Abstract
A 50-year-old male visited the outpatient clinic and complained of fever, poor oral intake, and weight loss. A chest X-ray demonstrated streaky and fibrotic lesions in both lungs, and chest CT revealed multifocal peribronchial patchy ground-glass opacities with septated cystic lesions in both lungs. Cell counts in the bronchoalveolar lavage fluid revealed lymphocyte-dominant leukocytosis, and further analysis of lymphocyte subsets showed a predominance of cytotoxic T cells and few T helper cells. Video-assisted wedge resection of the left upper lobe was performed, and the histologic examination was indicative of a Pneumocystis jirovecii infection. Trimethoprim-sulfamethoxazole (TMP-SMX) was orally administered for 3 weeks; however, the patient complained of cough, and the pneumonia was aggravated in the follow-up chest X-ray and chest CT. Molecular studies demonstrated mutations at codons 55 and 57 of the dihydropteroate synthase (DHPS) gene, which is associated with the resistance to TMP-SMX. Clindamycin-primaquine was subsequently administered for 3 weeks replacing the TMP-SMX. A follow-up chest X-ray showed that the pneumonia was resolving, and the cough was also alleviated. A positive result of HIV immunoassay and elevated titer of HCV RNA indicated HIV infection as an underlying condition. This case highlights the importance of careful monitoring of patients with P. jirovecii pneumonia (PCP) during the course of treatment, and the molecular study of DHPS mutations. Additionally, altering the anti-PCP drug utilized as treatment must be considered when infection with drug-resistant P. jirovecii is suspected. To the best of our knowledge, this is the first case of TMP-SMX-resistant PCP described in Korea.
-
Key words: Pneumocystis jirovecii, trimethoprim, sulfamethoxazole, drug resistance, clindamycin, primaquine
