Abstract
This study aimed to investigate the infection status, worm development, and phylogenetic characteristics of the intestinal trematode, Stellantchasmus falcatus. The metacercariae of S. falcatus were detected only in the half-beak (Dermogenus pusillus) out of the 4 fish species examined. Their prevalence was 90.0%, and the intensity of infection was 919 metacercariae on average. Worms were recovered from 33 (97.1%) of 34 chicks that were experimentally infected with 200 S. falcatus metacercariae each, and the average recovery rate was 43.0%. The body size and inner organs of S. falcatus quickly increased in the experimental chicks over days 1-2 post-infection (PI). In addition, ITS2 sequence data of this parasite were analyzed to examine the phylogenetic relationships with other trematodes using the UPGMA method. The results indicated that the ITS2 sequence data recorded from trematodes in the family Heterophyidae appeared to be monophyletic. This study concluded that D. pusillus serves as a compatible second intermediate host of S. falcatus in Thailand and that S. falcatus can develop rapidly in the experimental chicks. Data collected from this study can help to close the gap in knowledge regarding the epidemiology, biology, and phylogenetic characteristics of S. falcatus in Thailand.
-
Key words: Stellantchasmus falcatus, worm development, ITS2, phylogenetic analysis, prevalence
INTRODUCTION
Several kinds of mammals, i.e., humans, rats, cats, and dogs, as well as chicks, have been reported as definitive hosts of this fluke. This trematode is known to be the possible cause of clinical problems in some Asian countries [
1,
2]. In addition, metacercariae (=the infective stage) are mainly found in brackish water or marine fish, which serve as the second intermediate host, especially the group of mullets known as
Mugil spp. and
Liza spp. [
3,
4]. However, several previous studies have reported that
S. falcatus metacercariae can also be found in freshwater fish, such as the half-beak (
Dermogenys pusillus) [
5] and climbing perch (
Anabas tertudinaus) [
6], for which a high infection rate has been reported, and the rate of infection has been found to be high in the northern region of Thailand. Even though the symptomatic features of human
S. falcatus infection have not been clearly determined, the first clinical reports have revealed that
S. falcatus can be found at autopsy and many embryonated eggs remained in the blood vessels of the cardiac muscle of the host [
7]. Hence, it should be reminded that
S. falcatus has the potential to retain its clinical importance as a great foodborne zoonotic trematode.
Nowadays, molecular approaches are the most efficient and accurate tools used to understand the relationships that exist in an organism and for screening the genetic variations that occur among many populations of parasites. The high annealing temperature-randomly amplified polymorphic DNA (HAT-RAPD) PCR technique had been applied to study the phylogenetic relationships of heterophyid trematodes [
8,
9]. However, currently, this technique is not commonly used because the HAT-RAPD PCR technique does not involve a DNA profile and exhibits a lower level of stability than other methods. The internal transcribed spacer 2 (ITS2) of nuclear rDNA is far more variable in the sequence, and because of the relatively rapid rate at which new mutants are fixed, these regions may reveal closely distinguished related species that otherwise would show little genetic divergence [
10]. Therefore, phylogenetic analyses of ITS2 rDNA sequences have successfully been used to resolve evolutionary relationships among closely related species. Differing sequences of a given region are often assumed to be homogenized within a population of the same species by concerting evolution [
11].
PCR based-methods targeting ITS2 have been applied for phylogenetic studies of various trematodes, such as
Haplorchis taichui and
H. pumilio [
12,
13],
Clonorchis sinensis [
14],
Fasciola hepatica [
15,
16], and trematodes in the family Paramphistomidae [
17]. Recently, the phylogenetic analysis of this gene was performed by more appropriate methods than the HAT-RAPD procedure applied in previous reports [
5,
8,
9,
18,
19]. The purpose of this study was to investigate the prevalence of
S. falcatus infection in the half-beaks (
D. pussilus) and the worm recovery in experimentally infected chicks. In addition, phylogenetic relationships of
S. falcatus with other heterophyid trematodes were analyzed for additional information to the recent reports [
8,
9].
