Abstract
Giardia is a major public health concern and considered as reemerging in industrialized countries. The present study investigated the prevalence of giardiosis in 202 sheltered dogs using PCR. The infection rate was 33.2% (67/202); Gyeongsangbuk-do and Daejeon showed 25.7% (39/152, P<0.0001) and 56% (28/50), respectively. The prevalence of infected female dogs (46.7%, P<0.001) was higher than in male dogs (21.8%). A higher prevalence (43.5%, P<0.0001) was observed in mixed breed dogs than purebred (14.1%). Although most of the fecal samples collected were from dogs of ≥1 year of age which showed only 27.4% positive rate, 61.8% (P<0.001) of the total samples collected from young animals (<1 year of age) were positive for G. intestinalis. A significantly higher prevalence in symptomatic dogs (60.8%, P<0.0001) was observed than in asymptomatic dogs (23.8%). Furthermore, the analysis of nucleotide sequences of the samples revealed that G. intestinalis Assemblages A and C were found in the feces of dogs from Gyeongsangbuk-do and Daejeon. Since G. intestinalis Assemblage A has been known to infect humans, our results suggest that dogs can act as an important reservoir of giardiosis in Korea. Hence, hygienic management should be given to prevent possible transmission to humans.
-
Key words: Giardia intestinalis, dog, PCR
Giardia is a major public health concern of water utilities worldwide, and is considered as reemerging in industrialized countries due to its role in diarrheal, and in water and foodborne outbreaks [
1-
3]. It is an aerotolerant enteric protozoan parasite which can cause giardiosis, infecting a wide range of vertebrate hosts including humans characterized by diarrhea, bloating, abdominal cramps, weight loss, and malabsorption [
4,
5]. It is one of the most common enteric parasites of companion animals and livestock, and has been observed as one of the most frequently observed parasites infecting domestic dogs [
2,
6]. A recent study reported that during the period of 2004-2010, 70 out of 199 published protozoal disease outbreaks were caused by
Giardia [
7].
G. intestinalis, also known as
G. lamblia and
G. duodenalis, is the only species that has been recovered from both humans and dogs, hence regarded as potentially zoonotic [
8]. Furthermore, it has several genotypes such as assemblages C and D, which are the most commonly found in dogs, and assemblages A and B, which are the most commonly found in humans and have also been isolated in dogs, suggesting a potential source of infection to humans [
9].
Microscopic fecal examination has been the routine detection method of
G. intestinalis in dogs; however, this method is considered limited particularly in the presence of concurrent infections with multiple parasite species and insufficient to detect the parasites and many cases of
Giardia infections go undetected [
10]. Molecular diagnostic tools are thought to provide higher sensitivity and specificity compared to either microscopic or immunological assays and have been used recently in characterizing the epidemiology of human giardiosis, and in assessing the taxonomy, zoonotic potential, and transmission of giardiosis in humans and animals [
2,
5,
11]. PCR assay is a sensitive and specific method for detecting microorganisms by amplifying the target nucleic acids, and molecular detection methods based on this assay have been shown to be highly rapid, sensitive, and specific for detection of
G. intestinalis cysts [
12]. Consequently, the present study examined 202 canine fecal samples to determine the presence of giardiosis in dogs from animal shelters in Korea using PCR.
A total of 202 canine fecal samples collected from each animal shelter in Gyeongsangbuk-do (Province) (n=152) and Daejeon city (n=50) from February 2013 to February 2015 were used in the study. All cases were grouped according to gender, breed (mixed/pure), age group (≥1 year/<1 year), and health status (symptomatic/asymptomatic) (
Table 1). Health status was based on main clinical manifestations of giardiosis which can range from asymptomatic (without diarrhea), to acute or chronic diarrheal disease (symptomatic) [
13]. Moreover, the positive genomic DNA of
G. intestinalis (ATCC no. 30957) was kindly provided by Dr. S. E. Lee in Division of Malaria and Parasite Diseases, Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Korea. Total genomic DNA extraction of each fecal sample was performed using the QIAamp DNA Stool Mini Kit in accordance with manufacturer’s instruction (Qiagen, Hilden, Germany).
