Abstract
Theileria annulata is a tick-borne intracellular protozoan parasite that causes tropical theileriosis, a fatal bovine lymphoproliferative disease. The parasite predominantly invades bovine B lymphocytes and macrophages and induces host cell transformation by a mechanism that is not fully comprehended. Analysis of signaling pathways by quantitative real-time PCR (qPCR) could be a highly efficient means to understand this transformation mechanism. However, accurate analysis of qPCR data relies on selection of appropriate reference genes for normalization, yet few papers on T. annulata contain evidence of reference gene validation. We therefore used the geNorm and NormFinder programs to evaluate the stability of 5 candidate reference genes; 18S rRNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ACTB (β-actin), PRKG1 (protein kinase cGMP-dependent, type I) and TATA box binding protein (TBP). The results showed that 18S rRNA was the reference gene most stably expressed in bovine PBMCs transformed and non-transformed with T. annulata, followed by GAPDH and TBP. While 18S rRNA and GAPDH were the best combination, these 2 genes were chosen as references to study signaling pathways involved in the transformation mechanism of T. annulata.
-
Key words: Theileria annulata, quantitative real-time PCR (qPCR), reference gene, signaling pathway
INTRODUCTION
From the first report in 1993 describing real-time PCR detection [
1], quantitative real-time PCR (qPCR), as the “gold standard” technology, has been used to quantify nucleic acids in many fields of biological research [
2]. It has advantages in high sensitivity, outstanding accuracy, and a broad dynamic range compared with reverse transcription PCR (RT-PCR) [
3]. It can quantify both the absolute and relative amounts of a gene [
4,
5]. In the absolute quantification method, the copy number of the gene is calculated by a standard curve generated using a target gene with known copy number. In relative quantification, expression level is measured relative to a reference gene whose expression is presumed to be stable under different experimental conditions [
6,
7]. The use of the right reference genes can correct deviations caused by variations in template quality, experimental conditions, or amplification efficiency. However, no single reference gene can be used under all experiment conditions [
8]. Therefore, in order to acquire perfect experimental results, the choice of reference gene is critically important.
In recent years, increasing numbers of reference genes are being screened, such as 18S rRNA [
9], GAPDH [
10], ACTB [
11], PRKG1 [
12], TBP [
12], and so on. However, previous research has shown that the expression of many reference genes varied with tissue, developmental stage, and experimental conditions [
13,
14]. Thus, demands were placed on researchers to determine suitable reference genes according to different experimental material. The Microsoft Excel-based software programs, geNorm (ver. 3.5) [
15], and NormFinder (Ver 0.953) [
16] can be used to assess candidates of reference genes. For geNorm and NormFinder, data were analyzed by transforming raw cycle threshold (Ct) values into relative quantities using the delta-Ct method.
Theileria annulata is a tick-borne intracellular protozoan parasite that is the causal agents of tropical theileriosis [
17]. When a bovid host is bitten by ticks,
T. annulata sporozoites rapidly invade B lymphocytes and macrophages, and then differentiate into schizonts [
18]. The infected cells acquire characteristics of transformed cells, and induce continuous proliferation of infected cells and parasites in vitro [
19]. These parasitized cells are considered immortalized. To date,
T. annulata and the closely related parasites
Theileria parva and
Theileria lestoquardi are the only known eukaryotes to induce uncontrolled host cell proliferation. More interestingly, this parasite-induced transformation is reversible by killing the parasite with a specific drug (buparvaquone) with no effect on non-infected host lymphocytes [
20]. This provides us with a model for studying the pathogenesis of immortalized cells.
A valid method to understand the transformation mechanism of
T. annulata transformed host cells could be to explore signal transduction pathways of the immortalized mechanism in transformed and non-transformed cells by relative qPCR analysis. Nevertheless, no reference genes have ever been evaluated in
T. annulata-transformed bovine cell lines. Therefore, 5 reference genes, 18S rRNA, ACTB, GAPDH, TBP, and PRKG1 (
Table 1), that are known to be stably expressed in other organisms in previous studies, were selected and evaluated for stability using 2 software programs: geNorm and NormFinder. These programs can provide information for selecting suitable reference genes and normalizing gene expression in
T. annulata-transformed and non-transformed bovine host cells.
MATERIALS AND METHODS
Ethical approval
Ethical approval was given by Lanzhou Veterinary Research Institute Ethics Committee, CAAS (no. LVRIAEC2013-010). The use of experiment samples was approved by the Animal Ethics Procedures and Guideline of China.
Cell line and cell culture
Establishment and maintenance of
T. annulata Inner Mongolia (TaIM) transformed bovine cell line was performed as previously described [
21]. Cells were cultured at 37˚C in an atmosphere of 5% CO 2 using RPMI 1640 (Gibco-BRL, Shanghai, China), 10% heat-inactivated fetal bovine serum (Invitrogen, Paisley, UK), 100 U/ml penicillin (Sigma-Aldrich, Shanghai, China), and 100 mg/ml streptomycin (Sigma-Aldrich). Cells were passaged every 2-3 days.