INTRODUCTION
Pneumocystis jirovecii is an opportunistic pathogen, and is a major cause of serious pneumonia in immunocompromised conditions, including congenital immunodeficiency, organ transplantation, or acquired immune deficiency syndrome (AIDS) [
1]. The standard regimen of treatment or prophylaxis for pneumonia caused by
P. jirovecii is cotrimoxazole which contains sulfamethoxazole (SMX) and trimethoprim (TMP) [
2]. However, a few studies have reported therapeutic failure of the regimens used to treat pneumonia caused by
P. jirovecii [
3-
10], and no reports occurred in Korea. Here, we describe a case in which TMP-SMX, in sufficient dose and duration, had failed to treat pneumonia caused by
P. jirovecii which was then successfully treated with clindamycin-primaquine thereafter.
CASE RECORD
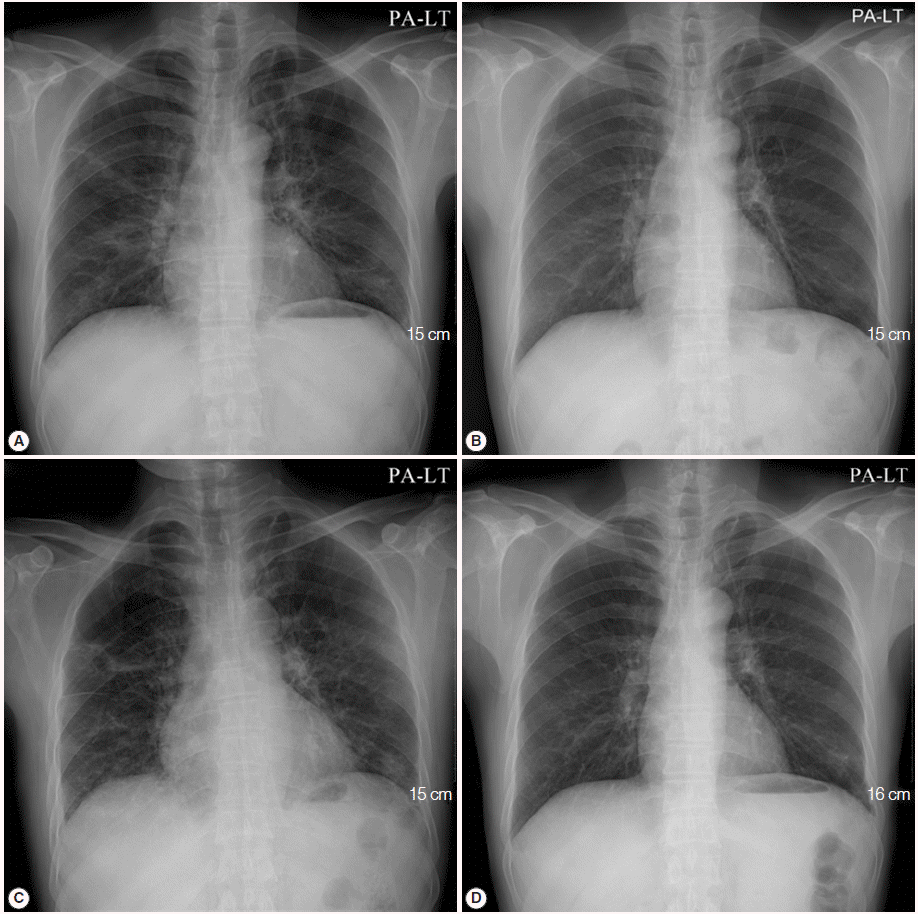
A 50-year-old male visited the outpatient clinic and complained of fever, poor oral intake, and weight loss (6 kg loss in 2 months). Routine laboratory tests and a chest X-ray were performed. Complete blood counts revealed mild leucopenia (WBC 3,720/μl), mild anemia (Hb 11.2 g/dl), and mild eosinophilia (830/μl), however, a normal platelet level (246,000/μ) was observed. Liver function tests showed elevated liver enzymes (AST 61 IU/L, ALT 74 IU/L, and GGT 215 IU/L), and serologic studies for hepatitis B virus, hepatitis C virus, human immunodeficiency virus (HIV), and syphilis were negative. A routine chest X-ray yielded streaky and fibrotic lesions in both lungs (
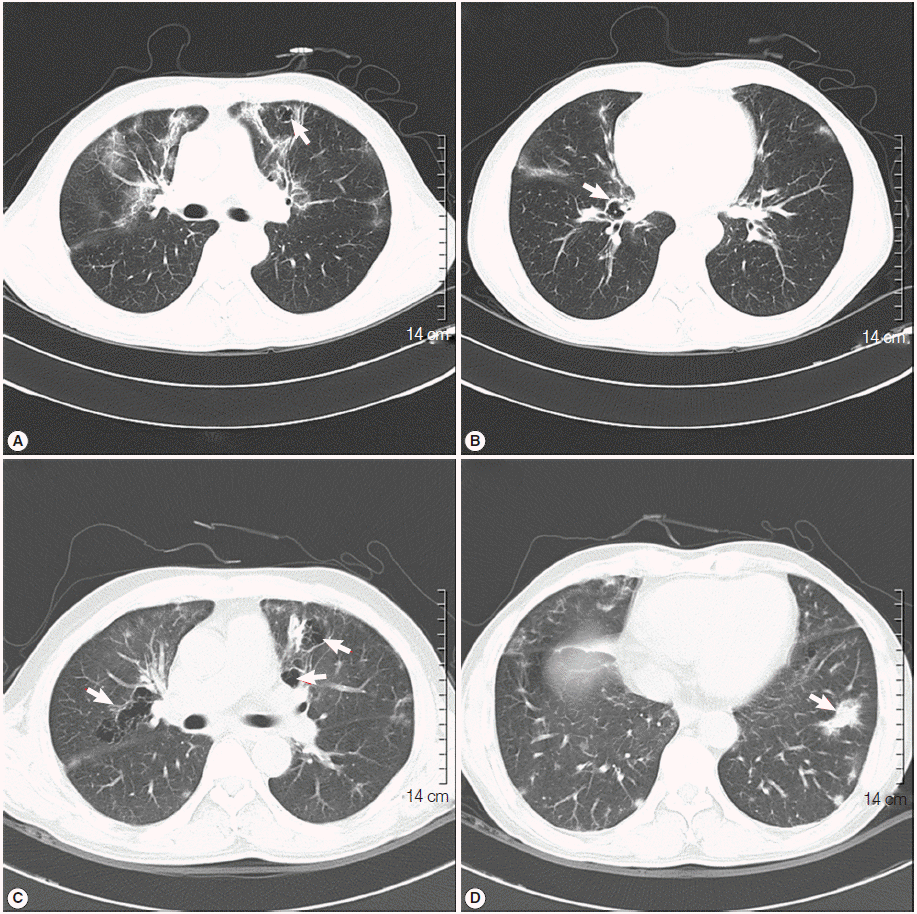
Fig. 1A), which led to further evaluation using contrast chest computed tomography (CT). The chest CT revealed multifocal peribronchial patchy ground-glass opacities in both lungs with septated cystic lesions in the left upper lobe, and the right lower lobe (
Fig. 2A,
B).