MATERIALS AND METHODS
Investigation of metacercarial infections in the fish host
Thirty individual specimens of 4 species of freshwater fish, including
D. pusillus, Henicorhynchus siamensis, Puntius brevis, and
Cyclocheilichthys armatus, were subjected to analyses by surveying the metacercarial infections of heterophyid trematodes. Each individual fish was digested using an acid pepsin solution (conc. HCl 1 ml: pepsin 1 g: 0.85% sodium chloride solution 99 ml) for 3 hr at 37˚C. The digested material was then rinsed with 0.85% sodium chloride solution and examined for metacercariae under a light microscope. Some metacercarial specimens were excysted and fixed in 4% formalin under the pressure of a cover slip. Specimens were then stained with hematoxylin, dehydrated in alcohol series, cleared with xylene, and mounted in Permount
®. The metacercariae were confirmed in terms of species, based on Pearson and Ow-Yang [
2] and Chai and Sohn [
3].
The half-beaks (D. pusillus) were collected from Chiang Mai province, Thailand and were examined and isolated for the metacercarial stage of S. falcatus infection. The fish were digested by 1% acid pepsin solution at 37˚C for 2 hr. The digested material was passed through graded sieves, rinsed in 0.85% saline solution, and then collected for metacercariae under a light microscope. Two hundred metacercariae of S. falcatus were orally infected to 34 chicks, Gallus gallus domesticus. The exposed hosts were killed daily and their small intestines, which were roughly divided into the duodenum, jejunum, and ileum, were opened in 0.85% saline solution. Worms were collected in a modified Baermann’s apparatus, and then examined and observed under a stereomicroscope. The specimens of S. falcatus were fixed in 4% formalin under the cover slip pressure, stained with hematoxylin, dehydrated in alcohol series, cleared with xylene, and mounted in permount. The specimens were measured and drawn under a compound microscope for morphological studies.
Parasite preparation for phylogenetic analysis
The metacercariae of S. falcatus, H. taichui, and C. formosanus were collected from different fish hosts; D. pusillus, H. siamensis, and P. brevis, respectively. Meanwhile, the adult specimens of Haplorchoides sp. were gathered from the intestines of the Yellow cat fish, Hemibragus filamentus, and the adult stages of other trematodes, namely the giant liver fluke, Fasciola gigantica and the rumen fluke, Orthocoelium streptocoelium, were recovered from cows (Bos taurus).
Total genomic DNA extraction
The genomic DNA of all parasites was extracted and purified from the adult worms using a DNeasy Tissue Kit (Vivantis, Oceanside, California, USA) according to the instructions of the manufacturer. All extracted genomic DNA were diluted to a working concentration of 30 ng/μl and stored at -20˚C until used.
Amplification of ITS2 region
The PCR amplification of partial ITS2 fragments was done using a pair of primers as has been described in the previous report [
20]. This consisted of (ITS3) 5´GCA TCG ATG AAG AACGCA GC 3´ as a forward primer and (ITS4) 5´TCC TCCGCT TAT TGA TAT GC 3´ as a reverse primer. The reaction was carried out at a final volume of 20 μl containing 1× PCR buffer, 2 mM MgCl
2, 10 μM of each dNTP, 1 μM of each primer, and 1 U of Vivantis Tag DNA polymerase. The analysis was then performed in a MyCycler
TM Thermocycler (BioRAD, Hercules, California, USA). Recommended PCR protocols were as follows; 1 cycle of 95˚C for 2 min, 35 cycles of 94˚C for 45 sec, 50˚C for 45 sec, 72˚C for 1 min, and 1 cycle of final extension at 72˚C for 7 min. Specific PCR products were separated on 1.4% TBE agarose gel electrophoresis, stained with ethidium bromide, and photographed using a Kodak digital camera Gel Logic 100 and direct sequenced by the dideoxy terminator method (First Base Company). The sequence was checked using the BLAST program in the NCBI (National Center for Biotechnology Information, Bethesda, Maryland, USA) database, to confirm the PCR target. The eletropherograms of each sequence were examined for sequence accuracy using a Sequence Scanner version 1.0 and Bioedit version 7.1. All sequences were aligned automatically using Clustal X version 2.0. The phylogenetic relationships of 11 trematodes were constructed using MEGA version 5.0. All molecular data were analyzed by UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method (
Table 1). The reliability of the internal branches was assessed using the bootstrap method per 1,000 replicates.