Calves, 0.5 to 1 year old, were purchased from Lintao County, Gansu province, China. Blood samples from calves were tested for the presence of
Theileria using microscopic examination and a PCR assay using 989/990 primers [
22]. Only those that tested negative were used in this experiment. PBMCs of the calves were isolated using Ficoll-Hypaque solution (Haoyang Biological Manufacture Co., Ltd, Tianjin, China) by density gradient centrifugation as described [
23]. PBMCs were cultured in RPMI 1640 complete medium, i.e., RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin (Sigma-Aldrich) and 100 mg/ml streptomycin (Sigma-Aldrich). After 3 days, cells were collected by centrifugation and lysed in 1 ml of TRIZOL reagent (Takara, Dalian, China)/5×10
6 cells by repetitive pipetting. The cell lysates were stored at -80˚C until needed.
A total of 5 candidate genes were selected from previous reports. These have been selected as reference genes in cattle and lymphoid malignancies and included 18S rRNA, ACTB, GAPDH, PRKG1, and TBP. The Primer Premier 5 software was used to design the primers. All the primers were synthesized in Sangon Biotech (Shanghai, China). The full gene name, accession ID, primers, product size, and melting temperature are shown in
Table 1.
Total RNA was extracted from 5×106 cells of T. annulata transformed cell lines and PBMCs of uninfected calves using TRIZOL reagent (Takara) according to the manufacturer’s instructions. Each sample was represented by 3 biological replicates. RNA was treated with DNase I (Invitrogen) to remove contaminating genomic DNA. The purity and concentration of the total RNA were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware, USA). Integrity of total RNA samples was also assessed by 1% ethidium bromide agarose gel electrophoresis. First-strand cDNA synthesis was performed with Random hexamer primed Reverse Transcription Superscript III (Invitrogen) according to the manufacturer’s instructions. cDNA was quantified using a NanoDrop 2000 spectrophotometer. All cDNA samples were diluted to 0.5 µg/μl using DEPC-treated water and stored at 80˚C until needed for qPCR analysis.
Quantitative real-time PCR
Samples of cDNA from
T. annulata-transformed cells and PBMCs of uninfected calves were serially diluted (10
-1 to 10
-6 dilution) and used as a template. The qPCR was performed using a model MX3000 P real-time PCR machine (Stratagene, La Jolla, California, USA). The PCR mixture (20 μl total volume) consisted of 0.8 μl forward/reverse PCR primers (10 μM), 2 μl diluted cDNA, 0.4 μl ROX Reference Dye II, 6 μl nuclease-free water, and 10 μl SYBR Premix Ex Taq II (Takara). PCR cycling conditions were as follows: 95˚C for 30 sec, 45 cycles of 95˚C for 5 sec, 56˚C for 34 sec. Each reaction was performed in triplicate, and the mean Ct values were used for further analysis. Expression levels of 5 candidate reference genes were analyzed using Prism software. The linear relationship between the Ct values and -log
10(concentration) was used to generate a standard curve. The PCR amplification efficiency (E) was determined based on the slopes of the standard curves: E (%)=(10
1/|slope| − 1)×100% [
24]. The correlation coefficient (R
2) was calculated by the linear regression equation of the Ct and the negative logarithmic of the template concentration.
The cDNA samples of
T. annulata transformed cells and PBMCs of uninfected calves (10
-1-10
-6) were used as the template to evaluate the stability of candidate reference genes (18S rRNA, GAPDH, ACTB, TBP, and PRKG1). Average Ct values from 3 replicates were exported to Microsoft Excel 2007 and analyzed using geNorm (version 3.5) and NormFinder (version 0.953). For geNorm and NormFinder analysis, raw Ct values were transformed to relative quantities using the 2
-delta Ct equation [
25], where delta Ct=Ct sample-Ct min. Ct sample is the raw Ct value for each gene, and Ct min is the minimal raw Ct value over a range of samples.
RESULTS
The quality of total RNAs
The ratio OD 260/280 and concentration of total RNA obtained from T. annulata-transformed cells were 2.00 and 2,500 ng/μl, respectively. The corresponding values for PBMCs of uninfected calves were 1.92 and 2,300 ng/μl. The ratio of 28S and 18S rRNA products was roughly 2:1 by electrophoresis on 1% (w/v) agarose gels, indicating that the degradation of RNA was minimal. Therefore, the purity and integrity of all the RNA samples were appropriate for synthesizing the cDNA template.