Bronchoscopy was performed, but no endobronchial lesions were observed. Bronchoalveolar lavage (BAL) was performed in the right middle lobe. Cell counts in BAL revealed a lymphocyte-dominant leukocytosis (white cells 342/μl, neutrophils 9%, lymphocytes 37%, eosinophils 5%, and macrophages 49%). Further analysis of lymphocyte subsets showed a predominance of cytotoxic T cells (cytotoxic T cells 98.3%, T helper cells 1.7%, natural killer cells 3%, and B cells 0.1%). Cytologic studies, acid-fast bacillus stain, and PCR for tuberculosis and non-tuberculotic mycobacteria in the BAL fluid exhibited negative results. A tuberculin skin test and interferon-γ release assay (Quantiferon
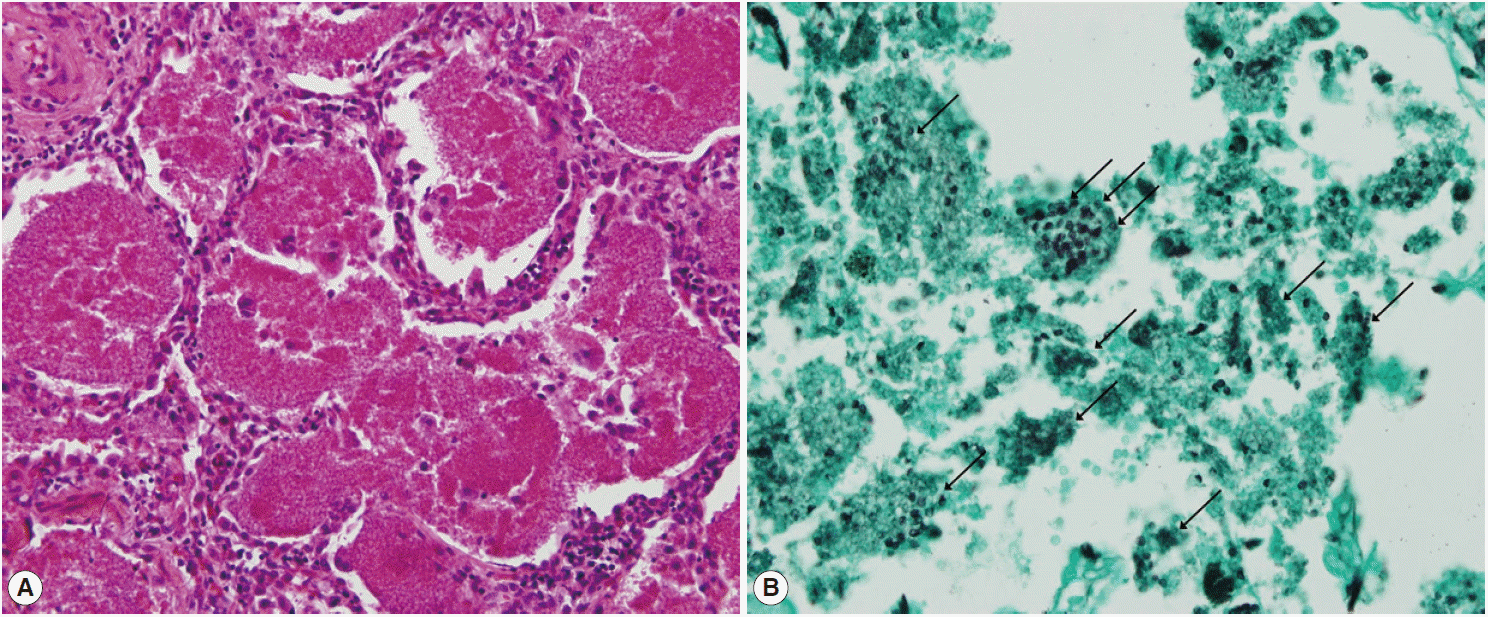
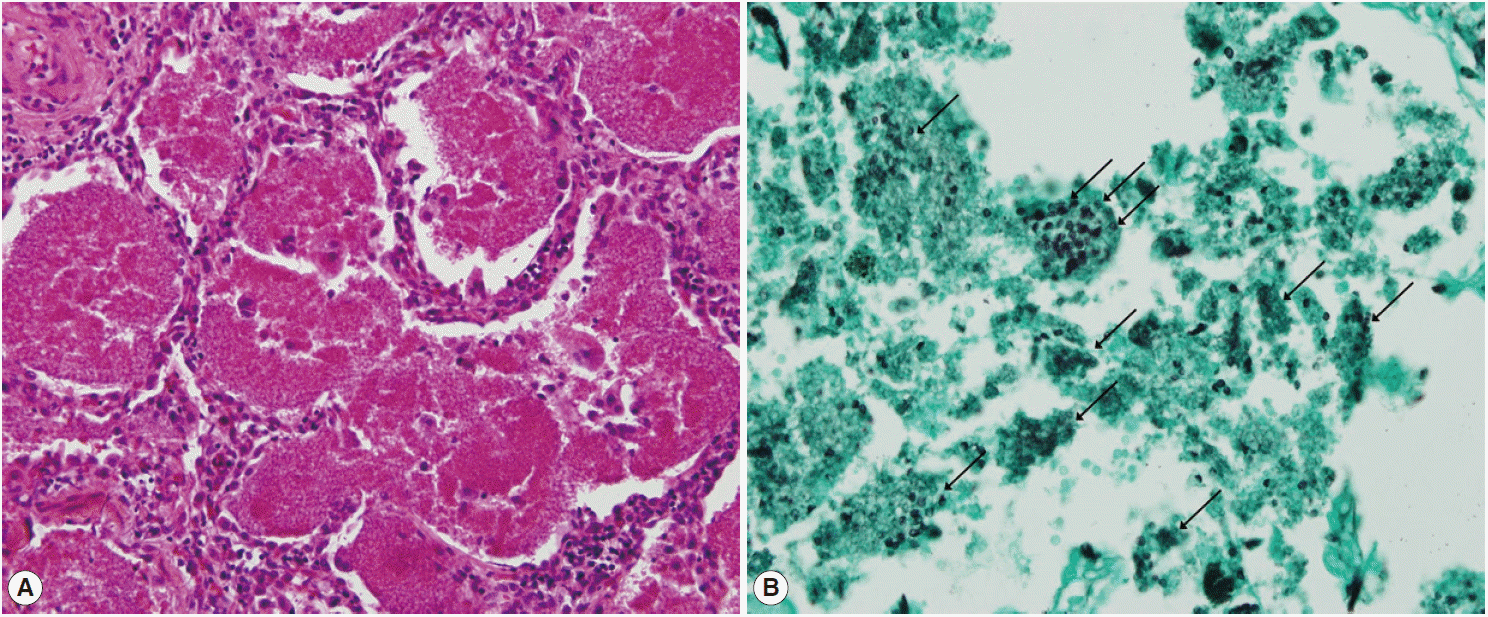
®; Carnegie, Victoria, Australia) were also negative. A video-assisted wedge resection of the left upper lobe was performed. Upon histological examination, hematoxylin and eosin (H&E) staining revealed eosinophilic frothy exudates in alveolar spaces accompanied by mild interstitial inflammation, and Grocott-Gomori’s methenamine silver (GMS) stains, performed during the histological examination, revealed many cystic- and trophic-form organisms (arrows) in the alveolar exudate, consistent with
P. jirovecii infection (
