Abstract
Clonorchis sinensis is a Group-I bio-carcinogen, associated with cholangiocarcinoma (CCA). The hamster is the only experimental model of C. sinensis-mediated CCA, but we oblige another animal model. The present study intended to develop a C. sinensis (Cs) mediated CCA model using C3H/He mice, co-stimulated with N-nitrosodimethyl-amine (NDMA) and dicyclanil (DC). The mice were divided into 8 groups with different combinations of Cs, NDMA, and DC. Six months later the mice were sacrificed and subjected to gross and histopathological examination. The body weights were significantly reduced among the groups treated with 2 or more agents (eg. Cs+NDMA, Cs+DC, NDMA+DC, and Cs+NDMA+DC). In contrast, liver weight percentages to body weight were increased in above groups by 4.1% to 4.7%. A Change of the spleen weight was observed only in Cs+NDMA group. Though C. sinensis infection is evident from hyperplastic changes, only 1 worm was recovered. T wo mice, 1 from Cs and the other from Cs+DC group, showed mass forming lesions; 1 (281.2 mm3) from the Cs group was a hepatocellular adenoma and the other (280.6 mm3) from the Cs+DC group was a cystic mass (peliosis). Higher prevalence of gray-white nodules was observed in Cs group (42.9%) followed by Cs+NDMA+DC group (21.4%). The mice of the Cs+NDMA+DC group showed hyper-proliferation of the bile duct with fibrotic changes. No characteristic change for CCA was recognized in any of the groups. In conclusion, C3H/He mice produce no CCA but extensive fibrosis when they are challenged by Cs, NDMA, and DC together.
-
Key words: Clonorchis sinensis, C3H/He mice, NDMA, dicyclanil, cholangiocarcinoma, fibrosis, hepatocellular adenoma, peliosis
INTRODUCTION
Clonorchis sinensis is a carcinogenic liver fluke to induce cholangiocarcinoma (CCA) in human [
1-
3]. Although a number of studies suggested the association between
C. sinensis and CCA, the underlying mechanism is currently unknown [
4]. The study of mechanism inevitably requires a suitable in vivo animal model. Syrian golden hamsters have been used for experimental CCA studies induced by liver flukes,
C. sinensis and
Opisthorchis viverrini. Even though hamsters mimic the human CCA in many aspects [
5], there are lack of genetic information and antibodies [
6]. Therefore, a more suitable experimental model having genetic features and antibodies available is required to investigate molecular mechanisms of CCA development.
Mice are usually less susceptible to infection with
C. sinensis compared to rats, rabbits, and other experimental mammals. In our previous study, we examined 6 strains of mice namely ICR, BALB/c, C57, DDY, CBA, and C3H/He and found that C3H/He mouse strain was relatively susceptible to
C. sinensis infection [
6]. The worm recovery rate of C3H/He mice was 20.7% after 4 weeks of infection which was highest among the strains. It is well known that mice are suitable for many carcinogenic studies for having well documented genetic information, associated molecular products, and cost effectiveness. Thus despite of their relative low susceptibility, we considered C3H/He mice to establish as a CCA model animal.
C. sinensis infection (Cs) alone is unable to induce CCA in experimental animals and the worm is considered as a promoting agent for CCA [
7]. The worm lives in the biliary tree for decades and act as an agent for chronic inflammation. Chronic inflammations are account for about 25% of all human cancers and most of the cases which might leads to reactive oxygen species (ROS) or reactive nitrogen species (RNS) mediated DNA damage [
8]. However, in short term experiment such DNA damage was not evident from
C. sinensis. In this context, we have used a well-known animal hepatocarcinogen N-nitrosodimethylamine (NDMA) as an initiator of CCA at sub-carcinogenic level to develop 2-steps carcinogenesis model [
7]. In addition, we have used another tumor promoting agent, dicyclanil (4,6-diamino-2-cyclopropylaminopyrimidine-5-carbonaitrite; DC) which was used earlier to determine its oxidative damage in mice [
9]. In the present study, the C3H/He mice which were challenged with Cs, NDMA, and DC alone or different combination and were evaluated for hepatobiliary histopathological changes, whether this 2-steps carcinogenesis process could induce CCA.