RESULTS
Infection status of metacercariae in fish hosts
The prevalence of infection in 4 heterophyid trematodes;
S. falcatus,
H. taichui,
Haplorchoides sp., and
C. formosanus, were recorded individually from their fish hosts which revealed the prevalence of 90.0%, 73.3%, 33.3%, and 33.3%, respectively, while only
D. pusillus was infected by
S. falcatus with the highest value of intensity (919 metacercariae/fish) (
Table 2).
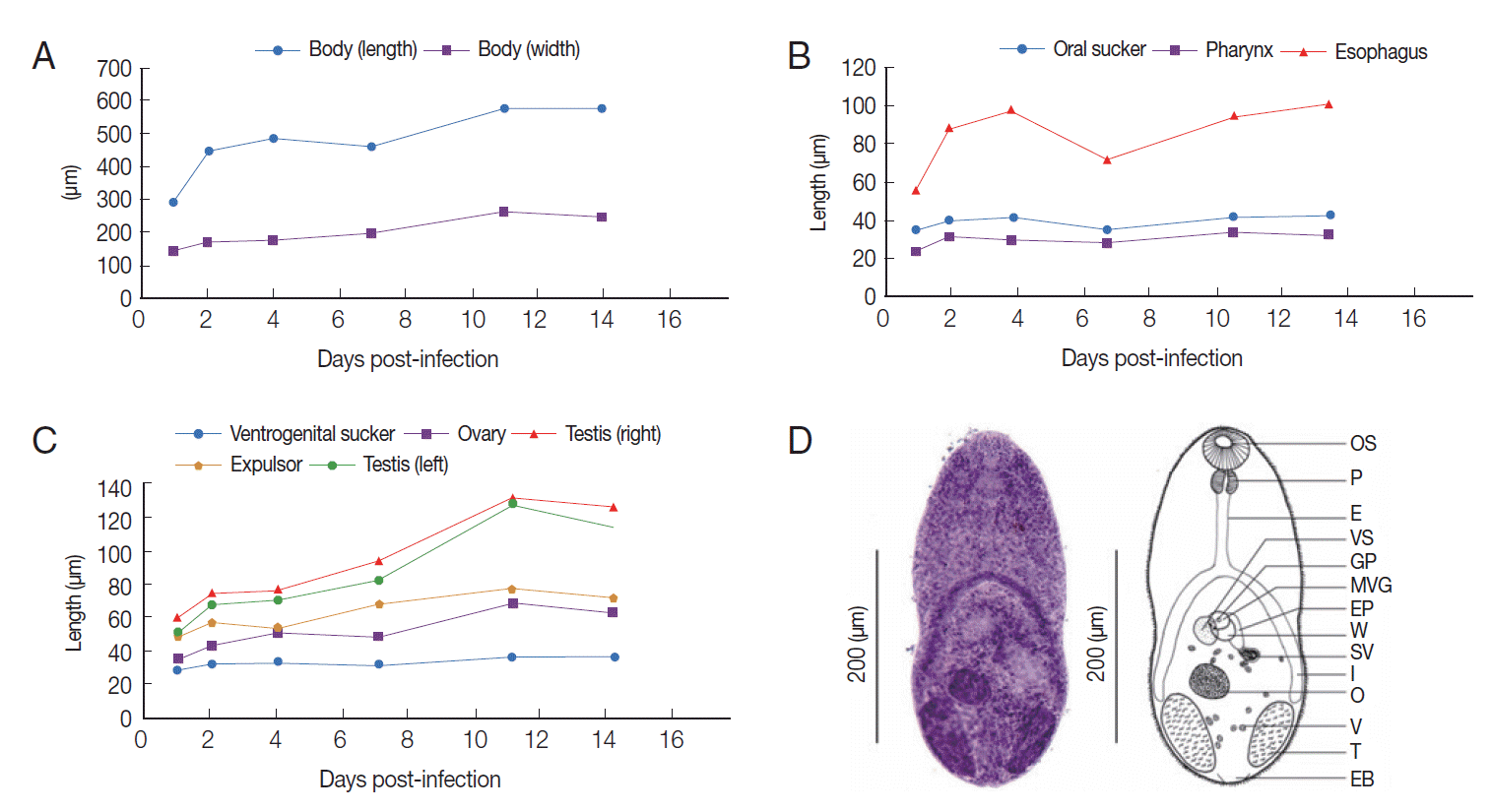
Worms were recovered in 33 (97.1%) of 34 chicks that were experimentally infected with 200
S. falcatus metacercariae each. The average worm recovery rate was 43.0%. Measurements of worms recovered from the chick are summarized in
Fig. 1. The body length and width were remarkably increased from days 1-2 post-infection (PI), and then gradually increased until day 14 PI (
Fig. 1A). The oral sucker, pharynx, esophagus, and ventrogenital sucker also showed a similar developmental pattern to the body size over days 1-2 PI, before stabilizing at a plateaued level (
Fig. 1B). The left testis was a little bit larger than the right one in terms of both length and width. The size of 2 testes was dramatically increased from day 1 to day 2 PI, and gradually increased until day 11 PI. The ovary and the expulsor developed at an equivalent level, as was also observed in the testes (
Fig. 1C). Uterine eggs were recognized in 87.5% of the specimens at day 2 PI. Eggs in the uterine duct were constant in size during days 2-14 PI, with a length of 20.0-22.5 μm and a width of 7.5-10.5 μm.
