [
1]. Nevertheless, clinicians have difficulties in clinical diagnosis of imported malaria as well as having limited accessibility to effective anti-malarial drugs [
2]. In particular, falciparum malaria is becoming multi-drug resistant and often shows clinical severity, resulting in mortality.
Babesiosis is a malaria-like acute febrile infectious disease caused by
Babesia spp., a genus of protozoa found as parasites in red blood cells. Human babesiosis is a worldwide emerging zoonosis, requiring increased medical awareness. Imported human babesiosis has previously been reported in Korea, including a case mixed with falciparum malaria [
3]. Although a peripheral blood smear using Giemsa stain has been widely used for a simple and rapid diagnosis of both babesiosis and malaria, it is sometimes difficult to distinguish clearly between
Babesia spp. and malaria because of morphologic similarities, often requiring
Babesia-specific PCR tests [
4].
We experienced an interesting case of severe falciparum malaria which was initially treated with a full treatment dose of artesunate and Malarone (atovaquone/proguail); however, his condition became complicated with unexplained prolonged severe hemolytic anemia. On the basis of the clinical similarity of malaria and babesiosis, Malarone and azithromycin were given, with a resultant clinical improvement. However, the PCR assays on the early blood samples were positive only for P. falciparum and failed to detect Babesia species. We report here an unusual case of severe falciparum malaria complicated with unexplained prolonged hemolytic anemia, clinically mimicking a coinfection with babesiosis.
CASE REPORT
A 48-year-old Korean man was admitted to a hospital in France due to intermittent high fever for 2 weeks and a mental change for 1 day. Before coming to the hospital, he went on a business trip to the Republic of Benin in West Africa for 14 days. He exhibited a confused mental state and pulmonary edema. He was diagnosed with falciparum malaria and treated with a combination of intravenous artesunate for 1 day and oral Malarone for 7 days. His supportive treatment included blood transfusions and hemodialysis. After a 12-day hospitalization period, he was discharged with clinical improvement.
Five days after the discharge, he was admitted to our hospital in Korea because of recurrent high fever, jaundice, and abdominal discomfort. He was a businessman and his residence is in Seoul, Korea. He had no specific medical history or surgical operations except for the current attack of falciparum malaria. His family history was unremarkable. On admission, his vital signs were blood pressure 130/90 mmHg, pulse rate 106/min, body temperature 38℃ and respiration rate 20/min. He was alert, but acutely ill. He showed anemic conjunctiva and mild icteric sclera. Other findings were unremarkable except for mild splenomegaly and abdominal tenderness. The initial laboratory findings were as follows: Hb 8.0 g/dl, HCT 23.6%, WBC 4,600/mm
3, platelet 116,000/mm
3, PT 86%, ESR 22 mm/hr, CRP 32.4 mg/dl, BUN 17.5 mg/dl, creatinine 1.36 mg/dl, LDH 2,021 IU/L, Na 132 mmol/L, K 4.0 mmol/L, Cl 99 mmol/L, total protein 6.2 g/dl, albumin 3.1 g/dl, total/direct bilirubin 1.74/0.40 mg/dl, AST 55 IU/L, ALT 64 IU/L. In day 3 of his hospital stay, the PCR assay for
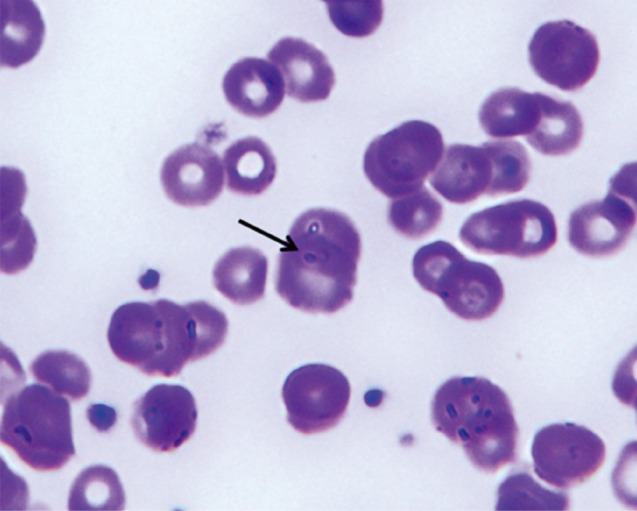
P. falciparum was positive. A peripheral blood smear demonstrated normocytic normochromic anemia with anisocytosis, poikilocytosis with spherocytes (1-5/HPF), polychromasia, and nucleated RBCs (1/100 WBCs) with presence of a few protozoa of
P. falciparum (
Fig. 1). The direct Coombs' test was positive; urinalysis showed blood (+2), proteinuria (+1), and hemoglobinuria was positive; serology was negative for HIV, HBsAg, and HCV, and positive for HBsAb. Cultures of blood, sputum, and urine samples were all negative; chest radiography was normal; abdominal CT scan demonstrated splenomegaly with multiple infarctions.
