Abstract
Plants used for traditional medicine contain a wide range of substances that can be used to treat various diseases such as infectious diseases. The present study was designed to evaluate the antileishmanial effects of the essential oil and methanolic extract of Myrtus communis against Leishmania tropica on an in vitro model. Antileishmanial effects of essential oil and methanolic extract of M. communis on promastigote forms and their cytotoxic activities against J774 cells were evaluated using MTT assay for 72 hr. In addition, their leishmanicidal activity against amastigote forms was determined in a macrophage model, for 72 hr. Findings showed that the main components of essential oil were α-pinene (24.7%), 1,8-cineole (19.6%), and linalool (12.6%). Findings demonstrated that M. communis, particularly its essential oil, significantly (P<0.05) inhibited the growth rate of promastigote and amastigote forms of L. tropica based on a dose-dependent response. The IC50 values for essential oil and methanolic extract was 8.4 and 28.9 μg/ml against promastigotes, respectively. These values were 11.6 and 40.8 μg/ml against amastigote forms, respectively. Glucantime as control drug also revealed IC50 values of 88.3 and 44.6 μg/ml for promastigotes and amastigotes of L. tropica, respectively. The in vitro assay demonstrated no significant cytotoxicity in J774 cells. However, essential oil indicated a more cytotoxic effect as compared with the methanolic extract of M. communis. The findings of the present study demonstrated that M. communis might be a natural source for production of a new leishmanicidal agent.
-
Key words: Leishmania tropica, cutaneous leishmaniasis, myrtle, promastigote, amastigote, J774-A1 cell
INTRODUCTION
Cutaneous leishmaniasis (CL) is a protozoan infection caused by protozoa of the genus
Leishmania. This disease is characterized by chronic skin lesions and leaves permanent scars with deformation of the infected area [
1]. CL is a public health problem at a global level because of affecting annually 1.5 million people worldwide [
2]. Both epidemiological forms of this skin disease are present In Iran; anthroponotic CL (ACL) and zoonotic CL (ZCL) caused by
Leishmania tropica and
Leishmania major, respectively [
3]. Current first choice chemotherapy of CL with antimonial drugs such as meglumine antimoniate and sodium stibogluconate is a challenge because of limited efficacy, toxic side effects, and drug resistance [
4]. Since there is no effective vaccine for prevention, maintenance, and improvement of existing treatment regimens, combined with new drug discovery initiatives, appear to be the only ways to guarantee continued control of this important tropical disease.
Since ancient time, plant extracts and plant-derived compounds, due to having fewer side effects, low cost, and high availability, are valuable sources that are commonly used to treat a wide range of disease conditions including infectious diseases [
5].
Myrtus communis L. (Myrtaceae) with the common name "myrtle" is native to Southern Europe, North Africa, and Asia. Different parts of this plant such as its berries, branches, leaves, and fruits have been used extensively as a folk medicine for the treatment of diarrhea, peptic ulcers, bleeding, headache, palpitation, leucorrhoea, urethritis, conjunctivitis, and pulmonary and skin diseases [
6]. Moreover, reviews have reported
M. communis as having anti-inflammatory, antinociceptive, antioxidant, neuro-protective, anti-diabetic, and antimicrobial effects [
6-
9]. Previous studies have also shown that the main constituents of the essential oil of
M. communis are terpenoid compounds (1,8- cineole, α-pinene, myrtenyl acetate, limonene, linalool, and α-terpinolene) [
6]. However, composition of the essential oil is quite variable depending on the geographic region of production, the season of harvest, and the length of distillation [
10]. The present study aimed to evaluate leishmanicidal effects of methanolic extract and essential oil of
M. communis against promastigote and amastigote forms of
L. tropica and their cytotoxic activities against J774-A1 cells on an in vitro model.
MATERIALS AND METHODS
Chemicals
All the chemicals and solvents used were of the highest purity commercially available. Meglumine antimoniate (MA) (Glucantime) as a control was purchased from Aventis, France. Penicillin and streptomycin were obtained from Alborz Pharmacy, Karaj, Iran, and were stored at room temperature (25˚C) until testing. MTT powder [3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide)], fetal calf serum (FCS), RPMI-1640 medium with L-glutamine, and Tween 20 were prepared from Sigma-Aldrich (St. Louis, Missouri, USA).