On day 1 PI, the worms differed morphologically from the metacercariae particularly in terms of their body size. The body measured 240.0-375.0 μm in length and 130.0-160.0 μm in width. The ovary was 30.0-37.50 μm in length and 27.5-45.0 μm in width. The right testis was found to be 42.5-90.0 μm long and 20.0-42.5 μm wide, while the left testis was 37.5-70.0 μm long and 22.5-30.0 μm wide. On day 2 PI, mature worms were observed. The body measured 360.0-510.0 μm in length and 130.0-210.0 μm in width. The ovary enlarged to 30.0-62.5 μm long and 27.5-50.0 μm wide. The right testis was 55.0-87.5 μm long and 32.5-52.5 μm wide, while the left testis was 47.5-80.0 μm long and 30.0-52.5 μm wide. The expulsor was oval and relatively large, with a length of 32.5-82.5 μm and a width of 25.0-37.5 μm. The ventrogenital sucker was slightly submedian to the right side and contained a ventral sucker. The ventral sucker armed with 2 dense lateral groups of small spines on the lip. The ovary was submedian and located slightly to the right side between the ventral sucker and right testis and was 30.0-62.5 μm long and 27.5-50.0 μm wide. The vitellaria extended dorsally into the ovariotesticular zone (
Fig. 1D).
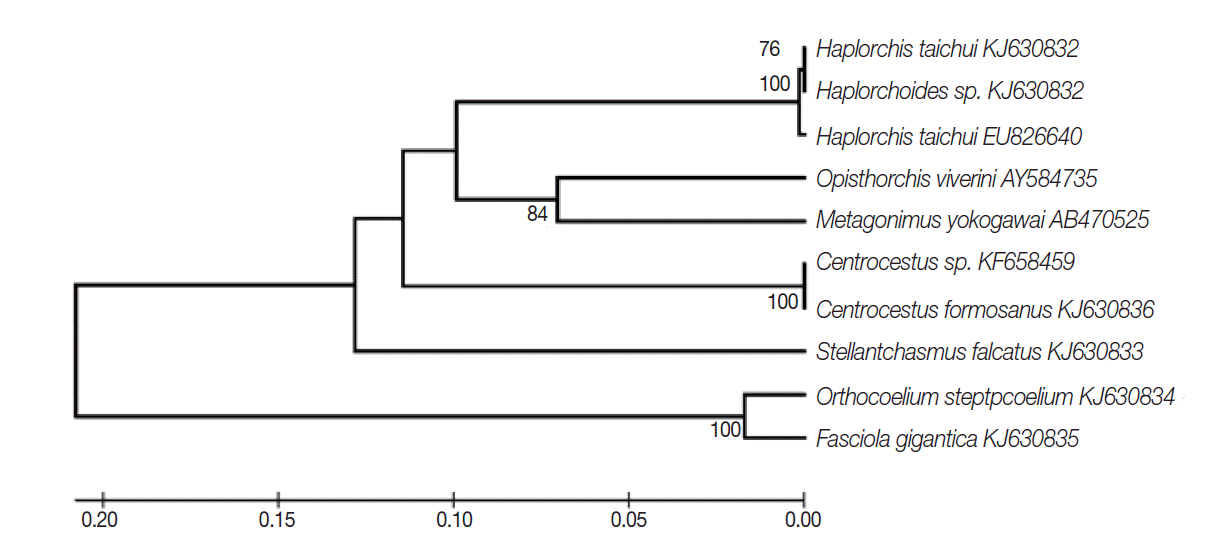
The partial sequence data of the ITS2 region were used to understand their phylogenetic relationships. The length of this fragment was 410-590 bp. The trematodes in the family Heterophyidae appeared to be in a monophyletic clade. The heterophyids were separated into 2 sister groups including
S. falcatus group and the other heterophyid group (
Fig. 2).