RT-qPCR amplification of reference genes
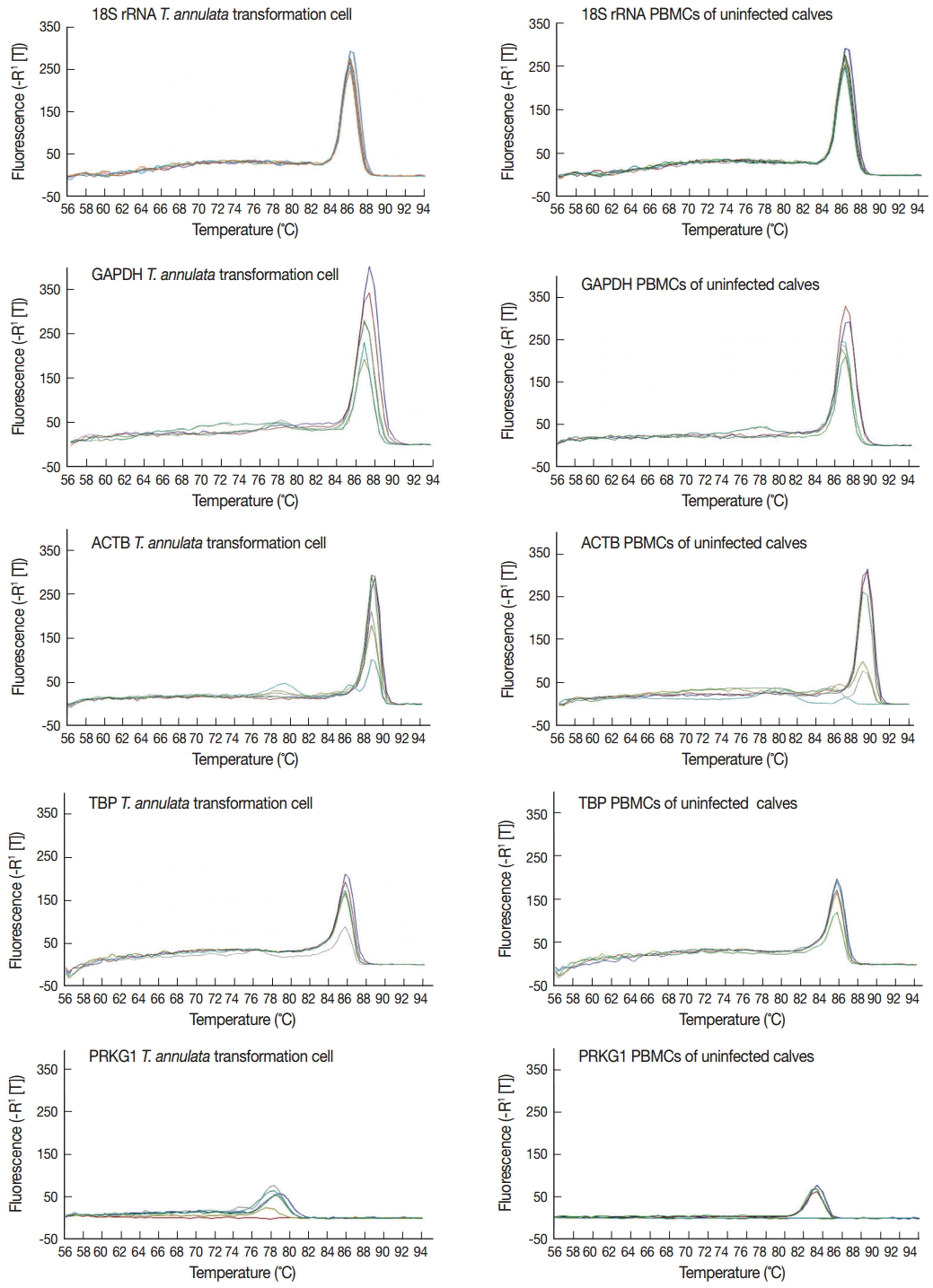
The specificity of the primers was evaluated by the dissociation curve of the RT-qPCR in serially diluted cDNA samples of
T. annulata-transformed cells and PBMCs of uninfected calves (10
-1-10
-6). The results showed that all reference genes produced a single peak that confirmed a unique amplification (
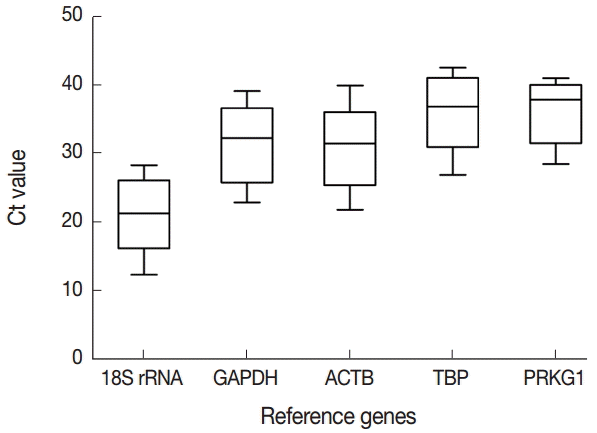
Fig. 1). Expression levels of 5 candidate reference genes were analyzed using Prism software. Transcript abundance was higher in 18S rRNA than in all the other candidate reference genes (
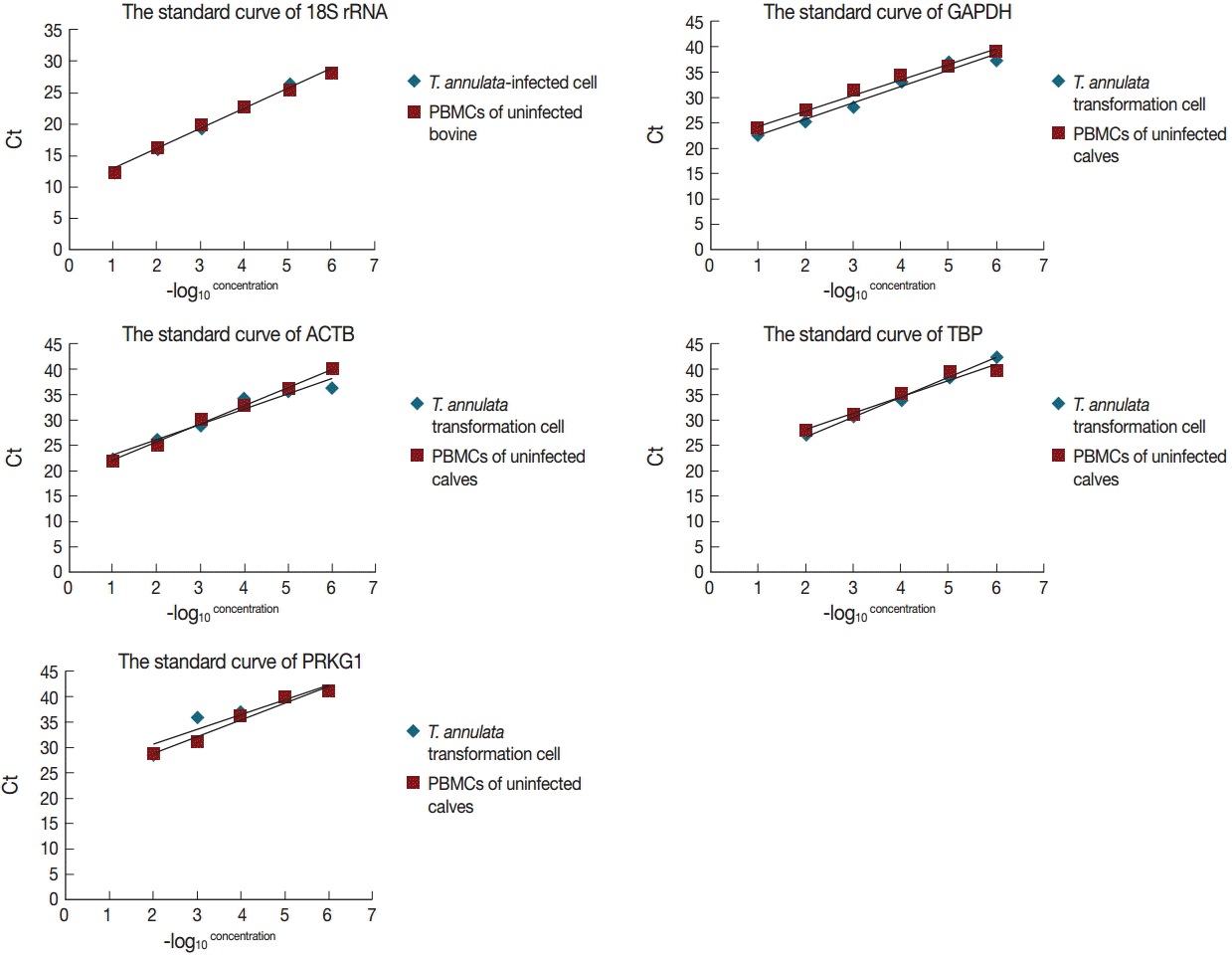
Fig. 2). The standard curve method was used to estimate amplification efficiency and parallelity of reference genes from 10-fold serially diluted cDNA samples of bovine PBMCs transformed and non-transformed with