MATERIALS AND METHODS
Ethics statement
The animal experiment protocol was reviewed and approved by the institutional animal care and use committee (IACUC) of Seoul National University, Seoul, Korea (SNU-091120-1) and the NIH Guide for the Care and Use of Laboratory Animals (ISBN 0-309-05377-3). It is accredited by the Ministry of Food and Drug Administration and also by the Ministry of Education, Science and Technology (LNL08-402) as an animal experiment facility. The laboratory has been monitored and inspected regularly by the Ministry and the IACUC of Seoul National University.
Mice and housing
Male C3H/He mice of 4-5 weeks of age weighing about 20 g were purchased from the Central Laboratory Animals Inc. (Seoul, Korea) and were assigned to different groups randomly. They were housed at biosafety level 2 (BCL-2) animal facilities in polycarbonate cages containing wood chip bedding (Samtako Bio Korea Inc., Gyeonggi, Korea) at 12 hr light-dark cycle. The room temperature was 21±2˚C. Animals were allowed to eat rat chow (Samtako Bio Korea Inc., Gyeonggi, Korea) and drink tap water ad libitum.
Reagents
N-nitrosodimethylamine (NDMA) was purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA) and dicyclanil (4,6-diamino-2-cyclopropylaminopyrimidine-5-carbonaitrite; DC) purchased from Novartis, China.
Collection of metacercariae
Metacercariae of
C. sinensis were collected from naturally infected freshwater fish
Pseudorasbora parva as described previously [
3]. The metacercariae were collected and then preserved in storing solution at 4°C until infection. Each of the mice was challenged with 30 metacercariae by intragastric intubation.
To determine the dose of NDMA, C3H/He mice were divided into 4 groups containing 5 mice each and subjected to intraperitoneal injection (i.p.) of NDMA 3, 5, and 7.5 mg/kg thrice for 3 consecutive days (day 1, day 2, and day 3). Phosphate buffered saline (PBS) was injected in the control mice. They were observed for 5 months to monitor their survival (
Supplementary Fig. S1).
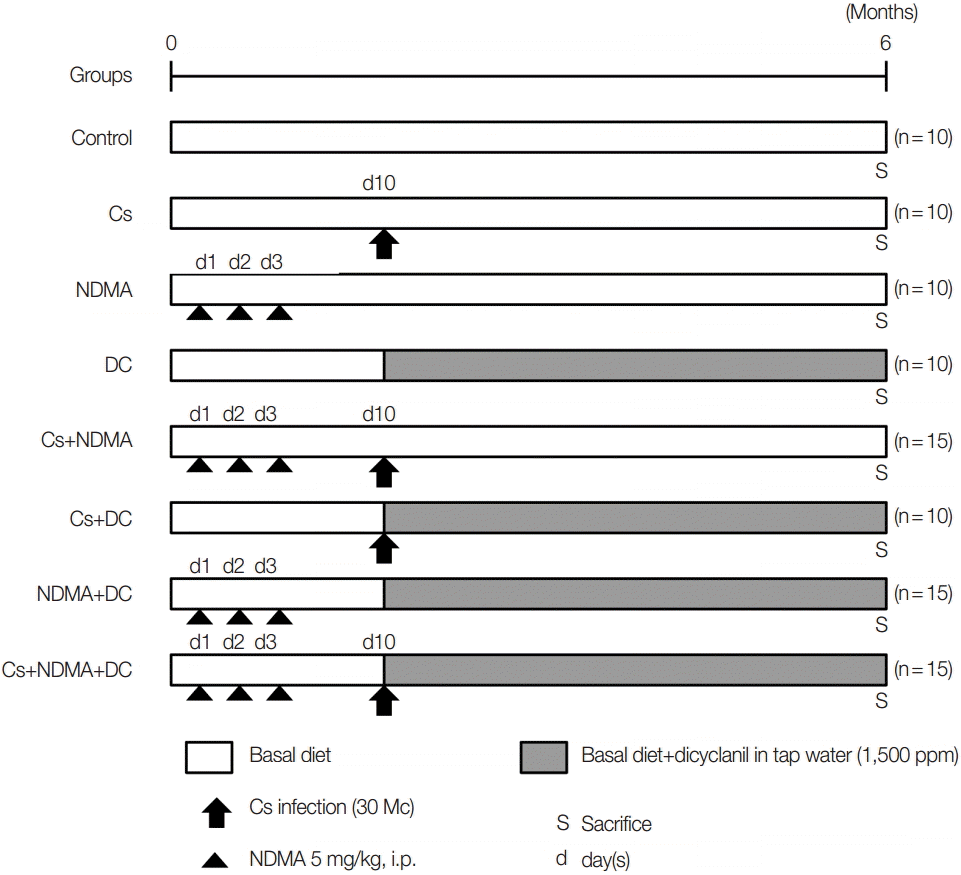
A total of 95 male C3H/He mice were divided randomly into 8 groups: Group I (Ctrl.) 10 mice as uninfected control, Group II (Cs) 10 mice challenged with 30 metacercariae of