A set of primers to amplify a 384 bp fragment of
β-giardin gene of
G. intestinalis was amplified using the forward primer (5ʹ CAT AAC GAC GCC ATC GCG GCT CTC AGG AA 3ʹ) and the reverse primer (5ʹ GAG GCC GCC CTG GAT CTT CGA GAC GAC 3ʹ) as previously described [
14]. The primers were designed based from the complete
β-giardin gene of the Portland-1 isolate (GenBank no. M36728). The amplification of PCR products was performed in a MyGenie96 Thermal Block thermal cycler (Bioneer, Seoul, Korea), resolved using 1.5% Tris-acetate-EDTA (TAE) in an electrophoresis chamber containing 0.5x TAE buffer, run at 100 V for 25 min or until the dye indicator reached the target lane, stained with ethidium bromide, and viewed under an ultraviolet trans-illuminator machine. The PCR products with the target band size were purified using MEGAquick-spin™ Total Fragment DNA Purification Kit (Intron Biotechnology, Seoul, Korea). The representative
β-giardin gene from 3 fecal samples and a positive control were submitted for sequencing (
Fig. 1). The DNA sequences were searched using BLASTN search algorithms (
http://www.ncbi.nml.nih.gov/blast/) (
Table 2) and aligned using CLUSTAL W2 software (
www.ebi.ac.uk/Tools/msa/clustalw2/) (
Fig. 2).
Giardins are a family of structural proteins of approximately 29-38 kDa in size and the advantage of using
giardin genes as targets for molecular detection of
Giardia cysts is that they are considered unique to this parasite [
14]. The prevalence according to the place of origin, gender, breed, age groups, and health status was compared using the chi-square test of Statistical Package for the Social Sciences (SPSS, IBM, Redmond, Washington, USA).
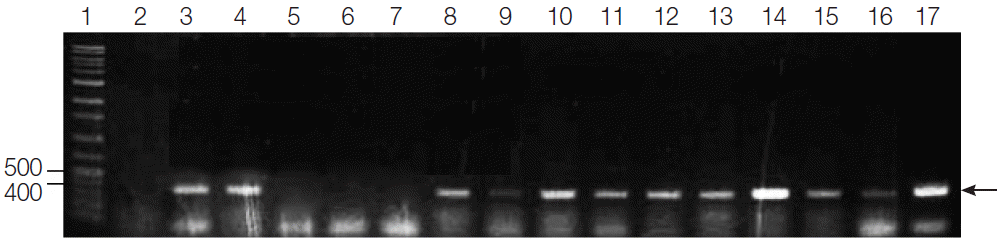
In the present study, the fecal samples collected from stray dogs in animal shelters of Gyeongsangbuk-do and Daejeon showed 25.7% (39 out of 152) and 56% (28 out of 50), respectively, showing that the positive rate was significantly higher in Daejeon (
P<0.0001) as compared to Gyeongsangbuk-do. Sixty-seven out of 202 canine fecal specimens sampled yielded the PCR products of the expected 384 bp fragment of
β-giardin gene of
G. intestinalis, showing an overall
Giardia infection rate of 33.2% (67/202) (
Fig. 1). Three of these positive samples and a positive control were sequenced, and all of the nucleotide sequences obtained belonged to
β-giardin sequences of
G. intestinalis based on BLASTN search algorithms of the GenBank database (
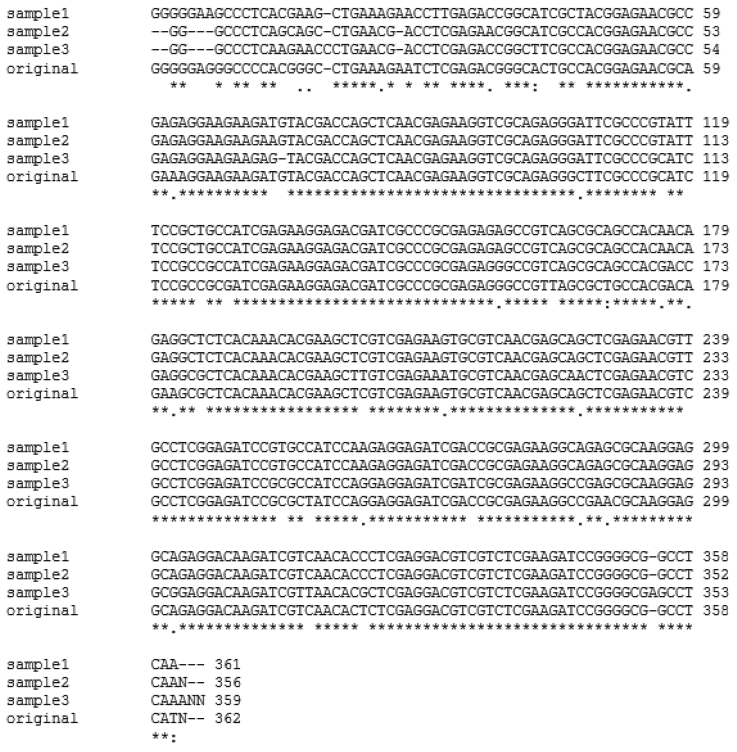
Table 2). The multiple sequence alignment using Clustal W2 software and sequence analysis using BLASTN search algorithms revealed that
β-giardin gene from the 3 representative positive samples shared 91-92% identity with that of the positive sample (
Fig. 2;
Table 2). The positive control showed 98% homology to
G. intestinalis Assemblage A subtype AI, 2 of the positive samples showed 98% homology to
G. intestinalis Assemblage A, and 1 positive sample showed 99% homology to
G. intestinalis isolate BRAdogD15/C2 which was reported by Paz e Silva et al. [
15] to have a 100% homology to
G. intestinalis Assemblage C (
Table 2).
