Abstract
Pyronaridine and artesunate have been shown to be effective in falciparum malaria treatment. However, pyronaridine is rarely used in Hainan Island clinically, and artesunate is not widely used as a therapeutic agent. Instead, conventional antimalarial drugs, chloroquine and piperaquine, are used, explaining the emergence of chloroquine-resistant Plasmodium falciparum. In this article, we investigated the sensitivity of P. falciparum to antimalarial drugs used in Hainan Island for rational drug therapy. We performed in vivo (28 days) and in vitro tests to determine the sensitivity of P. falciparum to antimalarial drugs. Total 46 patients with falciparum malaria were treated with dihydroartemisinin/piperaquine phosphate (DUO-COTECXIN) and followed up for 28 day. The cure rate was 97.8%. The mean fever clearance time (22.5±10.6 hr) and the mean parasite clearance time (27.3±12.2 hr) showed no statistical significance with different genders, ages, temperatures, or parasite density (P>0.05). The resistance rates of chloroquine, piperaquine, pyronarididine, and artesunate detected in vitro were 71.9%, 40.6%, 12.5%, and 0%, respectively (P<0.0001). The resistance intensities decreased as follows: chloroquine>piperaquine>pyronarididine>artesunate. The inhibitory dose 50 (IC50) was 3.77×10-6 mol/L, 2.09×10-6 mol/L, 0.09×10-6 mol/L, and 0.05×10-6 mol/L, and the mean concentrations for complete inhibition (CIMC) of schizont formation were 5.60×10-6 mol/L, 9.26×10-6 mol/L, 0.55×10-6 mol/L, and 0.07×10-6 mol/L, respectively. Dihydroartemisinin showed a strong therapeutic effect against falciparum malaria with a low toxicity.
-
Key words: Plasmodium falciparum, antimalarial, sensitivity, in vivo and in vitro test
INTRODUCTION
Plasmodium falciparum is currently the main species of
Plasmodium that is resistant to antimalarial drugs. Drug resistance is defined as the ability to survive or reproduce at a concentration, which can kill or inhibit the reproduction of the same kind of species [
1]. The resistant strains eventually spread with a growing intensity and develop into multidrug-resistant varieties [
2]. Cases of chloroquine-resistant falciparum malaria in a hyperendemic area of Hainan Island were first identified in 1973 [
3]. Until 1978, chloroquine-resistant
P. falciparum was found throughout the island, approximately 1/3 of which belonged to R1-R2 level of resistance. In 1979, piperaquine completely replaced chloroquine in malaria prophylaxis and treatment. However, in 1982, piperaquine-resistant
P. falciparum emerged with a resistance rate of 10-20%, 5% of which belonged to R2/R3 level of resistance [
4]. A new in vitro test was established by Seiji Waki in Hainan Island [
2] to monitor the sensitivity of
P. falciparum between 1987 and 1990. Results showed that isolates from
P. falciparum were associated with a declined chloroquine resistance but still presented piperaquine resistance. No cross-resistance between chloroquine and piperaquine was found, and the original
P. falciparum acquired resistance to other antimalarial drugs such as amodiaquine, quinine, mefloquine, pyronaridine, and artesunate.
In recent years, although the morbidity and mortality of malaria in china were significantly decreased [
5], the incidence of malaria remained high in Africa and Southeast Asia. Over 207 million people suffer from this disease every year with the number of deaths higher than 627,000 [
6]. The primary reason for the deaths is related to the resistance of
P. falciparum strains to conventional antimalarial drugs such as chloroquine, mefloquine, quinine, and sulfadoxine/pyrimethamine [
7]. Multidrug-resistant falciparum malaria is epidemic in Southeast Asia and South America. Therefore, in order to prevent the spread of drug resistance of
P. falciparum, WHO in 2006, recommended that the production and sales of single artemisinin preparations as well as the use of artemisinin monotherapy in malaria treatment, should be stopped. The guidelines for malaria treatment involving artemisinin-based combination therapies were modified accordingly [
8].