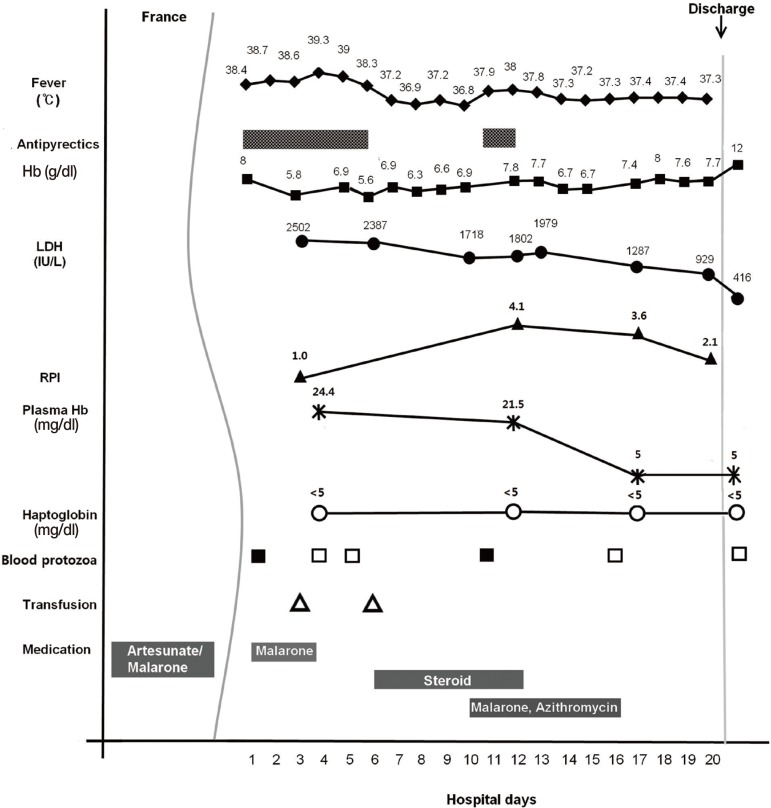
From day 1 to day 3 of his hospitalization, he was additionally given Malarone (atovaquone/proguail 1,000 mg/400 mg once daily for 3 days) for falciparum malaria. However, a daily spiking fever as high as 40℃ persisted, and the laboratory findings related to hemolytic anemia showed Hb 5.8 g/dl, LDH 2,502 IU/L, plasma Hb 24.4 mg/dl, haptoglobin <5 mg/dl, RPI (reticulocyte production index) 1.0, and total/direct bilirubin 2.78/0.63 mg/dl. FANA was checked; cytoskeletal pattern 1:40 and nucleolar pattern 1:320. ANCA and anti-ENA profiles were all negative. Blood transfusion and steroids were given for severe hemolytic anemia. The high fever subsided with steroid administration for 6 days (1 mg/kg for the first day and then 0.6 mg/kg on the following 5 days), but the hemolytic anemia profiles exhibited little change.
On day 10 of hospitalization, babesiosis was included as an additional suspected diagnosis, although he denied a tick bite during his stay in Benin. For the suspected babesiosis, a 7-day course of Malarone (750 mg/day) and azithromycin (500 mg/day) was started empirically, along with a parasitology expert's opinion. A repeated blood smear on the following day demonstrated the presence of intraerythrocytic ring forms of parasites. Blood samples were sent to the reference laboratories for detection of parasite DNA by the PCR assay (
Fig. 2). The high fever that recurred after the cessation of steroids was normalized within 3 days of the treatment.