T. annulata. The results indicated that only 18S rRNA and GAPDH exhibited good linear relationships between the Ct values and -log
10 (concentration) in a variety of diluted concentrations (
Fig. 3). The amplification efficiencies of 5 reference genes were determined and ranged between 81.2% and 120.1%, and correlation coefficients (R
2) ranged from 0.8689 to 0.9979 (
Table 1).
GeNorm analysis
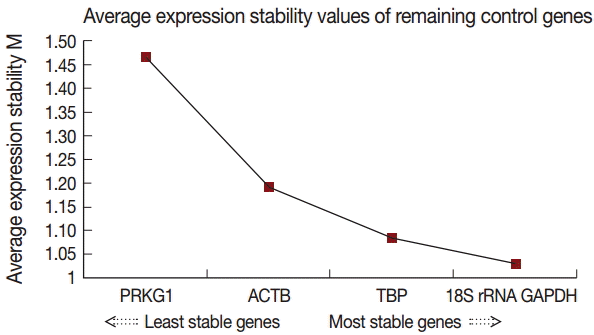
GeNorm was used to evaluate the stability of candidate reference genes as previously described by Vandesompele et al. [
15]. Our results showed that the 18S rRNA gene had the highest stability, followed by GAPDH, TBP, ACTB, and PRKG1. The individual M (average expression stability) values were 1.207, 1.332, 1.423, 1.500, and 1.885, respectively (
Table 2). The geNorm analysis determined that 18S rRNA and GAPDH were the best combination (
Fig. 4).