Dihydroartemisinin is a derivative of artemisinin with a strong therapeutic effect against falciparum malaria. Dihydroartemisinin/piperaquine phosphate (DUO-COTECXIN) is a new antimalarial drug developed and produced in China, in accordance with the guidelines of WHO. Pyronaridine and artesunate have been shown to be effective in falciparum malaria treatment. However, pyronaridine is rarely used in Hainan Island clinically, and artesunate is not widely used as a therapeutic agent. Instead, conventional antimalarial drugs such as chloroquine and piperaquine were used, explaining the emergence of chloroquine-resistant
P. falciparum. The sensitivity of
P. falciparum to conventional antimalarial drugs in Hainan Island was investigated before [
9,
10]. However, the recent incidence of resistance to antimalarial drugs along the Thai-Cambodian and Thai-Myanmar border areas [
11], has arisen the interest in
P. falciparum once again. As an endemic area of falciparum malaria in China, especially after 1998, falciparum malaria was already restricted in 2 provinces (Yunnan and Hainan). Hainan Island is of great significance to monitor the sensitivity of
P. falciparum to antimalarial drugs to guide rational therapeutic interventions. In this study, we investigated the sensitivity of
P. falciparum to antimalarial drugs used in Hainan Island for rational drug therapy.
MATERIALS AND METHODS
The drugs, patients, and methods in this study were reviewed and approved by the Ethics Committee of the Center for Disease Control in Hainan Province, China.
Study sites and patients
Hainan malaria endemic areas have been divided into 3 layers; higher, middle, and lower by corresponding to their incidence. In this present study, 2 counties were selected from high endemic areas and 2 middle areas, respectively. Four cities and counties (Dongfang City, Sanya City, Baisha Country, and Lingshui County) with a high incidence of falciparum malaria in Hainan Island were selected by stratified randomization (
Fig. 1). A few (1-2) townships were selected in each city or country, with the rural hospitals as the survey sites.
Patients aged between 4 and 70 years were selected ensuring that the microscopic density of asexual parasites was 1,000-8,000 parasites per μl of blood. The patients were required to have no history of antimalarial drug usage 15 days prior to the onset, and to be negative for urinary 4-aminoquinoline. The body temperature of these patients was ≥37.5˚C or manifesting fever within 24 hr. Patients with severe heart, kidney, liver, and hematopoietic diseases or severe malnutrition, or patients who had taken antimalarial drugs 15 days prior to the onset, pregnant and lactating women, those with a history of allergy for DUO-COTECXIN, and critical cases, were excluded from this survey. All the patients voluntarily participated in this survey and provided signed informed consent.
In vivo and in vitro tests
The in vivo sensitivity of P. falciparum was determined by means of the WHO standard 4-week test. The total dose of DUO-COTECXIN (lot no. 061606 produced by Chongqing Holley Pharmaceutical Co., China) for adults was 8 tablets for 3 days. Each tablet contains 40 mg dihydroartemisinin and 320 mg piperaquine. Under medical supervision, on the first day, 4 tablets were administered and on the second and third days, 2 tablets were given, respectively. The dosage for children was calculated as weight (kg)×adult dose/50. Patients’ temperature and the parasite density were monitored during hospitalization and on days 7, 14, 21, and 28.
The in vitro test was performed using a modification of the 96-well microplate technique recommended by WHO [
12]. Briefly, adds the drug coating to be measured in 8×12 well (WHO plate) on a flat-bottomed tissue culture plates, each plate only 1 drug, set contrast, 7 concentrations, and aseptic processing. Blood mixed with the culture medium joined the training conducted in the well. Training is over, smear test.
Patients involved in the in vivo test were hospitalized for observation and discharged after asexual parasite clearance, body temperature recovery, and symptom relief, were followed up to 28 day. The resistance rate, inhibitory dose 50 (IC50) and the mean concentration for complete inhibition (CIMC) of schizont formation were measured in vitro, to observe the treatment effect.
The time of parasite clearance was defined as the time until first of 3 consecutive counts of negative parasites after administration. The mean parasite clearance was calculated using the arithmetic average method. The axillary temperature was measured every 4 hr before and after drug administration. Fever clearance was defined as the time for the body temperature to return to normal over 2 consecutive times. The mean fever clearance time was calculated using the arithmetic average. A cure was indicated if asexual parasite clearance was observed in patients’ blood test 7 days after drug administration without relapse after 28 days. The cure rate was expressed as a percentage. Parasite recrudescence (expressed as a percentage) was monitored on days 7, 14, 21, and 28 after the drug administration. The adverse reaction rate was expressed as a percentage. IC50, the dose required to inhibit half of the tested subjects, was calculated by ED50 regression. CIMC, the concentration required to inhibit all the tested subjects, was calculated using the arithmetic average method. Drug resistance rate was expressed as a percentage.