Parasite and cell culture
Standard strain of L. tropica (MHOM/IR/2002/Mash2) was kindly prepared from Center for Research and Training in Skin Diseases and Leprosy (Tehran, Iran). The parasites were cultured in NNN medium and subcultured in RPMI 1640 supplemented with penicillin (200 IU/ml), streptomycin (100 μg/ml) and 15% heat-inactivated fetal calf serum (FCS). Murine macrophage cells (J774-A1) were obtained from Pasteur Institute of Iran (Tehran, Iran). The cells were cultured and maintained in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% FCS at 37˚C in 5% CO2.
Collection of plant materials
The leaves of M. communis were collected from rural regions of Baft district of Kerman province in September 2013. The identity was confirmed by the botanist at the Botany Department of Shahid Bahonar University, Kerman, Iran. A voucher specimen of the plant materials was deposited at the Herbarium of Department of Pharmacognosy of School of Pharmacy, Kerman University of Medical Science, Kerman, Iran (KF1356).
Preparing methanolic extract
The dried aerial parts of the plant (100 g) were extracted by percolation method with methanol (80%) successively for 72 hr in room temperature. The extracts were passed through filter paper (Whatman No. 3, Sigma, Germany) to remove plant debris. Finally, they were concentrated in vacuum at 50˚C using a rotary evaporator (Heidolph, Germany) and stored at -20˚C until use.
Phytochemical analysis of the methanolic extract
The preliminary phytochemical analysis of the
M. communis methanolic extract was carried out to determined the presence of tannins, saponins, alkaloids, phenols, and glycosides as described elsewhere [
11].
Air-dried plant materials (200 g) were subjected to hydro-distillation for 3 hr using an all-glass clevenger-type apparatus. The essential oil obtained was dried over anhydrous sodium sulfate and stored in darkness at 4˚C in air-tight glass vials closed under nitrogen gas until testing.
Gas chromatography/mass spectrometry (GC/MS) analysis of essential oil
GC analysis: GC analysis was carried out by a Hewlett-Packard 6890 (Hewlett-Packard, Palo Alto, California, USA) with a HP-5MS column (30m×0.25 mm, film thickness 0.25 mm). The column temperature was maintained at 55˚C for 3 min and programmed to 280˚C at a rate of 15˚C per min, and kept constant at 280˚C for 5 min. Injector and interface temperatures were 220˚C and 260˚C, respectively. The flow rate of helium as carrier gas was 1 ml/min C.F. The percentages were calculated by electronic integration of FID peak areas without the use of response factors correction. Linear retention indices for all components were determined by coinjection of the samples with a solution containing homologous series of C8–C24 n-alkanes.
GC/MS analysis: GC/MS analysis was performed using a Thermoquest-Finnigan gas chromatograph equipped with fused silica capillary DB-5 column (30 m×0.25 mm, film thickness 0.25 mm) coupled with a TRACE mass (Manchester, UK). Helium was used as carrier gas with ionization voltage of 70 eV. Ion source and interface temperatures were 220˚C and 280˚C, respectively. Mass range was from 30 to 450 unit. Oven temperature program was the same as given above for the GC.
Identification of the essential oil components
The components of the essential oil were identified by comparison of their relative retention time and mass spectra with those of standards Wiley 2001 library data of the GC-MS system or with those reported in the literature data [
12].
For the preparation of dilutions of the essential oil of M. communis, 0.1 ml of the essential oil was dissolved in 9.7 ml of normal saline. In addition, to enhance the dispersal of the essential oil in normal saline, 0.3 ml of Tween 20 (Sigma-Aldrich) was added to the test tube. The resulting solution was mixed adequately by a magnetic stirrer. Serial dilution was then made to obtain the essential oil at 3.125, 6.25, 12.5, 25, 50, and 100 μg/ml concentrations. To prepare the dilutions of methanolic extract, 10 mg of methanolic extract was dissolved in 10 ml normal saline. Then, serial dilution was made to obtain the essential oil at 3.125, 6.25, 12.5, 25, 50, and 100 μg/ml concentrations. The selection of dilutions of the essential oil was based on initial experiments, which also revealed that normal saline plus Tween 20 (3%) had no inhibitory effect on the growth of the parasite.