NormFinder analysis
The stability of the reference genes can also be evaluated by the NormFinder program. This program is based on a statistical and mathematical model. The stability value of reference genes was calculated by intra- and intergroup variation. The most stably expressed gene has the lowest expression stability value. The calculated stability values of the 5 candidate reference genes are listed in
Table 2. According to NormFinder analysis results, the most stable reference gene was 18S rRNA, with an M value of 0.206, followed by TBP, ACTB, GAPDH, and PRKG1. The best combination of 2 genes was 18S rRNA and GAPDH with a stability value of 0.1560 (
Table 2).
The optimal reference genes have been evaluated by data analysis. GeNorm confirmed that 18S rRNA was the most stably expressed gene, and 18S rRNA and GAPDH were the best combination. Normfinder gave the same results (
Table 2). Moreover, by dissociation curve analysis, it was shown that 5 reference genes had better primer specificity. Standard curve analysis results indicated that only 18S rRNA and GAPDH exhibited good linear relationships under various degrees of dilution. On the basis of the above analyses, we proposed that the combination of 18S rRNA and GAPDH could be chosen to analyze the signaling pathways involved in the transformation mechanism of
T. annulata.
DISCUSSION
To our knowledge, this is the first study to validate a set of candidate reference genes for RT-qPCR in bovine PBMCs transformed and non-transformed with
T. annulata. In this report, 5 candidate reference genes were assessed for expression stability using variously diluted cDNA samples of
T. annulata-transformed cells and PBMCs from uninfected calves. Although an accurate and sensitive quantification method for gene expression analysis, the results of qPCR are affected by many factors, including the selection of reference genes. An ideal reference gene should be expressed at a constant level among different samples, and should be unaffected by experimental treatment. To date, 18S rRNA, GAPDH, and ACTB are the most common reference genes used in qPCR, and suitable for most samples [
9]. In addition, Lossos et al. [
12] demonstrated that TBP and PRKG1 were more stable as reference genes with qPCR of lymphoma malignancies. Therefore, 5 candidate reference genes were selected to evaluate their stability in qPCR studies of signaling pathway analysis of the transformation mechanism using samples of
T. annulata-transformed cells and PBMCs of uninfected calves.
In order to obtain reliable experimental results from qPCR, the purity and integrity of all RNA samples are of crucial importance. In this study, the absorbance ratio of each sample at OD 260/280 was close to 2.0. In addition, the integrity of the total RNA was determined by electrophoresis; this showed that the ratio of 28S and 18S rRNA products was roughly 2:1. Also, before synthesizing the cDNA template, RNA was treated with DNase I to remove contaminating genomic DNA. All of these factors have contributed to acquiring reliable results.
The quality of results obtained from qPCR was related to the specificity and efficiencies of the amplification of reference genes. The specificity of the reference genes was evaluated by the dissociation curve of the qPCR. The dissociation curve of the qPCR analysis results showed that all primers produced a single peak with a specific amplification. Beside this, it should exhibit good linear relationships under degrees of various dilutions [
26]. In this study, by analyzing the standard curve, only 18S rRNA and GAPDH exhibited good linear relationships under different degrees of serial dilutions of bovine PBMCs transformed and non-transformed with
T. annulata. The amplification efficiencies of each reference gene were determined through the standard curve from RT-qPCR. The results showed that the amplification efficiencies of 5 reference genes ranged between 81.2% and 120.1%, and the amplification efficiencies of 18S rRNA, GAPDH, and PRKG1 (more than 91%) were higher than those of ACTB and TBP. Only genes with amplification efficiency between 90% and 120% were used for further statistical analysis. Therefore, in view of the results of the specificity and efficiencies of amplification of reference genes, it was considered that 18S rRNA and GAPDH can be used as reference genes in signaling pathway studies of the transformation mechanism of
T. annulata.
In this study, the stability of reference genes was evaluated using geNorm and NormFinder. These 2 programs are commonly used algorithms to determine the stability of reference genes [
15]. The geNorm program uses a pair-wise comparison approach based on the assumption that none of the genes being analyzed are co-regulated, whereas NormFinder uses an intra- and inter-group variation approach to evaluate reference genes, thereby avoiding the influence of gene co-regulation [
27]. Because different programs have different mathematical algorithms, respectively, there may be discrepancies in the results of best reference genes and the stability rankings between them. The results obtained in our study showed a similar phenomenon. The stability rankings of the reference genes in the 2 programs were 18S rRNA, GAPDH, ACTB, TBP, and PRKG1 for geNorm, and 18S rRNA, TBP, ACTB, GAPDH, and PRKG1 for NormFinder. The most stable reference gene was 18S rRNA, and the best combination of 2 genes was 18S rRNA and GAPDH in both analyses.