Evaluation criteria for drug efficiency
Evaluation of the in vivo test was based on the WHO evaluation criteria of susceptibility testing for P. falciparum to chloroquine in 28 days in 1976. The level of resistance was classified as sensitivity (S), markedly effective (R1), effective (R2), and ineffective (R3). It is considered markedly effective (R1) when asexual parasite clearance was observed in blood test 7 days after drug administration and recrudescence occurred within 28 days. It is considered to be effective (R2) when the amount of parasite was significantly reduced more than 75% after the drug administration but without parasite clearance within 7 days. If reduction of the parasite was less than 75% or the amount of parasite increased at 48 hr after drug administration, it is considered ineffective (R3). Resistance rate in vivo=(R1 + R2 + R3)/T × 100%.
A successful in vitro test shows more than 10% rate of schizont (with more than 3 nuclei) development among the parasites in the control well of the test plates (provided by National Institute of Parasitic Diseases, Chinese Center for Diseases Control and Prevention). If the parasites developed to schizonts in the wells containing 0.000008 μmol chloroquine, 0.000032 μmol piperaquine, 0.000008 μmol pyronaridine, and 0.000004 μmol artesunate, respectively. P. falciparum is considered resistant to these drugs. Resistance rate in vitro is no. of cases with resistance/no. of cases tested × 100%.
Statistical analysis
The statistical analysis was performed using software Excel and SPSS13.0. The rank sum test was conducted for data analysis and the chi-square test was used to compare the data.
RESULTS
In vivo test
A total of 58 patients were admitted to this test, and 46 patients were finally enrolled, among which 34 were from Dongfang City (Jiangbian Township), 6 were from Sanya City (Tianya and Fenghuang Township), 4 were from Baisha Country (Qifang and Xishui Township), and 2 were from Lingshui Country (Zuguan Township). The eligible patients included 37 males and 9 females aged from 5 to 67 years, with an average age of 27.8±15.7 years. Fourteen patients were ≤15 years and 32 were ≥16 years. Before administration, patients’ body temperature ranged from 35-40˚C, with an average of 38.5±1.0˚C. The parasite density was 1,000-149,280 parasites per μl blood, and the mean density was 16,663±24,134 parasites per μl blood.
Among 6 patients from Sanya City, 1 was a case of P. falciparum resistance level R3, and in 2 patients, parasite clearance was observed until 48 hr after treatment without recrudescence during the follow-up. Symptoms of other patients were quickly brought under control with a rapid parasite clearance and recovery of temperature. The cure rate of 46 patients was 97.8%.
Among 46 patients, the mean fever clearance time was 22.5±10.6 hr and the mean parasite clearance time was 27.3±12.2 hr. The mean fever clearance time for males was 23.6±11.2 hr and 21.2±7.8 hr for females. The difference of mean parasite clearance time between men and women was insignificant, 27.6±12.6 hr and 27.8±14.9 hr, respectively. Based on the rank sum test, the mean fever clearance time (
P=0.07) and the mean parasite clearance time of different genders (
P=0.07) were not significantly different. In terms of age, the mean fever clearance time for adult group was 25.4±11.0 hr, which was 17.7±6.8 hr for minor group. The mean parasite clearance times for the 2 groups were similar. The difference between the mean fever clearance time and the mean parasite clearance time of different ages was not statistically significant (
Table 1).
The high fever group was defined as the body temperature was>39˚C before administration, the lower group was defined as the body temperature was ≤39˚C but above normal temperature. The mean fever clearance time for the high fever group was 28.8±14.8 hr, which was 21.9±8.4 hr for the low fever group. The mean parasite clearance times for the 2 groups were similar. The difference between the mean fever clearance time and the mean parasite clearance time at different body temperatures before drug administration was statistically not significant, by the rank sum test. The results are shown in
Table 2.
In terms of the parasite density, the mean fever clearance time for the group with a parasites density of ≤12,000 parasites per μl blood was 23.2±9.3 hr, which was 18.7±5.2 hr for the group with a density of 12,000 parasites per μl blood. The mean parasite clearance times in the 2 groups were similar, 26.5±14.8 hr and 26.8±6.4 hr, respectively. The difference between the mean fever clearance time (
P=0.07) and the mean parasite clearance time (
P=0.23) of different
Plasmodium density was statistically not significant, by the rank sum test (
Table 3).