Anti-promastigote assay
To evaluate antipromastigote effects of essential oil and methanolic extract on promastigotes of
L. tropica, colorimetric cell viability MTT assay was used as described elsewhere [
13]. Briefly, 100 μl of promastigotes (10
6 cells/ml) harvested from logarithmic growth phase were added to a 96-well tissue culture plate. Then, 100 μl of various concentrations of essential oil (3.125-50 μg/ml) and methanolic extract (0-100 μg/ml) was added to each well and incubated at 25˚C±1˚C for 48 hr. After incubation, 10 μl of MTT solution (5 mg/ml) was added to each well and incubated at 25˚C for 4 hr. Then, cold isopropanol was added as a solvent for formazan crystals to produce purple color. The absorbance was measured for each well at 490 nm using an ELISA reader (BioTek-ELX800, Winooski, Vermont, USA). Promastigotes were cultured in complete medium with no drug used as positive control and complete medium with no promastigotes and drugs as blank. The absorbance was measured for each well at 490 nm using the ELISA reader. Fifty % inhibitory concentrations (IC
50 values) were measured for all the tested extracts by Probit test in SPSS software.
This was carried out according to the method described by Mahmoudvand et al. [
14], Initially, before adding the macrophages to the plates, 1 cm
2 cover slips were placed in the wells of 6-chamber slides (Lab-Tek, Nalge Nunc International, New York, USA). In the next step, 200 μl of macrophage cells (10
5 cells/ml) were incubated at 37˚C in 5% CO
2 for 2 hr. Then, 200 μl (10
6 cells/ml) promastigotes in stationary phase were added to murine macrophages, so that the proportion of
Leishmania : macrophage was 10:1 and incubated again in a similar condition for 24 hr. Free parasites were removed by washing with RPMI 1640 medium and the infected macrophages were treated with 50 μl of various concentrations of (0-50 μg/ml) essential oil and methanolic extract at 37˚C in 5% CO
2 for 48 hr. At the end, the dried slides were fixed with methanol, stained by Giemsa, and studied under a light microscope. Also, the macrophages containing amastigotes without extract and those with no parasite and extract were considered positive and negative controls, respectively. Activity of anti-intramacrophage amastigotes of the extracts was evaluated by counting the number of amastigotes in each macrophage by examining 100 macrophages (% amastigotes viability) in comparison with those obtained with positive control. In addition, 50% inhibitory concentrations (IC
50 values) were measured for all the tested extracts by Probit test in SPSS software.
In this investigation, cytotoxic effects of
M. communis against J774-A1 cells were assessed by cultivating macrophages (5×10
5) with various concentrations of essential oil (0 to 100 μg/ml) and methanolic extract (0 to 500 μg/ml) in 96-well tissue culture plates at 37˚C in 5% CO
2 for 48 hr. Cell viability was determined by colorimetric MTT assay, and the results were displayed as percentage of dead cells compared to macrophages treated with MA and non-treated macrophages (100% of viability). Moreover, CC
50 (cytotoxic concentration for 50% of cells) was calculated by Probit test in SPSS software [
3].
All the tests in this study were carried out in triplicate. Selectivity index (SI), calculated based on the equation of CC
50 for murine macrophage cells/IC
50 for amastigote forms of
L. tropica, was used to compare toxicity and activity of essential oil and methanolic extract of
M. communis as described by Weninger et al. [
15]. Data analysis was carried out by using SPSS statistical package version 17.0 (SPSS Inc., Chicago, Illinois, USA). Differences between test and control groups were analyzed by
t-test. In addition,
P<0.05 was considered statistically significant.
RESULTS
GC/MS analysis of essential oil
Table 1 indicates the results obtained by GC/MS analysis of
M. communis essential oil (as the most effective extract). Twenty-five compounds were identified, representing 93.0% of the total oil. The main components were α-pinene (24.7%), 1,8-cineole (19.6%), and linalool (12.6%).
Phytochemical analysis of the methanolic extract
Evaluation of the primary phytochemical screening of the M. communis methanolic extract revealed the presence of high amount of terpenoid, flavonoids, tannins, phenols, and glycosides and lacking the alkaloids in this plant.