However, there is debate about 18S rRNA as a stable reference gene. Some researchers believe that 18S rRNA as a control gene reduces the ability and sensitivity to detect differentially expressed genes because of its very high abundance [
12]. Nevertheless, 18S rRNA has repeatedly been used to normalize gene expression in qPCR [
28]. ACTB and GAPDH have been validated as the most stable reference genes in many species [
29,
30], However, several studies have shown different results using these genes as references [
3,
31]. In our study, ACTB had lower efficiencies of amplification in PBMCs of uninfected calves by
T. annulata but GAPDH showed better performance than ACTB in these aspects. Therefore, GAPDH is more suited than ACTB as a reference gene in our research. PRKG1 and TBP are less frequently used as reference genes. However, Lossos et al. [
12] found that PRKG1 and TBP genes were the most suitable ones for RNA quality and quantity control in both B and T cells and tumors.
In our results, the efficiencies of TBP amplification were less than 91% in T. annulata-transformed cells, and PRKG1 had the least abundant mRNA. Therefore, PRKG1 and TBP are not suited as reference genes in this study.
In conclusion, 5 candidate reference genes (18S rRNA, GAPDH, ACTB, TBP, and PRKG1) were comprehensively assessed by dissociation curve, standard curve, geNorm, and Normfinder. The results showed that 18S rRNA and GAPDH could be used in studies to analyze the signaling pathways involved in the transformation mechanism of T. annulata.
Notes
-
The authors declare no conflicts of interest related to this study.
This study was financially supported by the National Basic Science Research Program (973 programs) of China (no. 2015 CB150300), the NSFC (no. 31372432, no. 31402189), the NBCIS CARS-38, ASTIP, the Specific Fund for Sino-Europe Cooperation, and PIROVAC (no. KBBE-3-245145) of European Commission.
Fig. 1.The dissociation curve of the 5 candidate reference genes in serial-diluted cDNA samples of T. annulata-transformed cells and PBMCs of uninfected calves.

Fig. 2.Expression levels of 5 candidate reference genes. The range of Ct values across the T. annulata-transformed cells and PBMCs of uninfected calves are exhibited in the boxplot. Ct values of candidate reference genes were widely distributed between 13 to 42 cycles. The horizontal line inside the box represents the median. The bars below and above the box represent the minimum and maximum values of the datasets, respectively.