One day before drug administration, 1 patient had symptoms of headache, dizziness, nausea, and vomiting, which were not significantly worsened after the drug administration. These may not be related to the drug. However, 2 patients manifested the above symptoms, which may be related to the drug and disappeared after the parasite clearance and the temperature returned to normal.
In vitro test
Total 32 patients were observed in this test. The resistance rate of
P. falciparum to chloroquine, piperaquine, pyronaridine, and artesunate was significantly different (χ
2=45.67,
P<0.01). The difference of the overall probability between these 4 drugs was statistically significant after pairwise comparisons (
P<0.001). The resistance intensities of
P. falciparum to these 4 drugs were as follows: chloroquine>piperaquine>pyronaridine>artesunate. Results are shown in
Table 4.
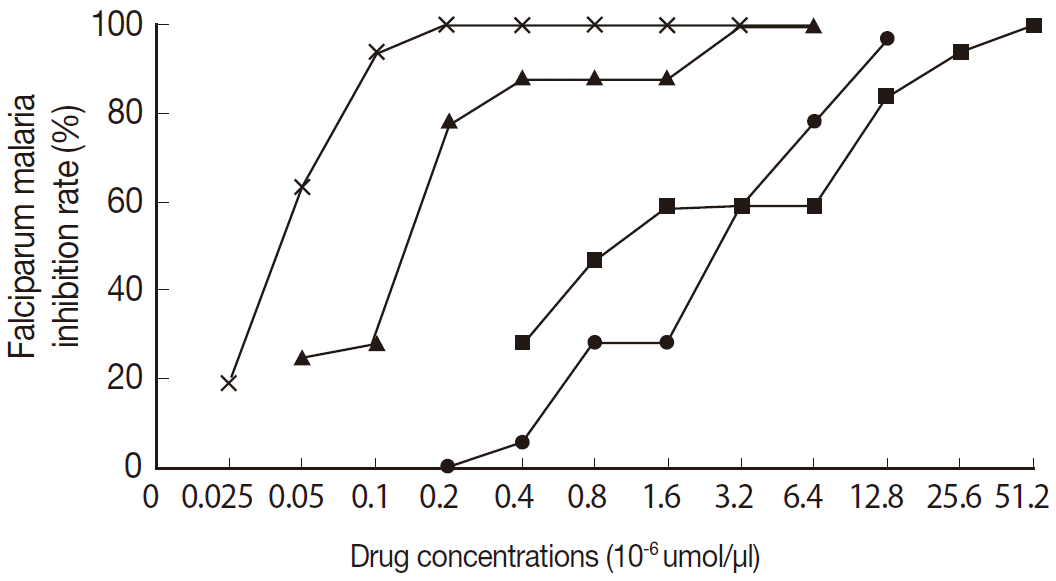
The antimalarial effect of the 4 drugs increased with the concentration. The relationship between the drug concentration and number of inhibited cases was similar to a S-curve (
Fig. 2). As shown in
Fig. 1, artesunate was effective at the lowest concentration of complete inhibition, followed by pyronaridine. Although the effective therapeutic concentrations of chloroquine and piperaquine were similar, piperaquine was more potent than chloroquine. Combined with the IC
50 values of these 4 drugs, the antimalarial effects associated with the 4 drugs varied as: artesunate>pyronaridine>piperaquine>chloroquine. Results are shown in
Table 5.
Statistical differences in CIMC were observed with these 4 antimalarial drugs (
P<0.0001). The total CIMC between different drugs was different. After pairwise comparison, CIMC of chloroquine and piperaquine was not significantly different (
P>0.48), and differences between the other comparison groups were statistically significant (
P<0.0001). CIMC of artesunate was lower than that of pyronaridine, both of which was lower than that of chloroquine and piperaquine. Results are shown in
Table 6.