Antileishmanial effects
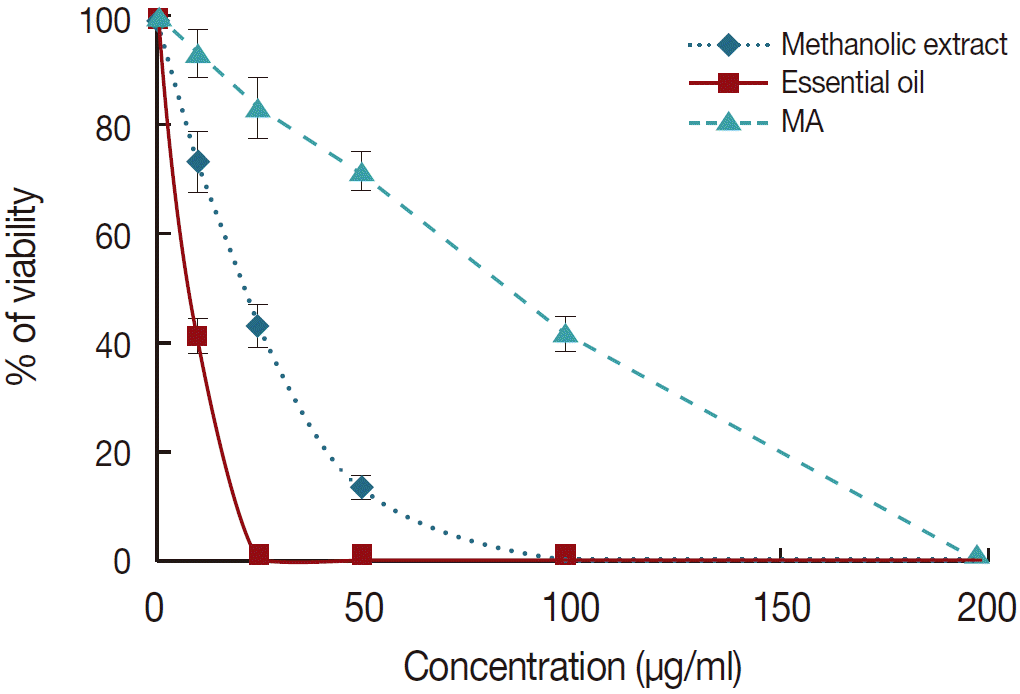
Anti-promastigote assay: In this study, to investigate the anti-promastigote activity, promastigotes of both species were incubated in the presence of various concentrations of essential oil and methanolic extract and cell viability was determined after 72 hr using MTT assay. The findings demonstrated that essential oil and methanolic extract of M. communis had potent antileishmanial activity against the promastigote forms based on a dose-dependent response (P<0.05). These results also revealed that essential oil in comparison with methanolic extract and MA had significantly (P<0.05) higher leishmanicidal effect on the promastigotes of L. tropica once it exhibited lower IC50 value for the tested promastigotes. The IC50 values for the essential oil and methanolic extract were 8.4 μg/ml and 28.9 μg/ml against promastigotes of L. tropica, respectively, whereas this value was 88.3 μg/ml for MA as control drug.
Anti-amastigote assay: The results showed that essential oil and methanolic extract of
M. communis significantly (
P<0.05) inhibited the growth rate of intramacrophage amastigotes as a dose-dependent manner (
Fig. 1). Similar to anti-promastigote assay, the essential oil was more effective on the amastigote forms than the methanolic extract and MA once it exhibited the lower IC
50 value for amastigote forms. The IC
50 value for the essential oil (11.6 μg/ml) against promastigotes of
L. tropica was significantly (
P<0.05) lower than those of MA (44.6 μg/ml) and methanolic extract (40.8 μg/ml).