Fig. 3.The linear relationships between the Ct values and -log10 (concentration) in 5 reference genes from 10-fold serial-diluted cDNA samples of T. annulata-transformed cells and PBMCs of uninfected calves.
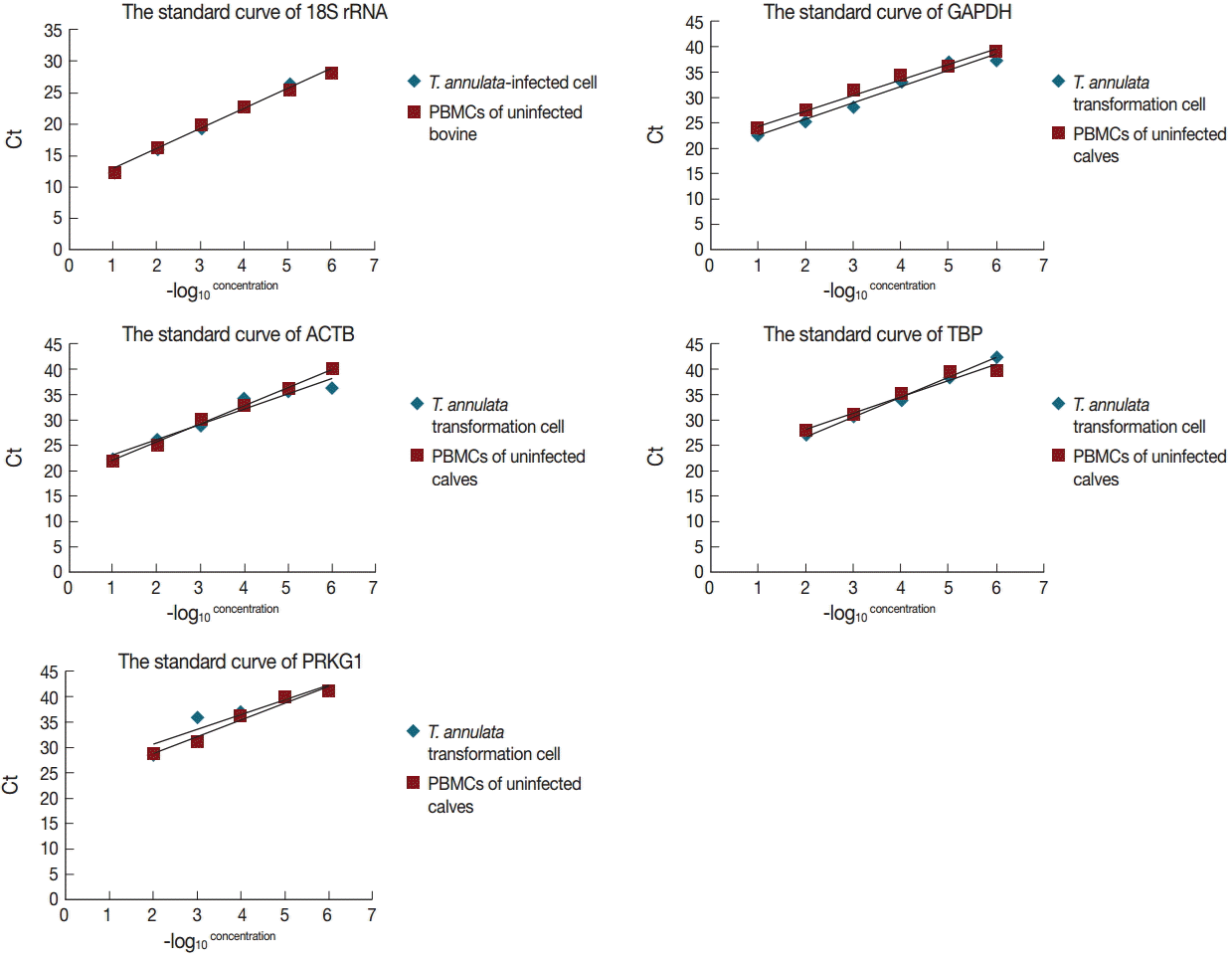
Fig. 4.Gene expression stability of the candidate reference genes analyzed by geNorm.
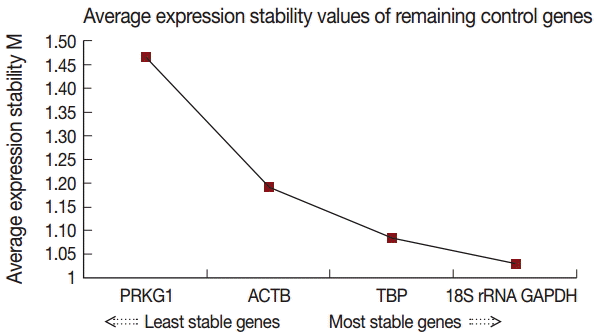
Table 1.Sequence information for 5 selected candidate reference genes
Table 1.
|
Gene symbol |
Full name [mRNA NCBI accession ID] |
Primer sequence (5´-3´) |
Product size (bp) |
Average Tm (˚C) |
Correlation coefficient (R2)
|
Amplification efficiency (E)
|
|
T. annulata transformation cell |
PBMCs of the uninfected calves |
T. annulata transformation cell |
PBMCs of the uninfected calves |
|
18S rRNA |
Ribosomal subunit |
Forward:TTCGATGGTAGTCGCTGTGC |
99 |
56 |
0.9950 |
0.9936 |
108.8 |
103.1 |
|
NR_036642.1 |
Reverse:TTGGATGTGGTAGCCGTTTCT |
|
GAPDH |
Glyceraldehyde-3-phosphate dehydrogenase |
Forward: GATGGTGAAGGTCGGAGTGAAC |
100 |
56 |
0.9674 |
0.9873 |
106.7 |
110.5 |
|
NM_001034034.2 |
Reverse:GTCATTGATGGCGACGATGT |
|
ACTB |
Cytoskeletal structural protein |
Forward:GATCTGGCACCACACCTTCTAC |
182 |
56 |
0.9474 |
0.9945 |
114.9 |
89.9 |
|
AY141970.1 |
Reverse: AGGCATACAGGGACAGCACA |
|
TBP |
TATA box binding protein |
Forward:TGAACGTCATGGATCAGAACAACA |
114 |
56 |
0.9979 |
0.9526 |
81.2 |
104.5 |
|
NM_001075742.1 |
Reverse:TGCCGTAAGGCATCATTGGA |
|
PRKG1 |
The protein kinase GMP-dependent, type I. |
Forward:AGCACAAATGGTTTGAGGGCTTTA |
140 |
56 |
0.8689 |
0.9670 |
120.1 |
99.4 |
|
NM_174436.2 |
Reverse:AGGTGGCGGTTCATCATTGTC |
Table 2.The candidate reference genes expression stability analysis by geNorm and NormFinder
Table 2.
|
Gene name |
geNorm
|
NormFinder
|
|
Stability value |
Ranking |
Best combination of two genes |
Stability value |
Ranking |
Best combination of 2 genes |
|
18S rRNA |
1.207 |
1 |
18S rRNA and GAPDH |
0.206 |
1 |
18S rRNA and GAPDH |
|
GAPDH |
1.332 |
2 |
|
0.338 |
4 |
|
|
ACTB |
1.500 |
4 |
|
0.319 |
3 |
|
|
TBP |
1.423 |
3 |
|
0.314 |
2 |
|
|
PRKG1 |
1.885 |
5 |
|
0.502 |
5 |
|
References
- 1. Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology 1993;11:1026-1030.