Laboratory findings at the completion of the treatment between days 16 and 17 of the hospitalization revealed Hb 7.4 g/dl, LDH 1,287 IU/L, plasma Hb 5 mg/dl, and haptoglobin <5 mg/dl. The RPI also decreased to 3.6 from 4.1 (day 12). He improved gradually and was discharged on day 20 of hospitalization. Three weeks after the discharge, Hb rose to 12 g/dl, and LDH and plasma Hb levels returned to normal ranges (
Fig. 3).
PCR tests for
Babesia spp. performed by the Korea Centers for Disease Control and Prevention, Osong, Korea, the Section of Microbiology, Hyogo University of Health Sciences, Kobe, Japan (Prof. A. Saito-Ito), and the Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, Seoul, Korea all exhibited negative results. Instead, real-time PCR assays performed in Hyogo University of Health Sciences (Prof. A. Saito-Ito) and Seoul National University (JY Chai) (
Fig. 2) revealed positive results for
P. falciparum. Regarding the PCR protocol to detect malarial DNA (
P. falciparum,
P. vivax, and
P. malariae) and
B. microti DNA, the procedures of Veron et al. [
5] and Teal et al. [
6], respectively, were followed in Seoul National University College of Medicine.
DISCUSSION
This case report provides an unusual case of imported severe falciparum malaria that was complicated with prolonged hemolytic anemia, clinically mimicking a coinfection with babesiosis. In this report, we reviewed the protracted clinical course of the case at hand to formulate a differential diagnosis of the unexplained prolonged hemolytic anemia.
Severe falciparum malaria may develop life-threatening complications or death, especially in non-immune travelers of industrialized countries who are returning from endemic areas. The major complications include cerebral malaria, pulmonary edema, acute renal failure, severe anemia, and/or bleeding [
7]. The reported case fatality rate of imported falciparum malaria varies from 0.6% to 3.8% and may exceed 22% even in intensive care unit patients [
8].
Our non-immune Korean traveler infected by
P. falciparum had manifested initially as serious complications; confused mentality, pulmonary edema, anemia, and renal failure, but improved with anti-malarial medication and supportive care in a hospital in France. When he presented as having recurrent high fever upon coming back to Korea, we first considered that it might probably be due to an insufficient treatment course for severe malaria and high parasitemia. Several factors associated with treatment failure in malaria might include drug resistance, a short treatment course being prescribed to treat a high parasite biomass, non-adherence to a full course of treatment, or pharmacokinetic factors such as an inadequate dose or oral absorption of artemether-lumefantrine [
9]. In our case, the treatment failure is very unlikely; he completed a full treatment dose in combination with a 1-day course of intravenous artesunate and a 7-day course of Malarone during his stay in the hospital. The regimen administered in combination is being recommended for severe and/or drug-resistant falciparum malaria [
10,
11]. In addition, he was contracted with progressive hemolytic anemia, and repeated blood smears revealed
Babesia-like ring forms (PCR revealed only
P. falciparum).
Babesiosis, transmitted by ixodid ticks, is increasingly gaining medical attention as an emerging worldwide zoonosis in humans. The spectrum of this disease ranges from an asymptomatic infection to a fulminating malaria-like disease, resulting in occasional death. Of more than 100
Babesia spp. identified from small mammals, including rodents and cattle,
B. microti and
B. divergens are commonly responsible for human cases reported in North America and Europe, respectively [
12]. In addition, cases are now being reported from geographic areas where babesiosis was not previously known to occur, and among travelers and immunocompromised individuals. The diagnosis of potential coinfection with
Babesia spp. and other tick-borne pathogens, or with malaria in endemic areas gives a serious challenge to clinicians as well as public health professionals [
13].
Malaria and babesiosis have clinical similarity as well as morphological similarity as blood parasites. The discerning point on Giemsa-stained blood smears can be as follows:
Babesia spp. appear as intraerythrocytic ring forms or pyriform inclusions with light blue cytoplasms, whereas
Plasmodium spp. present parasitic pigment (hemozoin) and gametocytes, and also intraerythrocytic rings. However, reliable identification of both parasites may not be possible in early stages of infection or in cases with a low level parasitemia [
13]. In addition,
Babesia spp. may be easily misdiagnosed as
Plasmodium spp. in cases of coinfection with both agents, especially in malaria endemic regions or in travelers returning from areas where malaria is known to occur, but where babesiosis has never been reported before [
14]. In such cases, PCR assays for detection of
Babesia DNA in blood should support the microscopic detection of parasites, as a highly sensitive and specific assay [
15].