C. sinensis at day 10 from the beginning of the experiment, Group III (NDMA) 10 mice with 3 intraperitoneal injections of NDMA (5 mg/kg) for 3 consecutive days, Group IV (DC) 10 mice with 1,500 ppm DC in drinking water from day 10, Group V (Cs+NDMA) 15 mice with Cs and NDMA, Group VI 10 mice with Cs and DC, Group VII (NDMA+DC) 15 mice with NDMA and DC, Group VIII (Cs+NDMA+DC) 15 mice with Cs, NDMA, and DC. The grouping and experimental design are summarized in
Fig. 1.
C. sinensis metacercariae were given to the respective groups with gavage intragastric intubation at day 10. DC was given with tap water and continues from day 10 to the end of the study. After 6 months (27 weeks), the mice were sacrificed and investigated for gross and histopathological changes. A mixture of xylazine (10 mg/kg; Bayer, Seoul, Korea) and zoletil-50 (30 mg/kg, Virbac, France) was injected into the muscle for anesthesia.
Measurement of body weight: Body weights of all mice were measured in every month and weight of the liver and spleen were measured at necropsy with an electronic balance (Sartorius, Germany).
Recovery of worms: After external observation, the livers were subjected to worm recovery process by applying gentle pressure from the periphery to the major bile duct then by slicing with 5 mm thickness.
Observation of mass forming lesions (MFLs): The livers of mice from all groups were observed grossly for the presence of any MFLs. After gross observation a representative portion of the liver was kept in neutral buffered formalin (buffered 10% formaldehyde, pH 7.2) for histopathologic analysis. Volumes of MFLs were calculated with following equation, V=1/2 (L×W
2) as used to measure tumor volume [
10], where, V=volume, L=length, and W=width of MFLs. In the present study, lesion’s volume higher than 100 mm
3 was considered as MFL, and lesion’s volume lower than the above considered as nodule.
Histopathology
A portion from each liver after fixation was prepared for histopathological analysis. Paraffin sections of 4 µm thickness were processed for routine hematoxylin and eosin (H-E) staining. Serial paraffin sections of 4 µm thickness were processed for periodic acid Schiff (PAS) and diastase periodic acid Schiff (DPAS) staining following standard procedure.
Data analysis and statistics
Data obtained from the experiment were analyzed by Microsoft Excel (Ed. 2007, USA), GraphPad Prism 5 and SPSS-19 statistical software. Comparisons of results were performed using the Student’s t-test. P-values<0.05 were considered as significant.