DISCUSSION
In this study, we reported the metacercarial infection status of 4 heterophyid flukes, i.e.,
S. falcatus,
H. taichui,
Haplorchoides sp., and
C. formosanus, which were collected individually from their fish hosts to be 90.0%, 86.7%, 33.3%, and 23.0%, respectively. Our findings corresponded well with previous reports that have confirmed a high prevalence of infection in
S. falcatus and
H. taichui, particularly in the northern part of Thailand [
21-
23]. Various reports have detected the presence of intestinal trematodes in the family Heterophyidae, such as,
H. taichui,
H. pumilio,
S. falcatus,
C. formosanus, and
Procerovum sp., which were found to be wildly distributed and had a high prevalence and intensity. Previous surveys have also revealed that the metacercariae, which were mostly found in Mae Taeng District of Chiang Mai Province, belonged to the family Heterophyidae [
21-
23]. Our findings corresponded well with those of Radomyos et al. [
24] who reported that certain heterophyid species were widely distributed in certain regions in the north of Thailand. Epidemiological surveys from 1997-2013 have shown an increase in trematode infections in the northern and central parts of Thailand. The surveyed region has been reported as an endemic area of heterophyid trematodes with high prevalences in a variety of intermediate and definitive hosts. For instance, Wongsawad et al. [
6] investigated on trematode infections in freshwater fish from Mae Sa stream, Chiang Mai Province, where 4 species of heterophyid trematodes were found, i.e., Haplorchis spp.,
Haplorchoides sp.,
C. formosanus, and
S. falcatus [
6].
From the present results, it is strongly suggested that the half-beaks (
D. pusillus) in northern Thailand typically serve as the highly compatible secondary host for infection with
S. falcatus. This result corresponded well with a previous report [
25]. In contrast, the metacercarial stage of
S. falcatus has been detected also in marine or brackish water fish [
3,
4]. However, further studies must be done to investigate
S. falcatus metacercariae in fish species from a larger area in order to confirm that this fish species (
D. pussilus) serves as the most popular second intermediate host of this parasite.
Based on the results of experimental infection, chicks seemed to be a susceptible host for
S. falcatus infection revealing much higher rates of infection and worm recovery than those observed in mice (
Mus musculus) (13.6%) and rats (
Rattus norvegicus) (20.2%) [
26]. These results are supported by the far higher worm recovery that was recorded in mice (13.6%) and chicks (33.8%). In our study, parasites were observed to have invaded the jejunum during days 1-6 PI and later they were typically observed in the ileum. A similar pattern was observed in
H. taichui and
H. pumilio which were found to have invaded the upper part of the small intestines during the first period of infection, but when they matured the parasites were more common in the middle and lower parts of the intestinal tract because the flukes became gradually excreted into the lumen of the intestinal canal along with mucus and other exudates. The flukes then reattached themselves further down the tract [
27]. However, host-parasite relationships in heterophyid infections have never been studied in detail. Since human infections with
S. falcatus and other heterophyid trematodes are expected to increase in areas where fresh and brackish water fish are popularly eaten raw, studies on host-parasite relationships are considered highly useful.
According to the phylogenetic analysis, the partial sequences of ITS2 of some heterophyid trematodes are declared to be known now. However, only a few studies have focused on the relationship that exists among the ITS2 of
S. falcatus in Thailand [
28]. Our sequence data of ITS2 agreed well with the sequence data reported in other recent reports [
28].
The UPGMA trees showed the monophyletic relationships of heterophyid trematodes. Heterophyid trematodes were separate in a same group except
S. falcatus which was separated as another group with a high bootstrap value. This agrees with the reports on phylogenetic relationships based on mitochondrial cytochrome c oxidase 1 (mtCOI) sequence data, which summarized that only
S. falcatus has a ventrogenital complex [
29], whereas the results of a phylogenetic study of
S. falcatus using the HAT-RAPD method showed a group of
S. falcatus together with other heterophyid trematodes [
8].