DISCUSSION
Dihydroartemisinin has a curative effect for falciparum malaria better than other antimalarial drugs. The inadequacy of the course is longer, and has recrudescence [
13]. The recrudescence rate was 63.6%, 54.8%, and 13.8%, respectively [
14]. A total of 480 mg dihydroartemisinin was administered over 5-7 days, to treat falciparum malaria. The fever clearance time was 28.9±13.0 hr and 25.4±12.3 hr. The parasite clearance time was 30.6±9.8 hr and 30.5±9.5 hr. The 28 day cure rate was 70.0% (21/30) and 97.7% (43/44), respectively [
15]. The therapeutic effect of dihydroartemisinin combined with phosphate naphthoquine or pyronaridine was better than that of dihydroartemisinin alone [
16,
17]. In this study, patients with falciparum malaria were treated with an oral compound of 320 mg dihydroartemisinin and 2,560 mg piperaquine, resulting in a cure rate of 97.8%. The fever and parasite clearance time were short and unaffected by gender, age, body temperature, and parasite density. The antimalarial efficacy was similar with other formulations but was better than artemisinin alone without significant adverse reactions. Dihydroartemisinin therapy controlled the parasites in erythrocyte stage. However, the treatment course was long with artemisinin-based drugs alone, leading to recrudescence. Piperaquine is also effective against erythrocytic parasitemia, with a sustained favorable outcome. Therefore, combination of these 2 drugs has a synergistic effect, shorter course, and reduced drug dosage and toxicity [
18]. The study of Jérôme Dormoi et al. [
19] also showed that atorvastatin in combination with dihydroartemisinin in a therapeutic scheme leads to a significant delay in mouse death, and it has an effect on the onset of cerebral malaria symptoms and on the level of parasitemia.
In 1974, resistance of
P. falciparum to chloroquine was widespread in Hainan. Following discontinuation of chloroquine use in 1979, the resistance rate decreased from 97.7% to 26.7% in 1997 [
20]. In our study, the resistance rate was 71.9%, indicating a considerable volatility. With increased and widespread use of piperaquine replacing chloroquine, piperaquine-resistant strains were identified in 1982. Piperaquine resistance increased from 18.4% in 1985 to 73.7% in 1994 [
9]. In this study, resistance rate to piperaquine was determined as 40.6%, which was consistent with the results measured by Lin et al. in Ledong County [
10]. Therefore, piperaquine sensitivity may be recovered through a reasonable use of antimalarial drugs. Results from in vitro sensitivity test of
P. falciparum in the China-Myanmar border region suggested that the resistance rates to chloroquine, piperaquine, and pyronaridine were 95.2%, 7.1%, and 54.8%, respectively, which were different from the rates in Hainan [
21]. In an in vitro sensitivity test of
P. falciparum to antimalarial drugs in Papua New Guinea, it was confirmed that
P. falciparum showed widespread resistance to chloroquine and was sensitive to artemisinin-based drugs [
22].
In the present study, artesunate showed the lowest effective concentration and the lowest concentration of complete inhibition while chloroquine had the highest. The relationship of antimalarial effect between the 4 drugs varied as follows: artesunate>pyronaridine>piperaquine>chloroquine. Artemisinin-based drugs showed a strong dose-dependent lethal effect against
P. falciparum. Statistical differences were observed in CIMC of the 4 antimalarial drugs tested in this study. After pairwise comparison, CIMC of chloroquine and piperaquine was not significantly different, probably due to the long-term use. The resistance rate to pyronaridine was 12.5% (4/32), which was identical with the rate reported in 1999. Compared with cross-resistance to other antimalarial drugs, no apparent resistance to artemisinin-based drugs has been reported so far. In 1997, resistance rate of these drugs was 19.6% (9/46), determined in vitro in Ledong County, Hainan Island, China. In the early 1990s, cases with clinically suspected resistance to artemisinin were reported in Thailand, India, and Sierra Leone. In 2006, it was also reported that
P. falciparum was resistant to artemisinin-based drugs in French Guiana and Senegal [
7]. The therapeutic efficacy study by Rithea Leang et al. [
23] demonstrated reasonable efficacy in an area of possible reduced artemisinin sensitivity in Cambodia. Although distinct artemisinin resistance has yet to be established, preventive measures are needed. Stringent drug susceptibility monitoring and surveillance mechanisms should guide the clinical interventions.
According to a previous study, antimalarial drugs had mild adverse reactions, with the adverse reaction rates of chloroquine 27% and of piperaquine 17% [
24]. As for pyronaridine and sulfadoxine-pyrimethamine, the adverse reaction rates of 32% and 36%, respectively were reported [
25]. Our suggestion is that DUO-COTECXIN compared to other antimalarial drugs has better efficacy and low toxicity characteristics.
Notes
-
The authors declare that they have no competing interests. There is also no conflict of interest with the drug (DUOCOTECXIN) producer.
This study received a financial support from the Hainan Provincial Scientific Research grant, China (grant nos. 813251 and 310174). We would like to thank the staff from Dongfang CDC, Sanya CDC, Baisha CDC, and Lingshui CDC, China.