Fig. 3). Trimethoprim-sulfamethoxazole (TMP-SMX) was administered orally (2 double strength TMP-SMX tablets every 8 hr) [
11]. The patient’s fever subsided within 3 days (from 38.5˚C to 37.0˚C), and the streaky and fibrotic lesions observed in both lungs on the chest X-ray were also markedly improved (
Fig. 1B) after 12 days of TMP-SMX administration, which enabled the patient to be discharged.
TMP-SMX treatment continued to be administrated for additional 17 days following the patient’s discharge from the hospital. However, the patient complained of cough again, and a follow-up chest X-ray showed aggravation of the streaky and fibrotic lesions in both lungs (
Fig. 1C). The patient was readmitted to the hospital, and chest CT was rechecked. A follow-up chest CT revealed an aggravation of multifocal peribronchial ground-glass opacity and septated cystic lesions in both upper lungs, and a newly appeared consolidation in the left lower lobe (
Fig. 2C,
D).
P. jirovecii was clinically suspected to be resistant to TMP-SMX, and molecular studies carried out using PCR with primers Dp15 (5´-TCTGAATTTTATAAAGCGCCTACAC-3´) and Dp800 (5´-ATTTCATAAACATCATGAACCCG-3´) demonstrated mutations at codons 55 and 57 of the dihydropteroate synthase (DHPS) gene as a previous report [
12].