ITS2 has been used to study the systematic analysis of some heterophyid trematodes, i.e.,
Haplorchis [
12,
13] and
Metagonimus [
30]. From our results, it is believed that the ITS2 locus could be used as a barcode for authenticating animal species, as well as a complementary locus to other barcoding genes for the purpose of identifying all organisms, including heterophyid trematodes. Several regions that have become known for a relevant presence of nuclear ribosomal DNA (nu rDNA) were selected and used for identification of various stages as well as for a study of the life cycle of trematodes (cercarial, metacercarial, and adult stages) in freshwater fish and snails because of the high levels of accuracy, sensitivity, and rapidity of this method. ITS2 has been revealed as a sensitive marker at the species level for trematodes. The PCR based-method targeting ITS2 has been applied for use in the phylogenetic study of various trematodes, such as
H. taichui and
H. pumilio [
12,
13],
C. sinensis [
14],
F. hepatica [
15], and trematodes in the family Paramphistomidae [
17]. Using the sequence data of the ITS2 region, the species-level identification could be achieved, and the ITS2 analysis could actually provide a phylogenetic study of these trematodes.
This study can conclude that D. pusillus fish serves as a second intermediate host for S. falcatus with the highest rate of prevalence and rapid development being found in the experimental host, chicks (G. gallus domesticus). Finally, the present study provided valuable and beneficial information that can be used to demonstrate the epidemiological situation, as well as to examine the development and phylogenetic relationships of S. falcatus with regard to the biological control and monitoring of this parasitic disease.
Notes
-
We have no conflict of interest related to this work.
The financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (grant no. PHD/0192/2543 and BGJ4580020) to student's initials and adviser's initials is acknowledged. We also thank the Biology Department, Faculty of Science, the Applied Parasitology Research Laboratory, Institute for Science and Technology Research, and Economic Plants Genome Research and Service Center, Chiang Mai University, Thailand. We thank them for providing the necessary facilities.
Fig. 1.Morphologic changes of Stellantchasmus falcatus recovered from experimental chicks (A-C) and illustrations demonstrating the permanent slide capture and line-drawing (D).

Fig. 2.The rooted phylogeny from partial ITS2 sequences of heterophyid trematodes based on the UPGMA method. Bootstrap values were computed independently for 1,000 resembling.
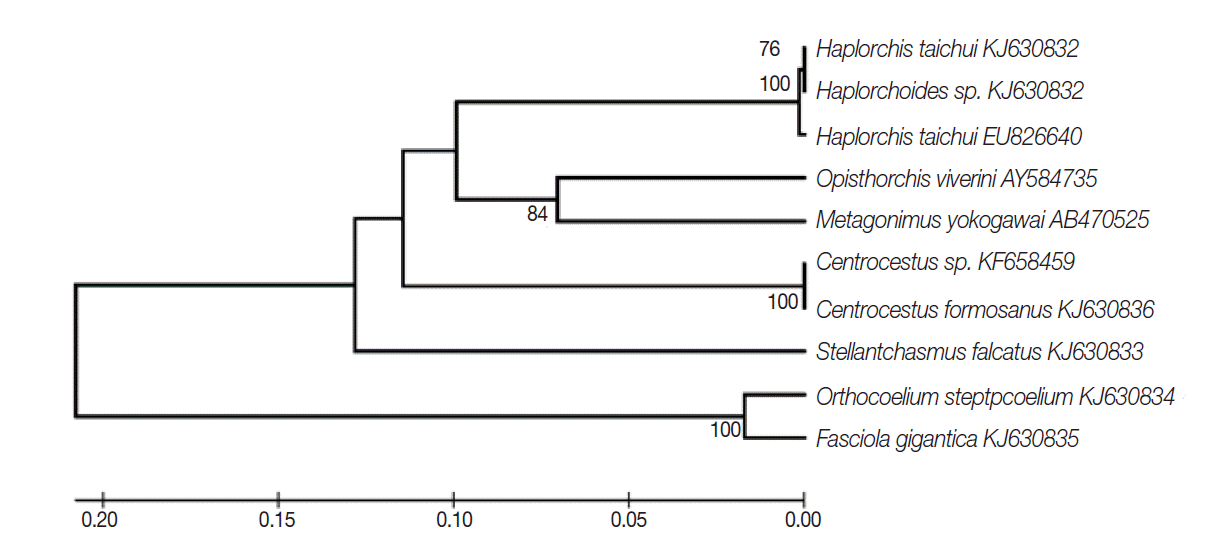
Table 1.List of materials and sequences of ITS2 used for phylogenetic analysis
Table 1.