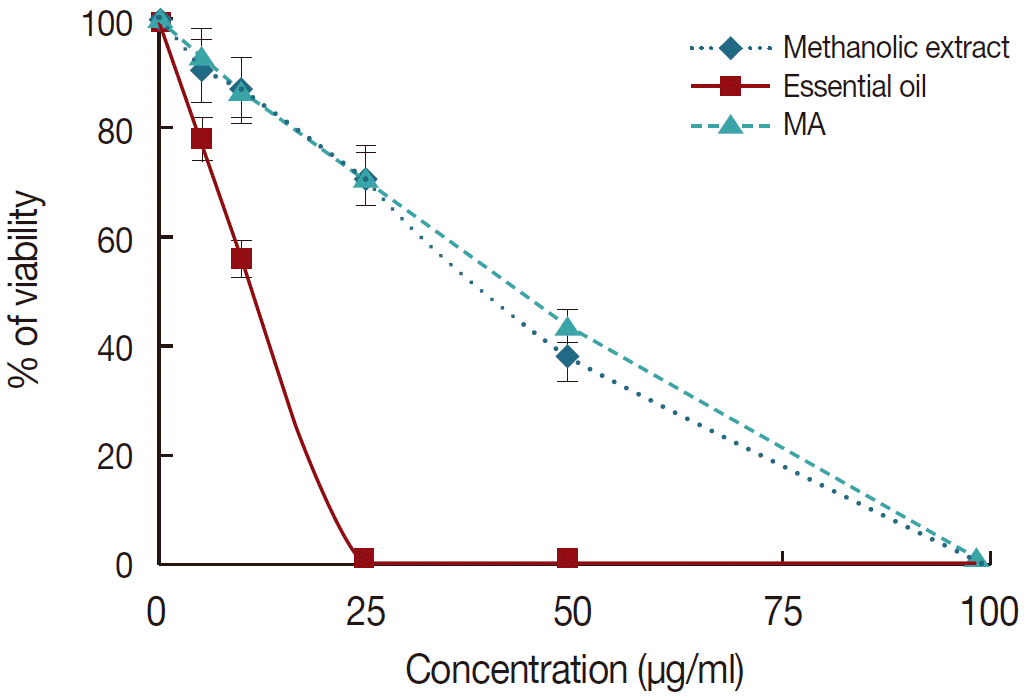
Cytotoxic effects of
M. communis were determined in J774-A1 cells using MTT assay. The obtained results indicated no significant cytotoxicity in J774-A1 cells. However, essential oil had a higher cytotoxic effect on cells as compared with the methanolic extract of
M. communis (
Fig. 2). The CC
50 values for essential oil and methanolic extract and also their SI values for amastigote forms of
L. tropica were shown in
Table 2.
DISCUSSION
Natural products, such as plants extract, either as pure compounds or as standardized extracts, provide unlimited opportunities for new and selective drug discoveries because of the unmatched availability of chemical diversity [
16]. According to the World Health Organization (WHO), more than 80% of the world's population relies on traditional medicine for their primary healthcare needs. The use of herbal medicines in Asia represents a long history of human interactions with the environment. Plants used for traditional medicine contain a wide range of substances that can be used to treat various diseases such as infectious diseases [
5]. In the present investigation, we evaluated antileishmanial activity of methanolic extract and essential oil of
M. communis and their cytotoxic activities against J774-A1 cells on an in vitro model.
Our findings demonstrated that
M. communis, particularly its essential oil, significantly (
P<0.05) inhibited the growth rate of promastigote and amastigote forms of
L. tropica based on a dose-dependent response. These results also displayed that amastigotes were more susceptible to
M. communis than promastigotes. This difference in susceptibility might be related to structural, biochemical, and morphological features as previously shown elsewhere [
17].
Previously, it has been proven that the activity of plant extracts could be influenced by the nature of the plant material or its origin as well as the climatic conditions in which plant grow, the plant part used, or the solvent used for extraction, because plants have different constituents depending on those factors [
18]. The chemical composition of
M. communis essential oil has widely been investigated; its composition is quite variable depending on the geographic region of production, the season of harvest, and the length of distillation [
8,
10]. However, in most regions, terpenoid compounds (1,8-cineole, α-pinene, myrtenyl acetate, limonene, linalool, and α-terpinolene) are the major constituents found in the essential oil obtained from the leaves of
M. communis. In the present study, in line with other study, we found that the main components of
M. communis essential oil are α-pinene, 1,8-cineole, and linalool. The phytochemical screenings of the
M. communis methanolic extract showed the presence of terpenoid, flavonoids, tannins, and phenols [
6]. So far, individual activities of these compounds have been demonstrated [
19]. Moreover, in several investigations, potent antibacterial, antifungal, and antiparasitic activities of these compounds and their derivatives such as α-pinene, 1,8-cineole, limonen, thymol, and carvacrole against some pathogenic strains have been proven [
20-
25]. Therefore, phytoconstituents in this plant could be responsible for their antileishmanial activity whereas their exact mechanism of action is unclear. However, in the case of antimicrobial mechanism of some terpenoid compounds such as monoterpens, Sikkema et al. [
26] revealed that they diffuse into pathogens and damage cell memberane structures. On the other hand, other reports suggested that the antimicrobial activity is related to ability of terpenes to affect not only permeability but also other functions of cell membranes; these compounds might cross the cell membranes, thus penetrating into the interior of the cell and interacting with critical intracellular sites [
27,
28]. Our findings also exhibited that SI of greater than 10 for essential oil and methanolic extract of
M. communis represent their safety to the mammalian cells and specificity to the parasite. In agreement with these results, no health hazards or side effects are reported as a result of the proper administration of designated therapeutic dosages of
M. communis [
6].