G. intestinalis Assemblages A and B have a wide range of hosts which include humans and animals such as livestock, dogs, cats, and other wild animals, while Assemblages C and D are dog-specific genotypes [
16]. Since
G. intestinalis Assemblage A has been found in canine fecal samples collected in this study which were similar to those that have been reported [
17], the results indicated potential transmission of
Giardia from dogs to humans or vice versa.
In a study done by Huber et al. [
18], neither gender nor age in clinically healthy dogs has a correlation with
Giardia positive rate although a slightly higher prevalence in male dogs but not significant was reported by Liu et al. [
19]. In contrast, the present study showed a significantly higher prevalence in female dogs (46.7%) than in male dogs (21.8%), which is in agreement with those reported by Pallant et al. [
20]. Most of the samples tested were obtained from dogs of ≥1 year of age (83.2%) but only 27.4% were infected with
Giardia as compared to the population of younger animals which showed a significant positive rate of 61.8%. This result was similar to those reported by Jacobs et al. [
21] where fecal samples from dogs under 1 year of age comprised approximately 73% of
Giardia cases. Mochizuki et al. [
22] reported that the rate of
Giardia-infected dogs was almost equal in fecal samples obtained from symptomatic or asymptomatic dogs. However, the results in our study showed that the prevalence of dogs with diarrhea was significantly higher than those without diarrhea which was similar to those reported by Liu et al. [
19].
In conclusion, G. intestinalis Assemblages A and C were found in the feces of dogs from animal shelters in Gyeongsangbuk-do and Daejeon. The prevalence of G. intestinalis infection was observed to be higher in females, mixed-breed, <1 year of age, and symptomatic dogs. Therefore, the role of dogs as a potential source of human giardiasis, though can be considered as minor, could not be excluded, and increasing awareness of this possible transmission to patients or clients is important. Further investigation is recommended to confirm the zoonotic potential of G. intestinalis detected in fecal samples from dogs in Korea.
Notes
-
The authors of this paper declare no conflict of interest.
Fig. 1.PCR amplification of G. intestinalis β-giardin genes from fecal samples of dogs from animal shelters in Gyeongsangbuk-do and Daejeon. Lane 1: 1,000 bp molecular weight marker, Lanes 2, 5-7: negative samples, Lanes 3-4, 8-16: positive samples, and Lane 17: positive control (G. intestinalis, arrow).

Fig. 2.Multiple alignments of representative β-giardin genes from fecal samples of dogs from animal shelters in Gyeongsangbuk-do and Daejeon (samples 1, 2, and 3) using CLUSTAL W2 software in comparison to the positive control (original). Asterisks (*) indicate homology among all samples.
Table 1.PCR results and statistical analysis according to origin, gender, breed, age, and health status of dogs
Table 1.
|
Historical factor |
Sample size |
No. of positive |
Positive rate (%) |
x2
|
P*
|
|
Origin |
|
|
|
|
|
|
Daejeon |
50 |
28 |
56 |
15.63 |
0.0001 |
|
Gyeongbuk |
152 |
39 |
25.7 |
|
|
|
Sex |
|
|
|
14.04 |
0.0002 |
|
Male |
110 |
24 |
21.8 |
|
|
|
Female |
92 |
43 |
46.7 |
|
|
|
Breed |
|
|
|
17.99 |
0.0000 |
|
Mixed |
131 |
57 |
43.5 |
|
|
|
Pure |
71 |
10 |
14.1 |
|
|
|
Age |
|
|
|
15.08 |
0.0001 |
|
≥ 1 year |
168 |
46 |
27.4 |
|
|
|
< 1 year |
34 |
21 |
61.8 |
|
|
|
Health status |
|
|
|
23.47 |
0.0000 |
|
Symptomatic |
51 |
31 |
60.8 |
|
|
|
Asymptomatic |
151 |
36 |
23.8 |
|
|
|
Total |
202 |
67 |
33.2 |
|
|
Table 2.Analysis and comparison of representative β-giardin gene from 3 canine fecal samples
Table 2.
|
Sample |
Homology to G. intestinalis(%) |
Homology to positive control (%) |
Description (GenBank accession no.) |
|
Positive control |
98 |
- |
G. intestinalis assemblage AI β-giardin gene (KF963547) |
|
Sample 1 |
98 |
91 |
G. intestinalis isolate A21 β-giardin gene (AY545647) |
|
Sample 2 |
98 |
92 |
G. intestinalis isolate A21 β-giardin gene (AY545647) |
|
Sample 3 |
99 |
92 |
G. intestinalis isolate BRAdogD15/BRAdogC2 β-giardin gene (JF422719) |
References
- 1. Nikaeen M, Mesdaghinia AR, Jeddi Tehrani M, Rezaian M, Vaezi F. Sensitive detection of Giardia cysts by polymerase chain reaction (PCR). Iran J Public Health 2003;32:15-18.