Primaquine (4 mg/kg/day intravenous once daily) and clindamycin (600 mg intravenous every 8 hr) were administrated for 3 weeks in place of the TMP-SMX. Consequently, the cough was resolved, and a follow-up chest X-ray showed improvement of the streaky and fibrotic lesions in both lungs (
Fig. 1D). We strongly suspected that HIV infection was an underlying condition, and rechecked the HIV immunoassay, which was determined to be positive. Further evaluation of HIV infection was performed; the titer of HCV RNA was determined to be 110,000 copies/ml, and the number of T helper cells in the peripheral blood was only 3/μl. Antiviral treatment regimen targeting the HIV was initiated, including lopinavir (800 mg/day), ritonavir (200 mg/day), lamivudine (300 mg/day), and zidovudine (600 mg/day), and the patient was discharged.
DICUSSION
P. jirovecii pneumonia (PCP) should be strongly suspected in HIV-infected patients that have a CD4+ T cell count less than 200 cells/µl, 1-3-β-d-glucan levels greater than 80 pg/ml (if available), and signs and symptoms that are typical of the infection; especially dyspnea, cough, and diffuse, bilateral, interstitial, or alveolar infiltrates present on a chest X-ray or chest CT [
13]. A confirmatory diagnosis of PCP requires the identification of cystic or trophic forms in appropriate specimens, both of which can be visualized using GMS, cresyl violet, Gram-Weigert, toluidine blue, Wright-Giemsa, and Diff-Quick staining. Pathologic confirmation is especially important when other infections or co-infections are suspected or need to be excluded [
14-
16].
Cotrimoxazole, namely TMP-SMX, is the most common drug used for prophylaxis and treatment of PCP, and has been utilized as an effective treatment option for many years [
5]. In addition to TMP-SMX, TMP-dapsone or clindamycin-primaquine can be used as an alternative regimen. In patients that do not exhibit any improvement after 4 to 8 days of therapy, treatment failure must be considered. Randomized trials have demonstrated that these treatments exhibited a comparable effectiveness in treating PCP. A previously published multicenter study randomly assigned 181 patients with mild to moderate PCP to therapy with TMP-SMX, TMP-dapsone, or clindamycinprimaquine for 21 days [
11], and no differences were observed in the therapeutic failure rate (approximately 5% at day 7, and 10% at day 21) among the treatment groups. Another randomized trial comparing TMP-SMX with TMP-dapsone in 60 PCP patients demonstrated no differences in the response to therapy; however, more TMP-SMX treated patients exhibited adverse reactions to the therapy [
4].
The current case was not initially diagnosed as having HIV infection as an underlying condition. The patient complained of fever, poor oral intake, and weight loss, and multifocal, peribronchial patchy ground-glass opacities in both lungs with septated cystic lesions were observed in chest CT. Bronchoscopy performed with BAL did not yield a confirmative diagnosis, so we could not but carry out a video-assisted wedge resection of the left upper lobe. In histological examinations, GMS staining of the specimen showed many cystic and trophic forms, which finally led to the diagnosis of PCP. We retrospectively suspected the presence of HIV infection as an underlying condition, and reassessed the HIV immunoassay, which turned out to be positive, and the HIV RNA titer was determined to be 110,000 copies/ml. The initial negative and subsequent positive HIV immunoassay and a positive virologic test, led us to suspect that the patient was in the initial stages of HIV infection, since the approximate time from infection to a positive immunoassay and viral load test is 15 to 45 days and 5 to 15 days, respectively [
17-
19].
To treat the PCP, high-dose TMP-SMX was orally administered, and the patient’s symptoms and radiologic findings were markedly improved within several days. However, PCP was aggravated 1 month later despite continuing the medication. The PCP was suspected to be resistant to TMP-SMX, and molecular studies confirmed mutations at codons 55 and 57 of the DHPS gene that are known to be associated with sulfaresistance of
P. jirovecii [
20].
Intravenous TMP-SMX, or pentamidine, is primarily recommended when oral TMP-SMX treatment failure is suspected, as demonstrated in a previous case report [
6]. However, these 2 drugs were unavailable in our hospital, so primaquine and clindamycin were substituted for TMP-SMX, which resulted in an improvement of PCP.