RESULTS
Body and organ weights of different mouse groups
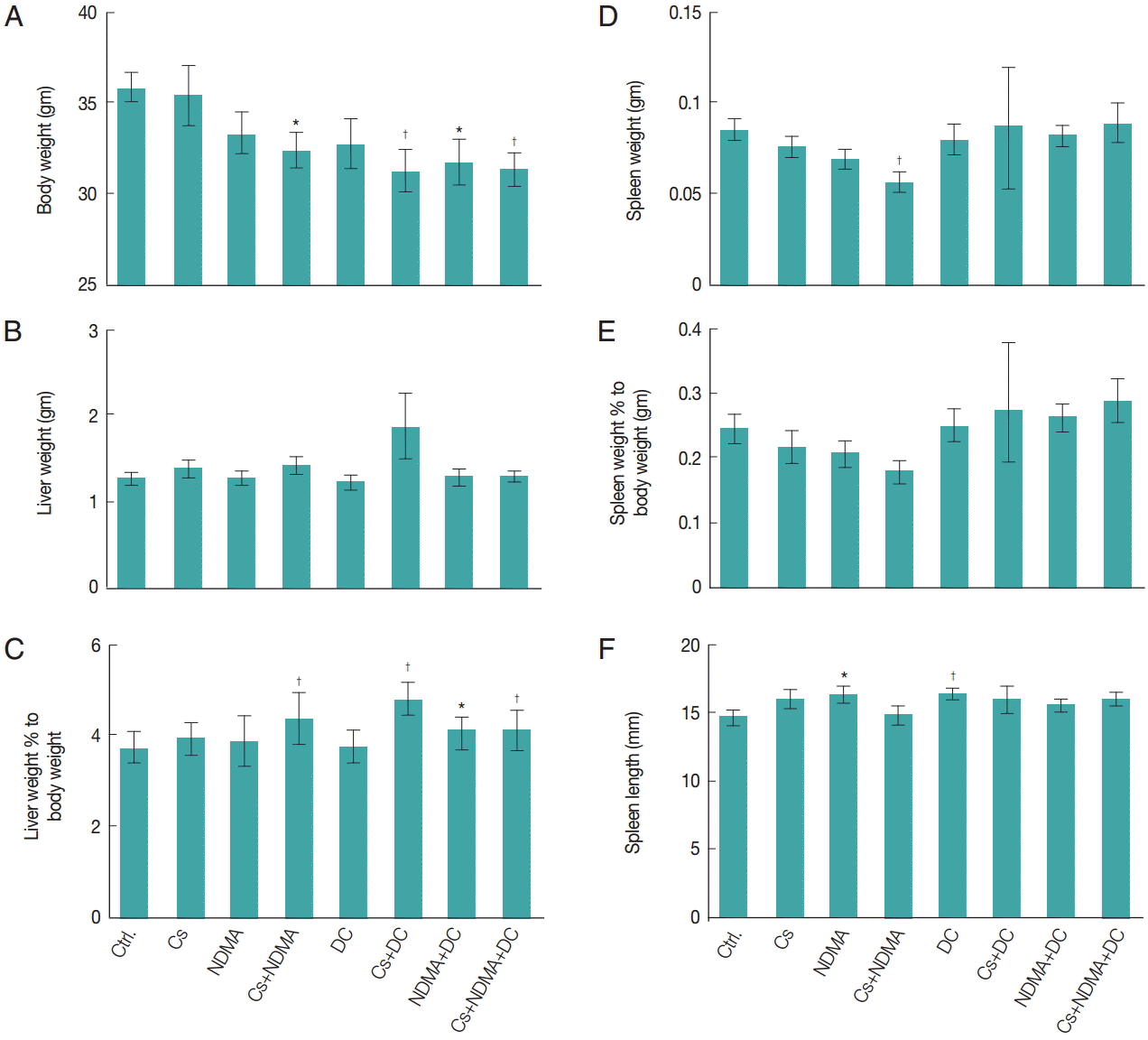
Body weights of mice were measured in every month (
Supplementary Fig. S2). No significant changes of body weights were observed at base line (0 month), however, after 6 months significant reduction of body weight were observed in different groups such as Cs+NDMA (32.4±0.9 gm), Cs+DC (31.3±1.1 gm), NDMA+DC (31.7±1.3 gm), and Cs+NDMA+DC (31.4± 0.9 gm) groups relative to control (35.9±0.8 gm) (
P<0.05;
P<0.01;
P<0.05 and
P<0.01, respectively). Mice groups challenged with 2 or more agents have shown reduced body weight indicates their combine effects (
Fig. 2A). No significant changes in liver weight were observed in any groups (
Fig. 2B) but when liver weight percentages to body weights considered, there were increases in liver weights in the combination groups: Cs+NDMA (4.4%±0.6%), Cs+DC (4.7%±0.4%), NDMA+DC (4.1%±0.3%), and Cs+NDMA+DC (4.1%±0.4%) (
P<0.01;
P<0.01;
P<0.05 and
P<0.01 respectively) (
Fig. 2C). The spleen weight and its percentage to body weight showed a significant decrease (0.1±0.0 gm,
P<0.01; 0.2%,
P<0.05 respectively) only in Cs+NDMA group (
Fig. 2D,
E). We also measured the length of spleen and observed average length 14.7±0.4 mm in control group but noticed enlargements in NDMA (16.3±0.5 mm,
P<0.05) and DC (16.5±0.4 mm,
P<0.01) groups.