- 2. Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA, Xiao L. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis 2003;9:1444-1452.
- 3. Read CM, Monis PT, Thompson RCA. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect Genet Evol 2004;4:125-130.
- 4. Ghosh S, Debnath A, Sil A, De S, Chattopadhyay DJ, Das P. PCR detection of Giardia lamblia in stool: targeting intergenic spacer region of multicopy rRNA gene. Mol Cell Probes 2000;14:181-189.
- 5. Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 2011;24:110-140.
- 6. Wang A, Ruch-Gallie R, Scorza V, Lin P, Lappin MR. Prevalence of Giardia and Cryptosporidium species in dog park attending dogs compared to non-dog park attending dogs in one region of Colorado. Vet Parasitol 2012;184:335-340.
- 7. Yang R, Jacobson C, Gardner G, Carmichael I, Campbell AJD, Ryan U. Development of a quantitative PCR (qPCR) for Giardia and analysis of the prevalence, cyst shedding and genotypes of Giardia present in sheep across four states in Australia. Exp Parasitol 2014;37:46-52.
- 8. Mohamed AS, Glickman LT, Camp JWJ, Lund E, Moore GE. Prevalence and risk factors for Giardia spp. infection in a large national sample of pet dogs visiting veterinary hospitals in the United States (2003–2009). Vet Parasitol 2013;195:35-41.
- 9. Uehlinger FD, Greenwood SJ, McClure T, Conboy G, O’Handley R, Barkema HW. Zoonotic potential of Giardia duodenalis and Cryptosporidium spp. and prevalence of intestinal parasites in young dogs from different populations on Prince Edward Island, Canada. Vet Parasitol 2013;196:509-514.
- 10. Li J, Wang P, Zhang A, Zhang P, Li G. Sensitive and rapid detection of Giardia lamblia infection in pet dogs using loop-mediated isothermal amplification. Korean J Parasitol 2013;51:237-241.
- 11. Ryan U, Cacciò SM. Zoonotic potential of Giardia. Int J Parasitol 2013;43:943-956.
- 12. Jung HH, Lee K. Detection of Giardia lambia cysts by polymerase chain reaction (PCR) coupled with immunomagnetic separation (IMS). J Ind Eng Chem 2004;10:102-106.
- 13. Halliez MCM, Buret AG. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol 2013;19:8974-8985.
- 14. Cacciò SM, Giacomo MD, Pozio E. Sequence analysis of the beta-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int J Parasitol 2002;32:1023-1030.
- 15. Paz e Silva FM, Monobe MM, Lopes RS, Araujo JP Jr. Molecular characterization of Giardia duodenalis in dogs from Brazil. Parasitol Res 2012;110:325-334.
- 16. Caccio SM, Thompson RC, McLauchlin J, Smith HV. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol 2005;21:430-437.
- 17. Ballweber LR, Xiao L, Bowman DD, Kahn G, Cama VA. Giardiasis in dogs and cats: update on epidemiology and public health significance. Trends Parasitol 2010;26:180-189.
- 18. Huber F, Bomfim TC, Gomes RS. Comparison between natural infection by Cryptosporidium spp., Giardia spp. in dogs in two living situations in the West Zone of the municipality of Rio de Janeiro. Vet Parasitol 2005;130:69-72.
- 19. Liu J, Lee SE, Song KH. Prevalence of canine giardiosis in South Korea. Res Vet Sci 2007;84:416-418.
- 20. Pallant L, Barutzki D, Schaper R, Thompson RCA. The epidemiology of infections with Giardia species and genotypes in well cared for dogs and cats in Germany. Parasit Vectors 2015;8:2.
- 21. Jacobs SR, Forrester CPR, Yang J. A survey of the prevalence of Giardia in dogs presented to Canadian veterinary practices. Can Vet J 2001;42:45-46.
- 22. Mochizuki M, Hashimoto M, Ishida T. Recent epidemiological status of canine viral enteric infections and Giardia infection in Japan. J Vet Med Sci 2001;63:573-575.