|
Species |
Accession no./ references |
|
Haplorchis taichui
|
KJ630831 |
|
Haplorchoides sp. |
KJ630832 |
|
Stellantchasmus falcatus
|
KJ630833 |
|
Orthocoelium sterptocoelium
|
KJ630834 |
|
Fasciola hepatica
|
KJ630835 |
|
Centrocestus formosanus
|
KJ630836 |
|
Haplorchis taichui
|
EU826640a
|
|
Metagonimus yokogawai
|
AB470525a
|
|
Centrocestus sp. |
KF658459a
|
|
Opisthorchis viverrini
|
AY584735a
|
Table 2.Summary of fish species, prevalence, and intensities of heterophyid trematodes examined in each fish species
Table 2.
|
Fish species |
No. of fish infected with each heterophyid species/No. of fish examined
|
Mean |
|
S. falcatus
|
H. taichui
|
Haplorchoides sp. |
C. formosanus
|
|
Dermogenys pusillus
|
27/30 |
0 |
0 |
0 |
90.0% |
|
919.0 (28-3,414)a
|
- |
- |
- |
919 |
|
Henicorhynchus siamensis
|
0 |
17/30 |
8/30 |
0 |
83.3% |
|
- |
37.0 (2-129)a
|
16.5 (3-44)a
|
- |
26.8 |
|
Puntius brevis
|
0 |
0 |
0 |
10/30 |
33.3% |
|
- |
- |
- |
213.5 (2-2,492) |
213.5 |
|
Cyclocheilichthys armatus
|
0 |
05/30 |
2/30 |
0 |
23.3% |
|
- |
29.0 (23-187)a
|
79.1a
|
- |
54.1 |
References
- 1. Pearson JC. A revision of the subfamily Haplorchinae Looss, 1899 (Trematoda: Heterophyidae). 1. The Haplorchis group. Parasitology 1964;54:601-676.
- 2. Pearson JC, Ow-Yang CK. New species of Haplorchis from Southeast Asia, together with keys to the Haplorchis-group of heterophyid trematodes of the region. Southeast Asian J Trop Med Public Health 1982;13:35-60.
- 3. Chai JY, Sohn WM. Identification of Stellantchasmus falcatus metacercariae encysted in mullets in Korea. Korean J Parasitol 1988;26:65-68.
- 4. Shin EH, Guk SM, Kim HJ, Lee HY, Chai JY. Trends in parasitic diseases in the Republic of Korea. Trends Parasitol 2008;24:143-150.
- 5. Sripalwit P, Wongsawad C, Wongsawad P, Anuntalabhochai S. High annealing temperature-random amplified polymorphic DNA (HAT-RAPD) analysis of three paramphistome flukes from Thailand. Exp Parasitol 2007;115:98-102.
- 6. Wongsawad C, Rojtinnakorn J, Wongsawad P, Rojanapaibul A. Helminths of vertebrates from Mae Sa Stream, Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health 2004;35(Suppl 1):140-146.
- 7. Kliks M, Tantachumrun T. Heterophyid (trematoda) parasites of cats in north Thailand, with notes on a human case found at necropsy. Southeast Asian J Trop Med Public Health 1974;5:547-555.
- 8. Wongsawad C, Wongsawad P. Molecular markers for identification of Stellantchasmas falcatus and a phylogenetic study using the HAT-RAPD method. Korean J Parasitol 2010;48:303-307.
- 9. Wongsawad C. Development of HAT-RAPD marker for detection of Stellantchasmas falcatus infection. Southeast Asian J Trop Med Public Health 2011;42:46-52.
- 10. Mas-Coma S, Bargues MD. Populations, hybrids and the systematic concepts of species and subspecies in Chagas disease triatomine vectors inferred from nuclear ribosomal and mitochondrial DNA. Acta Trop 2009;110:112-136.
- 11. Darissa OM, Iraki NM. Molecular identification of six Steinernema isolates and characterization of their internal transcribed spacers regions. Jordan J Biol Sci 2014;7:31-34.