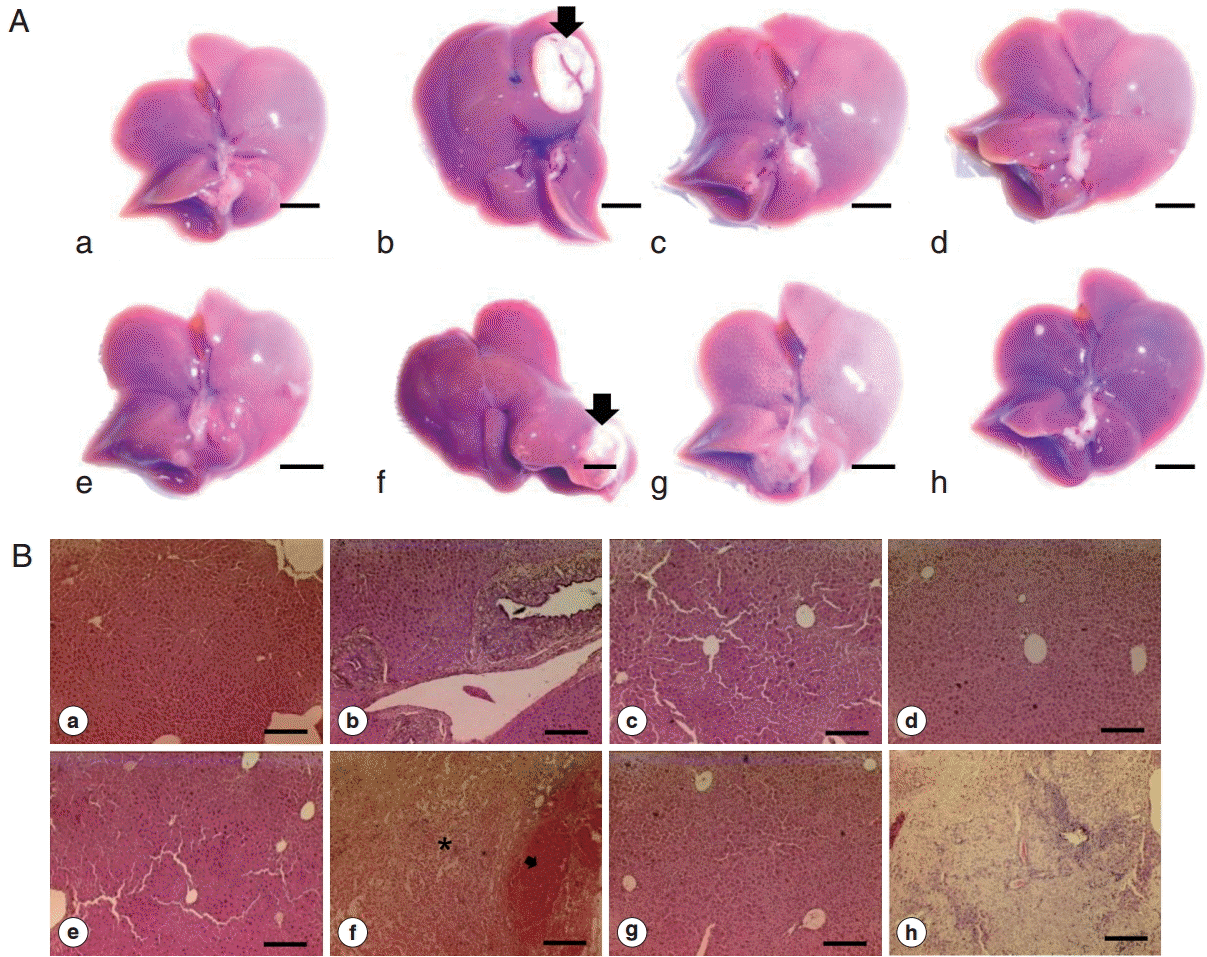
Grossly, all of the livers from all groups looked normal except 2 livers from 2 different groups. One liver from Cs and 1 from Cs+DC groups revealed large MFLs in the left (281.2 mm
3) and caudate lobe (280.6 mm
3) respectively (
Fig. 3A, arrows). A gray-white nodule (10.9 mm