Fig. 1.Map of China and Hainan Island and survey sites.

Fig. 2.Antimalarial drugs: dose-response curve.

Table 1.Comparative drug efficacy by age
Table 1.
|
Index |
16 |
< 16 |
Statistics |
P
|
|
Mean fever clearance time (hr) |
25.4 ± 11.0 |
17.7 ± 6.8 |
Z = -1.920 |
0.06 |
|
Mean parasite clearance time (hr) |
27.6 ± 11.9 |
27.6 ± 13.9 |
Z = -0.018 |
0.98 |
Table 2.Comparative drug efficacy by body temperature
Table 2.
|
Index |
> 39˚C |
≤ 39˚C |
Statistics |
P
|
|
Mean fever clearance time (hr) |
28.8 ± 14.8 |
21.9 ± 8.4 |
Z = -0.413 |
0.68 |
|
Mean parasite clearance time (hr) |
25.5 ± 6.9 |
27.4 ± 16.6 |
Z = -0.940 |
0.35 |
Table 3.Comparative drug efficacy by different parasitemia
Table 3.
|
Index |
≤ 1.2 × 104 p/μl |
>1.2 × 104 p/μl |
Statistics |
P
|
|
Mean fever clearance time |
23.2 ± 9.3 |
18.7 ± 5.2 |
Z = -1.820 |
0.069 |
|
Mean parasite clearance time |
26.5 ± 14.8 |
26.8 ± 6.4 |
Z = -1.210 |
0.23 |
Table 4.
Table 4.
|
Drugs |
Cases tested |
Cases with resistancea
|
Resistance rate (%) |
|
Chloroquine |
32 |
23 |
71.9 |
|
Piperaquine |
32 |
13 |
40.6 |
|
Pyronaridine |
32 |
4 |
12.5 |
|
Artesunate |
32 |
0 |
0 |
Table 5.Results of IC 50 in vitro
Table 5.
|
Drugs |
Cases tested |
Cases with resistance |
IC 50/(× 10-6 mol/L) |
|
Chloroquine |
32 |
23 |
3.77 ± 1.40 |
|
Piperaquine |
32 |
13 |
2.09 ± 3.30 |
|
Pyronaridine |
32 |
4 |
0.09 ± 0.05 |
|
Artesunate |
32 |
0 |
0.05 ± 0.01 |
Table 6.
Table 6.
|
Drugs |
Cases tested |
Cases with resistance |
CCIMC/(× 10-6 mol/L) |
|
Chloroquine |
32 |
23 |
5.60 ± 5.64 |
|
Piperaquine |
32 |
13 |
9.26 ± 13.67 |
|
Pyronaridine |
32 |
4 |
0.55 ± 1.02 |
|
Artesunate |
32 |
0 |
0.07 ± 0.04 |
References
- 1. Department of Endemic Disease, Ministry of Health (China). Manual for malaria control. Beijing, China. People’s Medical Publishing House; 1988, p 163.
- 2. Waki S, Li J, Zhu MY, Qian YL, Takagi T, Chen L, Gu HM, Suzuki M. A field trial of a fluorometric in vitro drug sensitivity test for Plasmodium falciparum in Hainan Island. Trans R Soc Trop Med Hyg 1989;83:165-166.
- 3. Chen L, Dai ZR, Cai XZ. Changes of prevalence of drug-resistant falciparum malaria in Hainan Province, China. Guangzhou Uni Trad Chin Med 1998;15:35-38.
- 4. Zhou MX, Chen L, Zhou YC, Mo QZ. Report of two chloroquine-resistant falciparum malaria cases in Hainan Island. Med J Chin PLA 1979;4:125.
- 5. Xia ZG, Yang MN, Zhou SS. Malaria situation in the Peoples’ Republic of China in 2011. Chin J Parasitol Parasit Dis 2012;30:419-422.
- 6. World Health Organization. World malaria report. Geneva, Switzerland. WHO Press; 2013, p 27.
- 7. Li XP, Yang YM. Research progress in drug resistance of Plasmodium falciparum to artemisinin. Chin Trop Med 2008;8:2250-2253.
- 8. World Health Organization. Call for ending using artemisinin alone in treatment of malaria by World Health Organization. Adv Drug Reaction J 2006;8:152.