Cotrimoxazole consists of TMP and SMX, and TMP seems to be inactive against pneumocystis [
6]. Consequently, cotrimoxazole acts as sulfa monotherapy [
5]. A common second-line agent is dapsone, which, like sulfamethoxazole, inhibits the enzyme DHPS [
5,
21]. These 2 DHPS inhibitors have been used as a prophylaxis or treatment for PCP in hundreds of thousands of patients over the past 2 decades, and the development of sulfa-resistant strains of
P. jirovecii was inevitable [
5]. Mutations in the
P. jirovecii DHPS gene have been described in 2 case reports [
6,
22], and a small cohort study that contained 27 patients [
23]. However, those studies did not demonstrate that the mutations caused the drug resistance, since human strains of
P. jirovecii cannot be cultured in vitro [
5]. Instead, Helwig-Larsen et al. [
12] found an association between the occurrence of DHPS mutations and the failure of PCP treatment with sulfa drugs in a large study. More importantly, DHPS mutations were linked to a higher risk of death within 3 months of the diagnosis, most likely due to inadequate responses to therapy. In a Japanese study, DHPS mutations were identified at amino acid positions 55 and/or 57 in isolates from 6 (25.0%) of 24 patients with
P. jirovecii pneumonia, and cotrimoxazole treatment failed more frequently in patients whose isolates had DHPS mutations than in those whose isolates had wild-type DHPS [
24]. Zingale et al. [
7] suggested that theses DHPS mutations were significantly more common in patients exposed to sulfa drugs than in those not exposed to sulfa drugs in their study of 70 bronchoalveolar lavage (BAL) specimens with positive PCR for
P. jirovecii. These phenomena were also confirmed in a study by Nahimana et al. [
8] who conducted BAL in 13 AIDS patients who had 2 separate episodes of pneumonia [
8]. While
P. jirovecii cannot be cultivated, subsequent
in vitro studies used
Saccharomyces cerevisiae and
Escherichia coli to further elucidate the association between DHPS polymorphisms and sulfa resistance, and suggested that mutations in DHPS correlate with sulfa drug resistance [
9,
10,
25-
27]. In the current case report, mutations at codons 55 and 57 of the DHPS gene were identified. In addition to DHPS, a recent study demonstrated the association of dihydrofolate reductase (DHFR) variants and resistance to trimethoprim in clinical isolates of
P. jirovecii [
28].
In summary, TMP-SMX was orally administered with a sufficient dose and duration in our patient. However, treatment failure was eventually detected in a follow-up chest X-ray and chest CT, and molecular analysis suggested mutations at codons 55 and 57 of the DHPS gene, which has been shown to confer sulfa resistance to P. jirovecii. Primaquine and clindamycin were substituted for TMP-SMX, which finally improved the PCP. The current case report indicates that PCP patients must be carefully monitored during treatment, and molecular analysis of DHPS mutations should be performed. Additionally, appropriate changes of anti-PCP drugs should be considered when a drugresistant P. jirovecii infection is suspected. To our knowledge, this is the first case of TMP-SMX-resistant PCP reported in Korea.
Notes
-
The authors of this article have no financial or other issues that might lead to a conflict of interest.
Fig. 1.Initial chest X-ray and follow-up chest X-rays after the initiation of treatment for Pneumocystis jirovecii pneumonia (PCP). (A) Initial chest X-ray showed streaky and fibrotic lesions in both lungs. (B) In the follow-up chest X-ray performed 12 days after the initiation of TMP-SMX treatment, the lesions were markedly improved. (C) In a follow-up chest X-ray carried out 29 days after the initiation of TMP-SMX treatment, streaky and fibrotic lesions in both lungs were aggravated. (D) In the follow-up chest X-rays, performed 21 days after changing the anti-PCP therapy from TMP-SMX to primaquine-clindamycin, the lesions were improved again.