In conclusion, the results of this study demonstrated potent antileishmanial effects of M. communis that might be a natural source for production of new antileishmanial agents against CL. However, further clinical studies are required to evaluate exact biological activity of M. communis in animal models as well as volunteer human subjects as a new therapeutic agent.
Notes
-
The authors declare that there is no conflict of interests in this study.
We would like to thank Ms. Fatemeh Ezzatkhah for cultivation of the parasites.
Fig. 1.The viability of Leishmania tropica promastigotes in the presence of various concentrations of the meglumine antimoniate (MA), essential oil, and methanolic extract of M. communis after 72 hr incubation. Data are expressed as the mean±SD (n=3).

Fig. 2.The effect of different concentrations of meglumine antimoniate (MA), essential oil, and methanolic extract of M. communis on the mean number of amastigotes in each macrophage in comparison with infected macrophages with no treatment as positive control. Data are expressed as the mean±SD (n=3).

Table 1.Essential oil composition of M. communis identified by GC/MS
Table 1.
|
No. |
Compound |
Percentage |
|
1 |
α-Thujene |
0.88 |
|
2 |
Camphene |
0.58 |
|
3 |
δ-3-Carene |
0.73 |
|
4 |
α-Pinene |
24.7 |
|
5 |
β-Pinene |
1.3 |
|
6 |
β-Myrcene |
0.61 |
|
7 |
α-Terpinene |
0.23 |
|
8 |
1,8-Cineole |
19.6 |
|
9 |
Methyl eugenol |
1.3 |
|
10 |
Linalool |
12.6 |
|
11 |
α-Terpinyl acetate |
3.8 |
|
12 |
Myrtenyl acetate |
8.3 |
|
13 |
α-Phellandrene |
0.1 |
|
14 |
β-Ocimene |
0.11 |
|
15 |
2,6-Octadien |
0.41 |
|
16 |
α-Phellandrene |
0.1 |
|
17 |
γ-Terpinene |
0.5 |
|
18 |
α-Terpinolene |
0.51 |
|
19 |
4-Terpineol |
0.6 |
|
20 |
α-Terpineol |
6.1 |
|
21 |
Linalyl Acetate |
5.9 |
|
22 |
Caryophyllene oxide |
1.4 |
|
23 |
α-Humulene |
1.2 |
|
24 |
Neryl acetate |
0.14 |
|
25 |
trans-Caryophyllene |
1.3 |
|
Total |
93.0 |
Table 2.The IC50 and CC50 values (μg/mL) determined for the essential oil and methanolic extract of M. communis and control drug (MA) and their SI against intramacrophage amastigote forms of Leishmania tropica
Table 2.
|
Tested material |
IC50 (µg/ml)a
|
CC50b
|
SI |
|
Promastigote |
Amastigote |
|
Essential oil |
8.4 ± 0.6 |
11.6 ± 1.2 |
136.3 ± 7.2 |
11.7 |
|
Methanolic extract |
28.9 ± 2.5 |
40.8 ± 3.1 |
578.6 |
14.2 |
|
MA |
88.3 ± 3.1 |
44.6 ± 2.5 |
1,225.6 ± 11.6 |
27.5 |
References
- 1. World Health Organization. Control of the leishmaniases. Geneva, Switzerland. WHO Tech Rep Ser 2010;(949):5-12.
- 2. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004;27:305-318.
- 3. Mahmoudvand H, Sharififar F, Sharifi I, Ezatpour B, Fasihi Harandi M, Makki MS, Zia-Ali N, Jahanbakhsh S. In vitro inhibitory effect of Berberis vulgaris (Berberidaceae) and its main component, berberine against different Leishmania species. Iranian J Parasitol 2014;9:28-36.
- 4. Santos DO, Coutinho CE, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC. Leishmaniasis treatment-a challenge that remains: a review. Parasitol Res 2008;103:1-10.