- 9. Lin SG, Cai XZ, Zeng LH, Wang SM. Drug sensitivity test of Plasmodium falciparum to chloroquine, piperaquine, amodiaquine, mefloquine and quinine in vitro in Ledong county. Hainan Med J 1995;6:71-72.
- 10. Lin SG, Liu DQ, Zhou KR. Test of Plasmodium falciparum to antimarials in vitro in Ledong county, Hainan province. China Trop Med 2005;5:1707-1708.
- 11. Bhumiratana A, Intarapuk A, Sorosjinda-Nunthawarasilp P, Maneekan P, Koyadun S. Border malaria associated with multidrug resistance on Thailand-Myanmar and Thailand-Cambodia borders: transmission dynamic, vulnerability, and surveillance. BioMed Res Int 2013;2013:1-13.
- 12. Rieckmann KH, Campbell GH, Sax LJ, Mrema JE. Drug sensitivity of Plasmodium falciparum. An in-vitro microtechnique. Lancet 1978;1:22-23.
- 13. Liu DQ, Lin SG, Feng XP, Chen WJ, Chen PL, Wu HM, Chen C, Liu J. Study on treatment of multi-drug resistant falciparum malaria by using a combination of dihydroartemisinin and pyronaridine. Chin J Parasitol Parasit Dis 2002;20:193-196.
- 14. Cai XZ, Wei RZ, Xing QL, Wang GZ, Lin RS, Wang FX. Efficacy of artemisinin tablets in treatment of 108 falciparum malaria cases. Chin J Prev Treat Parasit Dis 1994;7:175-178.
- 15. Che LG, Li XL, Yang CJ, Li CF, Zhang YL. Efficacy of dihydroartemisinin in treatment of falciparum malaria and vivax malaria. Chin J Parasitol Parasit Dis 1997;15:119.
- 16. Wang SQ, Meng F, Shen H, Wen Y, Zhou KR, Zhu QX, Pang XJ, Lin SG, Zheng LH. Therapeutic effect of dihydroartemisinin combined with naphthoquine phosphate in patients with falciparum malaria. Chin J Parasitol Parasit Dis 2002;20:180-182.
- 17. Cai XZ, Chen C, Zheng XY. Preliminary study on the treatment of falciparum malaria with combined use of dihydroartemisinin and piperaquine. J Prac Parasit Dis 1999;7:104-105.
- 18. Liu D, Cai X, Ren D, Liu R, Lin S, Zeng L, Tang X. Change in chloroquine resistance of Plasmodium falciparum in Hainan province. Chin J Parasitol Parasit Dis 1999;17:32-34.
- 19. Dormoi J, Briolant S, Pascual A, Desgrouas C, Travaillé C, Pradines B. Improvement of the efficacy of dihydroartemisinin with atorvastatin in an experimental cerebral malaria murine model. Malaria J 2013;12:302.
- 20. Liu D, Cai X, Ren D, Liu R, Lin S, Zeng L, Tang X. Changes in chloroquine resistance of Plamodium falciparum in Hainan province. Chin J Prasitol Parasit Dis 1999;17:32-34.
- 21. Zhang CL, Zhou HN, Wang J, Liu H. In vitro sensitivity of Plasmodium falciparum isolates from China-Myanmar border region to chloroquine, piperaquine and pyronaridine. Chin J Parasitol Parasit Dis 2012;30:41-44.
- 22. Wong RP, Lautu D, Tavul L, Hackett SL, Siba P, Karunajeewa HA, Ilett KF, Mueller I, Davis TM. In vitro sensitivity of Plasmodium falciparum to conventional and novel antimalarial drugs in Papua New Guinea. Trop Med Int Health 2010;15:342-349.
- 23. Leang R, Ros S, Duong S, Navaratnam V, Lim P, Ariev F, Kiechel JR, Menard D, Taylor WR. Therapeutic efficacy of fixed dose artesunate-mefloquine for the treatment of acute, uncomplicated Plasmodium falciparum malaria in Kampong Speu, Cambodia. Malaria J 2013;12:343.
- 24. Xu XY, Chen DL. Observation of Plasmodium vivax malaria on curative effect by chloroquine is combined with primaquine 4-days therapy. Hainan Med J 1994;5:133-135.
- 25. Huang ZS, Meng F, Fu SG. Comparative studies on the treatment of drug-resistant falciparum malaria with single-dose or two-day regimens of pyronaridine/sulfadoxine-pyrimethamine plus primaquine. Chin J Parasitol Parasit Dis 1996;14:314-317.