- 2. Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res 1996;6:986-994.
- 3. Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000;25:169-193.
- 4. Lee PD, Sladek R, Greenwood CM, Hudson TJ. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res 2002;12:292-297.
- 5. Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 2005;6:279-284.
- 6. Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 2004;313:856-862.
- 7. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101-1108.
- 8. Wang M, Wang Q, Zhang B. Evaluation and selection of reliable reference genes for gene expression under abiotic stress in cotton (Gossypium hirsutum L.). Gene 2013;530:44-50.
- 9. Yang CG, Wang XL, Tian J, Liu W, Wu F, Jiang M, Wen H. Evaluation of reference genes for quantitative real-time RT-PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus). Gene 2013;527:183-192.
- 10. Dhar AK, Bowers RM, Licon KS, Veazey G, Read B. Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Mol Immunol 2009;46:1688-1695.
- 11. Zhang X, Ding L, Sandford AJ. Selection of reference genes for gene expression studies in human neutrophils by real-time PCR. BMC Mol Biol 2005;6:4.
- 12. Lossos IS, Czerwinski DK, Wechser MA, Levy R. Optimization of quantitative real-time RT-PCR parameters for the study of lymphoid malignancies. Leukemia 2003;17:789-795.
- 13. Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Bioph Meth 2000;46:69-81.
- 14. Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem 2002;309:293-300.
- 15. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:RESEARCH0034.
- 16. Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 2004;64:5245-5250.
- 17. Chaussepied M, Langsley G. Theileria transformation of bovine leukocytes: a parasite model for the study of lymphoproliferation. Res Immunol 1996;147:127-138.
- 18. Li Y, Liu Z, Yang J, Chen Z, Guan G, Niu Q, Zhang X, Luo J, Yin H. Infection of small ruminants and their red blood cells with Theileria annulata schizonts. Exp Parasitol 2014;137:21-24.
- 19. Spooner RL, Innes EA, Glass EJ, Brown CG. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology 1989;66:284-288.
- 20. Ahmed JS, Rintelen M, Schein E, Williams RO. Effect of buparvaquone on the expression of interleukin 2 receptors in Theileria annulata-infected cells. Parasitol Res 1992;78:285-290.
- 21. Brown CG, Stagg DA, Purnell RE, Kanhai GK, Payne RC. Letter: Infection and transformation of bovine lymphoid cells in vitro by infective particles of Theileria parva. Nature 1973;245:101-103.
- 22. Yin H, Luo J, Schnittger L, Lu B, Beyer D, Ma M, Guan G, Bai Q, Lu C, Ahmed J. Phylogenetic analysis of Theileria species transmitted by Haemaphysalis qinghaiensis. Parasitol Res 2004;92:36-42.
- 23. Yamaguchi T, Yamanaka M, Ikehara S, Kida K, Kuboki N, Mizuno D, Yokoyama N, Narimatsu H, Ikehara Y. Generation of IFN-gamma-producing cells that recognize the major piroplasm surface protein in Theileria orientalis-infected bovines. Vet Parasitol 2010;171:207-215.
- 24. Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonak J, Lind K, Sindelka R, Sjoback R, Sjogreen B, Strombom L, Zoric N. The real-time polymerase chain reaction. Mol Aspects Med 2006;27:95-125.
- 25. Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 1999;270:41-49.
- 26. Ginzinger DG. Gene quantification using real-time quantitative PCR:An emerging technology hits the mainstream. Exp Hematol 2002;30:503-512.
- 27. Tong Z, Gao Z, Wang F, Zhou J, Zhang Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol Biol 2009;10:71.
- 28. Kuchipudi SV, Tellabati M, Nelli RK, White GA, Perez BB, Sebastian S, Slomka MJ, Brookes SM, Brown IH, Dunham SP, Chang KC. 18S rRNA is a reliable normalisation gene for real time PCR based on influenza virus infected cells. Virol J 2012;9:230.
- 29. Khurshid N, Agarwal V, Iyengar S. Expression of mu- and delta-opioid receptors in song control regions of adult male zebra finches (Taenopygia guttata). J Chem Neuroanat 2009;37:158-169.
- 30. Mukai M, Replogle K, Drnevich J, Wang G, Wacker D, Band M, Clayton DF, Wingfield JC. Seasonal differences of gene expression profiles in song sparrow (Melospiza melodia) hypothalamus in relation to territorial aggression. PLoS One 2009;4:e8182.
- 31. Dheda K, Huggett JF, Chang JS, Kim LU, Bustin SA, Johnson MA, Rook GA, Zumla A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem 2005;344:141-143.