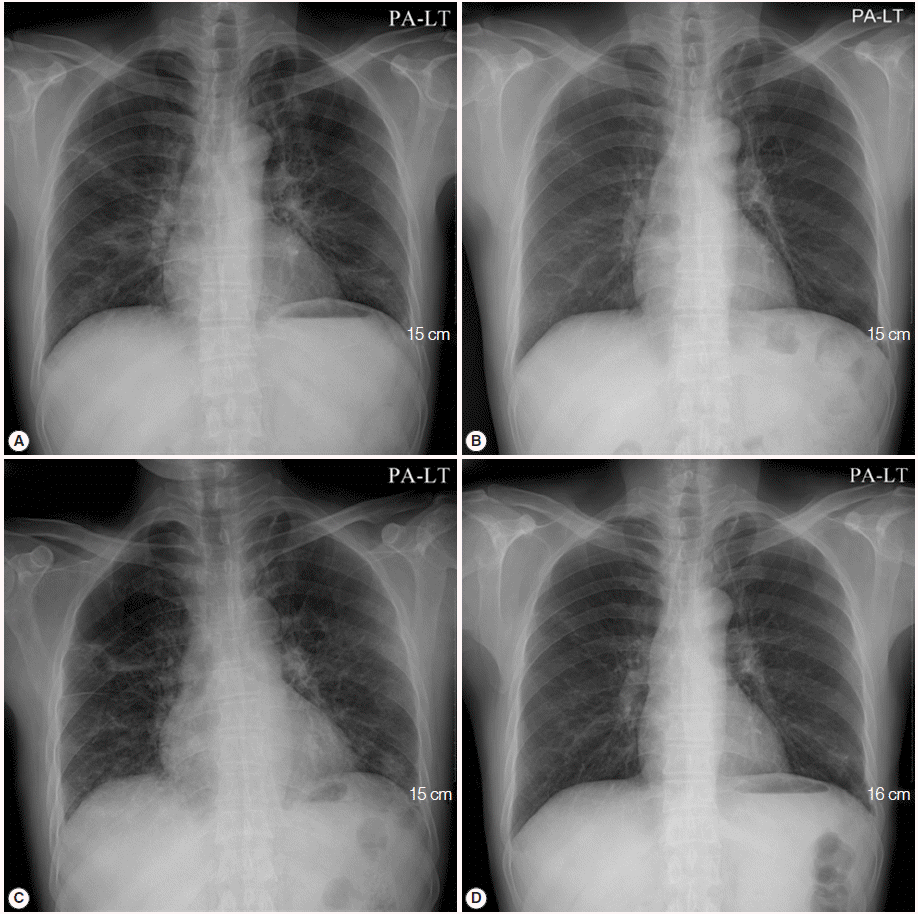
Fig. 2.Initial chest CT revealed multifocal, peribronchial patchy ground-glass opacities in both lungs with septated cystic lesions (arrow) in the left upper lobe (A), and the right lower lobe (B). A follow-up chest CT revealed the aggravation of multifocal, peribronchial ground-glass opacity, and septated cystic lesions (arrows) in both upper lungs (C), and the newly appeared consolidation (arrow) in the left lower lobe (D).

Fig. 3.Photomicrography of Pneumocystis jirovecii pneumonia. (A) Hematoxylin and eosin staining shows eosinophilic frothy exudates in alveolar spaces accompanied by mild interstitial inflammation (H&E, ×200). (B) Grocott-Gomori’s methenamine silver (GMS) stain visualized many cystic- and trophic-form organisms (arrows) in alveolar exudate, consistent with Pneumocystis jirovecii infection (GMS, ×400).
References
- 1. Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002;34:1098-1107.
- 2. Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep 2004;53:1-112.
- 3. Patterson JH, Lindsey IL, Edwards ES, Logan WD Jr. Pneumocystis carinii pneumonia and altered host resistance: treatment of one patient with pentamidine isethionate. Pediatrics 1966;38:388-397.
- 4. Medina I, Mills J, Leoung G, Hopewell PC, Lee B, Modin G, Benowitz N, Wofsy CB. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med 1990;323:776-782.
- 5. Meshnick SR. Drug-resistant Pneumocystis carinii. Lancet 1999;354:1318-1319.
- 6. Mei Q, Gurunathan S, Masur H, Kovacs JA. Failure of co-trimoxazole in Pneumocystis carinii infection and mutations in dihydropteroate synthase gene. Lancet 1998;351:1631-1632.
- 7. Zingale A, Carrera P, Lazzarin A, Scarpellini P. Detection of Pneumocystis carinii and characterization of mutations associated with sulfa resistance in bronchoalveolar lavage samples from human immunodeficiency virus-infected subjects. J Clin Microbiol 2003;41:2709-2712.
- 8. Nahimana A, Rabodonirina M, Helweg-Larsen J, Meneau I, Francioli P, Bille J, Hauser PM. Sulfa resistance and dihydropteroate synthase mutants in recurrent Pneumocystis carinii pneumonia. Emerg Infect Dis 2003;9:864-867.
- 9. Meneau I, Sanglard D, Bille J, Hauser PM. Pneumocystis jirovecii dihydropteroate synthase polymorphisms confer resistance to sulfadoxine and sulfanilamide in Saccharomyces cerevisiae. Antimicrob Agents Chemother 2004;48:2610-2616.