- 12. Van Van K, Dalsgaard A, Blair D, Le TH. Haplorchis pumilio and H. taichui in Vietnam discriminated using ITS-2 DNA sequence data from adults and larvae. Exp Parasitol 2009;123:146-151.
- 13. Skov J, Kania PW, Dalsgaard A, Jørgensen TR, Buchmann K. Life cycle stages of heterophyid trematodes in Vietnamese freshwater fishes traced by molecular and morphometric methods. Vet Parasitol 2009;160:66-75.
- 14. Müller B, Schmidt J, Mehlhorn H. Sensitive and species-specific detection of Clonorchis sinensis by PCR in infected snails and fishes. Parasitol Res 2007;100:911-914.
- 15. Erensoy A, Kuk S, Ozden M. Genetic identification of Fasciola hepatica by ITS-2 sequence of nuclear ribosomal DNA in Turkey. Parasitol Res 2009;105:407-412.
- 16. Wongsawad C, Wongsawad P. Anuntalaphochai S, Chai CJ, Sukontason K. Occurrence and molecular identification of liver and minute intestinal flukes metacercariae in freshwater fish from Fang-Mae Ai Agricultural Basin, Chiang Mai Province, Thailand. Asian Biomed 2013;7:97-104.
- 17. Loffy WM, Brant SV, Ashmawy KI, Devkota R, Mkoji GM, Loker ES. A molecular approach for identification of paramphistomes from Africa and Asia. Vet Parasitol 2010;174:234-240.
- 18. Chuboon S, Wongsawad C. Molecular identification of larval trematode in intermediate hosts from Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health 2009;40:1216-1220.
- 19. Wongsawad P, Wongsawad C. Development of PCR-based diagnosis of minute intestinal fluke, Haplorchis taichui. Southeast Asian J Trop Med Public Health 2009;40:919-923.
- 20. Barber KE, Mkoji GM, Loker ES. PCR-RFLP analysis of the ITS2 region to identify Schistosoma haematobium and S. bovis from Kenya. Am J Trop Med Hyg 2000;62:434-440.
- 21. Boonchot K, Wongsawad C. A survey of helminths in cyprinoid fish from the Mae Ngad Somboonchon Reservoir, Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health 2005;36:103-107.
- 22. Kumchoo K, Wongsawad C, Chai JY, Vanittanakom P, Rojanapaibul A. High prevalence of Haplorchis taichui metacercariae in cyprinoid fish from Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health 2005;36:451-455.
- 23. Nithikathkul C, Wongsawad C. Prevalence of Haplorchis taichui and Haplorchoides sp. metacercariae in freshwater fish from water reservoirs, Chiang Mai, Thailand. Korean J Parasitol 2008;46:109-112.
- 24. Thu ND, Dalsgaard A, Loan LTT, Murrell KD. Survey for zoonotic liver and intestinal trematode metacercariae in culture and wild fish in An Giang Province, Vietnam. Korean J Parasitol 2007;45:45-54.
- 25. Pubua J, Wongsawad C. Redescription of the trematode metacercariae from the mullet (Liza subviridis) and half-beak (Dermogenus pusillus). Southeast Asian J Trop Med Public Health 2007;38(Suppl 1):106-109.
- 26. Saenphet S, Wongsawad C, Saenphet K, Chai JY. Susceptibility of rodents to Stellanchasmus falcatus infection. Southeast Asian J Trop Med Public Health 2003;32(Suppl 2):123-127.
- 27. Africa CM, de Leon W, Garcia EY. Visceral complications in intestinal heterophyidiasis of man. Acta Med Philipina (Monogr Ser) 1940;1:1-132.
- 28. Chontananarth T, Wongsawad C. Prevalence of Haplorchis taichui in field-collected snails: a molecular approach. Korean J Parasitol 2010;48:343-346.
- 29. Chontananarth T, Wongsawad C, Chomdej S, Krailas D, Chai JY. Molecular phylogeny of trematodes in family Heterophyidae based on mitochondrial cytochrome c oxidase subunit I (mCOI). Asian Pac J Trop Med 2014;7:446-450.
- 30. Lee SU, Huh S, Sohn WM, Chai JY. Sequence comparisons of 28S ribosomal DNA and mitochondrial cytochrome c oxidase subunit I of Metagonimus yokogawai, M. takahashii and M. miyatai. Korean J Parasitol 2004;42:129-135.