In our case, the patient denied a tick bite while traveling in Benin of West Africa where epidemiologic information about babesiosis was completely unavailable. As hemolytic anemia can occur due to severe babesiosis for several days to a few months, empirical treatment for babesiosis suspected on clinical grounds was initiated without delay. Concurrently, follow-up blood smears showed the presence of intraerythrocytic ring forms morphologically indistinguishable from Babesia. His clinical course improved with the treatment, but the PCR assay failed to detect Babesia DNA from his blood.
On our retrospective literature review of malaria and hemolysis, there are some studies on severe malaria with prolonged hemolytic anemia asking whether or not the hemolysis is likely a result of severe malaria, and not the treatment itself. Interestingly, there are several reports of newly recognized delayed hemolytic anemia after intravenous artesunate treatment for severe malaria [
16,
17,
18]. The relevant 19 cases reported in the literature were summarized in the Morbidity and Mortality Weekly Report in 2013; worsening of hemolysis and anemia occurred 8-32 days after the completion of artesunate therapy, and an initial clinical improvement with the resolution of parasitemia, followed by the resolution of hemolysis within 4-8 weeks after artesunate treatment [
19]. Artesunate is the recommended first-line treatment for severe malaria since 2010, as it more rapidly clears parasitemia and decreases mortality. The mechanism of post-artesunate delayed hemolysis is not fully understood and is still debated; the delayed clearance of infected erythrocytes spared by pitting during artesunate treatment is an original mechanism of hemolytic anemia in malaria, suggesting an indirect role of artesunate [
20]. In addition, there is a concern that these delayed hemolytic events might be the direct toxicity of the non-GMP (good manufacturing practice) artesunate currently used outside of the United States. There are no reported cases of post-artesunate delayed hemolysis in the United States, the only country using the GMP artesunate [
18].
As in the cases of post-artesunate delayed hemolysis reported, our patient manifested progressive severe hemolytic anemia (Hb nadir, 5.6 g/dl) approximately 22 days after intravenous artesunate was given in France, and had hemolysis resolution and Hb recovery to 12 g/dl within 5-6 weeks. As shown in
Fig. 3, a low initial RPI 1.0 followed by a sustained increase in RPI may be consistent with delayed hemolysis reflected in the subsequent changes of the values of Hb, LDH, plasma Hb, and haptoglobin. Therefore, our case may be compatible with post-artesunate delayed hemolysis, if other potential causes of prolonged hemolytic anemia, including babesiosis are excluded.
In our case, an argument for/against a co-infection of falciparum malaria and babesiosis still remains as a limitation of this study. A potential false negativity of conventional PCR assays for detection of various Babesia spp. DNA remains to be evaluated. However, this case report would provide an important data for clinical practice in terms of differential diagnosis of imported malaria and babesiosis, especially in non-endemic countries.
In conclusion, we report a case of imported falciparum malaria complicated with prolonged hemolytic anemia, clinically mimicking a coinfection with babesiosis. What is more, there are growing numbers of travelers returning from areas where the diseases are potentially endemic. Consequently, it must be stressed that either malaria or babesiosis in the particular case of coinfection can be overlooked or misdiagnosed by clinicians of non-endemic countries who have little awareness of the 2 diseases, requiring a comprehensive evaluation of such cases to decrease morbidity and mortality.
Notes
-
We have no conflict of interest related to this work.
ACKNOWLEDGMENTS
A part of this study will be presented at the 32nd World Congress of Internal Medicine, Seoul, Korea, 2014. We appreciate Prof. Atsuko Saito-Ito, Hyogo University of Health Sciences, Kobe, Japan for her kind help in performing real-time PCR assays for P. falciparum and Babesia spp.