- 10. Iliades P, Meshnick SR, Macreadie IG. Dihydropteroate synthase mutations in Pneumocystis jirovecii can affect sulfamethoxazole resistance in a Saccharomyces cerevisiae model. Antimicrob Agents Chemother 2004;48:2617-2623.
- 11. Safrin S, Finkelstein DM, Feinberg J, Frame P, Simpson G, Wu A, Cheung T, Soeiro R, Hojczyk P, Black JR. Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. Ann Intern Med 1996;124:792-802.
- 12. Helweg-Larsen J, Benfield TL, Eugen-Olsen J, Lundgren JD, Lundgren B. Effects of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of AIDS-associated P. carinii pneumonia. Lancet 1999;354:1347-1351.
- 13. Tasaka S, Tokuda H. Recent advances in the diagnosis of Pneumocystis jirovecii pneumonia in HIV-infected adults. Expert Opin Med Diagn 2013;7:85-97.
- 14. Kim HW, Kim MG, Woo YS, Boo CS, Jo SK, Kim HK, Jo WY, Lee KG, Kim HO, Oh CR, Kim JH. A case of Pneumocystis jirovecii pneumonia following CMV duodenitis in a kidney transplant patient. Korean J Nephrol 2008;27:631-637.
- 15. Jung JY, Rhee KH, Koo DH, Park IN, Shim TS. Pneumocystis jirovecii pneumonia mimicking miliary tuberculosis in a kidney transplanted patient. Tubercul Respir Dis 2009;67:127-130.
- 16. Kim TY, Nam WJ, Kim SM, Shin JH, Lee KE, Kim SH, Oh DJ, Yu SH. Pneumonia caused by fungus, Pneumocystis jirovecii and cytomegalovirus coinfection in patient with renal transplantation: a case report. J Korean Soc Transplant 2009;23:161-165.
- 17. Branson BM, Stekler JD. Detection of acute HIV infection: we can't close the window. J Infect Dis 2012;205:521-524.
- 18. Owen SM. Testing for acute HIV infection: implications for treatment as prevention. Curr Opin HIV AIDS 2012;7:125-130.
- 19. Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis 2010;202(suppl 2):S270-S277.
- 20. Tyagi AK, Mirdha BR, Guleria R, Mohan A, Luthra K, Singh UB. Study of dihydropteroate synthase (DHPS) gene mutations among isolates of Pneumocystis jirovecii. Indian J Med Res 2008;128:734-739.
- 21. Castro M. Treatment and prophylaxis of Pneumocystis carinii pneumonia. Semin Respir Infect 1998;13:296-303.
- 22. Lane BR, Ast JC, Hossler PA, Mindell DP, Bartlett MS, Smith JW, Meshnick SR. Dihydropteroate synthase polymorphisms in Pneumocystis carinii. J Infect Dis 1997;175:482-485.
- 23. Kazanjian P, Locke AB, Hossler PA, Lane BR, Bartlett MS, Smith JW, Cannon M, Meshnick SR. Pneumocystis carinii mutations associated with sulfa and sulfone prophylaxis failures in AIDS patients. AIDS 1998;12:873-878.
- 24. Takahashi T, Hosoya N, Endo T, Nakamura T, Sakashita H, Kimura K, Ohnishi K, Nakamura Y, Iwamoto A. Relationship between mutations in dihydropteroate synthase of Pneumocystis carinii f. sp. hominis isolates in Japan and resistance to sulfonamide therapy. J Clin Microbiol 2000;38:3161-3164.
- 25. Iliades P, Meshnick SR, Macreadie IG. Mutations in the Pneumocystis jirovecii DHPS gene confer cross-resistance to sulfa drugs. Antimicrob Agents Chemother 2005;49:741-748.
- 26. Iliades P, Meshnick SR, Macreadie IG. Analysis of Pneumocystis jirovecii DHPS alleles implicated in sulfamethoxazole resistance using an Escherichia coli model system. Microb Drug Resist 2005;11:1-8.
- 27. Moukhlis R, Boyer J, Lacube P, Bolognini J, Roux P, Hennequin C. Linking Pneumocystis jirovecii sulfamethoxazole resistance to the alleles of the DHPS gene using functional complementation in Saccharomyces cerevisiae. Clin Microbiol Infect 2010;16:501-507.
- 28. Queener SF, Cody V, Pace J, Torkelson P, Gangjee A. Trimethoprim resistance of dihydrofolate reductase variants from clinical isolates of Pneumocystis jirovecii. Antimicrob Agents Chemother 2013;57:4990-4998.