- 5. Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine 2005;12:514-535.
- 6. Alipour G, Dashti S, Hosseinzadeh H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother Res 2014;28:1125-1136.
- 7. Hosseinzadeh H, Khoshdel M, Ghorbani M. Antinociceptive, anti-inflammatory effects and acute toxicity of aqueous and ethanolic extracts of Myrtus communis L. aerial parts in mice. J Acupunct Meridian Stud 2011;4:242-247.
- 8. Tuberoso CIG, Rosa A, Bifulco E, Melis MP, Atzeri A, Pirisi FM, Dessi MA. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem 2010;123:1242-1251.
- 9. Tumen I, Senol FS, Orhan IE. Inhibitory potential of the leaves and berries of Myrtus communis L. (myrtle) against enzymes linked to neurodegenerative diseases and their antioxidant actions. Int J Food Sci Nutr 2012;63:387-392.
- 10. Sumbul S, Ahmed MA, Asif M, Akhtar M. Myrtus communis Linn.-a review. Indian J Nat Prod Resour 2011;2:395-402.
- 11. Evans WC. Trease and Evans Pharmacognosy. 14th ed. WB Saunders Company Ltd; 1998, pp 15-16.
- 12. Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Carol Stream, Illinois, USA. Allured Publishing Corporation; 2004.
- 13. Mahmoudvand H, Tavakoli R, Sharififar F, Minaie K, Ezatpour B, Jahanbakhsh S, Sharifi I. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm Biol 2014;4:1-6.
- 14. Mahmoudvand H, Shakibaie M, Tavakoli R, Jahanbakhsh S, Sharifi I. In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against Iranian isolate of sensitive and glucan-time-resistant Leishmania tropica. Iran J Parasitol 2014;9(4):452-460.
- 15. Weniger B, Robledo S, Arango GJ, Deharo E, Aragón R, Muñoz V, Callapa J, Lobstein A, Anton R. Antiprotozoal activities of Colombian plants. J Ethnopharmacol 2001;78:193-200.
- 16. Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 2006;106:290-302.
- 17. Shokri A, Sharifi I, Khamesipour A, Nakhaee N, Harandi MF, Nosratabadi J, Hakimi Parizi M, Barati M. The effect of verapamil on in vitro susceptibility of promastigote and amastigote stages of Leishmania tropica to meglumine antimoniate. Parasitol Res 2012;110:1113-1117.
- 18. Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African J Biotechnol 2008;7:1797-1806.
- 19. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12:564-582.
- 20. Abbaszadeh S, Sharifzadeh A, Shokri H, Khosravi AR, Abbaszadeh A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Med Mycol 2014;24:e51-e56.
- 21. de Melo JO, Bitencourt TA, Fachin AL, Cruz EM, de Jesus HC, Alves PB, de Fátima Arrigoni-Blank M, de Castro Franca S, Beleboni RO, Fernandes RP, Blank AF, Scher R. Antidermatophytic and antileishmanial activities of essential oils from Lippia gracilis Schauer genotypes. Acta Trop 2013;128:110-115.
- 22. Mahboubi M, Kazempour N. The antimicrobial activity of essential oil from Perovskia abrotanoides Karel and its main components. Indian J Pharm Sci 2009;71:343-347.
- 23. Monzote L, García M, Pastor J, Gil L, Scull R, Maes L, Cos P, Gille L. Essential oil from Chenopodium ambrosioides and main components: activity against Leishmania, their mitochondria and other microorganisms. Exp Parasitol 2014;136:20-26.
- 24. Sokovic M, van Griensven LJLD. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur J Plant Pathol 2006;116:211-224.
- 25. Vardar-Unlu G, Candan F, Sökmen A, Daferera D, Polissiou M, Sökmen M, Dönmez E, Tepe B. Antibacterial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. var. pectinatus (Lamiaceae). J Agri Food Chem 2003;51:63-67.
- 26. Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Mol Biol Rev 1995;59:201-222.
- 27. Cristani M, D'Arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, Venuti V, Bisignano G, Saija A, Trombetta D. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem 2007;55:6300-6308.
- 28. Ismail A, Lamia H, Mohsen H, Samia S, Bassem J. Chemical composition and antifungal activity of three Anacardiaceae species grown in Tunisia. Science Int 2013;1:148-154.