References
- 1. Center for Disease Control and Prevention, Republic of Korea. Epidemiologic characteristics of imported malaria in Korea, 2003-2012. http://www.cdc.go.kr/CDC/contents/CdcKrContentLink.jsp?fid=31&cid=22134&ctype=6
- 2. Cheong HS, Kwon KT, Rhee JY, Ryu SY, Jung DS, Heo ST, Shin SY, Chung DR, Peck KR, Song JH. Imported malaria in Korea: a 13-year experience in a single center. Korean J Parasitol 2009;47:299-302.
- 3. Ahn MH. Imported parasitic diseases in Korea. Infect Chemother 2010;42:271-279. (in Korean).
- 4. Yoon KT, Kim YA, Ku NS, Kim JH, Jung SJ, Kim HJ, Song KH, Choi YK, Shin SY, Kim YK, Kim MS, Park YS, Choi JY, Song YG, Kim JM. A case of human babesiosis confirmed by polymerase chain reaction and treated with atovaquone and azithromycin. Infect Chemother 2006;38:300-303.
- 5. Veron V, Simon S, Carme B. Multiplex real-time PCR detection of P. falciparum, P. vivax, and P. malariae in human blood samples. Exp Parasitol 2009;121:346-351.
- 6. Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol 2012;50:903-908.
- 7. World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg 2000;94(suppl 1):S1-S90.
- 8. Lüthi B, Schlagenhauf P. Risk factors associated with malaria deaths in travelers: A literature review. Travel Med Infect Dis 2014;(doi: 10.1016/j.tmaid.2014.04.014)
- 9. Ashley E, McGready R, Proux S, Nosten F. Malaria. Travel Med Infect Dis 2006;4:159-173.
- 10. Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit Care 2003;7:315-323.
- 11. Jelinek T. Artemisinin based combination therapy in travel medicine. Travel Med Infect Dis 2013;11:23-28.
- 12. Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev 2000;13:451-469.
- 13. Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol 2008;38:1219-1237.
- 14. Bush JB, Isaäcson M, Mohamed AS, Potgieter FT, de Waal DT. Human babesiosis-a preliminary report of 2 suspected cases in South Africa. S Afr Med J 1990;78:699.
- 15. Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med 2006;13:65-70.
- 16. Rolling T, Schmiedel S, Wichmann D, Wittkopf D, Burchard GD, Cramer JP. Post-treatment haemolysis in severe imported malaria after intravenous artesunate: case report of three patients with hyperparasitaemia. Malar J 2012;11:169. doi: 10.1186/1475-2875-11-169
- 17. Kreeftmeijer-Vegter AR, van Genderen PJ, Visser LG, Bierman WF, Clerinx J, van Veldhuizen CK, de Vries PJ. Treatment outcome of intravenous artesunate in patients with severe malaria in the Netherlands and Belgium. Malar J 2012;11:102. doi: 10.1186/1475-2875-11-102
- 18. Zoller T, Junghanss T, Kapaun A, Gjorup I, Richter J, Hugo-Persson M, Mørch K, Foroutan B, Suttorp N, Yürek S, Flick H. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis 2011;17:771-777.
- 19. Centers for Disease Control and Prevention (CDC). Published reports of delayed hemolytic anemia after treatment with artesunate for severe malaria-worldwide, 2010-2012. MMWR Morb Mortal Wkly Rep 2013;62:5-8.
- 20. Jauréguiberry S, Ndour PA, Roussel C, Ader F, Safeukui I, Nguyen M, Biligui S, Ciceron L, Mouri O, Kendjo E, Bricaire F, Vray M, Angoulvant A, Mayaux J, Haldar K, Mazier D, Danis M, Caumes E, Thellier M, Buffet P. French Artesunate Working Group. Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood 2014;124:167-175.
Fig. 1A peripheral blood smear showing a ring form of Plasmodium falciparum (arrow) at the first day of hospitalization. Wright-Giemsa stain, ×1,000.

Fig. 2Relative expression (OD/mm) of Plasmodium falciparum and Babesia microti 18S rRNA genes in blood of our patient using quantitative real-time PCR. Positive results for P. falciparum and negative results for babesiosis (B. microti) are seen. Values are from OD/mm of each group (mean±SD of triplicated wells) divided by that of the housekeeping gene (β-actin). Significant differences (*P<0.05) were noted compared to negative controls.

Fig. 3A graph illustrating the clinical course of the falciparum malaria case, showing changes in fever, laboratory values, and medications during the course of illness.