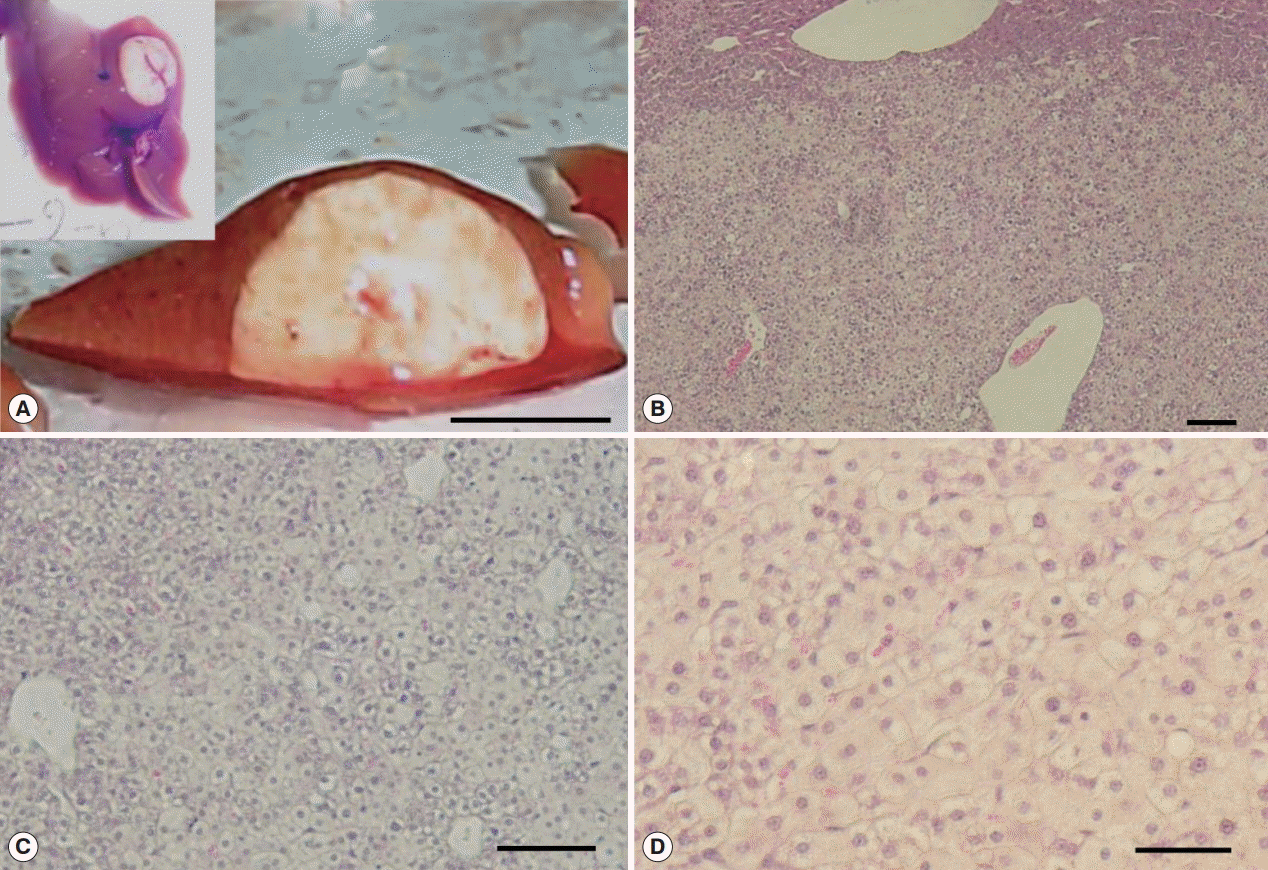
3) adjacent to large MFLs also observed in Cs group. The large mass forming lesions from Cs group were caused by hepatocellular adenoma (
Fig. 4). Small sized (range: 0.02-5.3 mm
3) gray-white nodules observed on the liver surface were recorded in the present study and shown in supplementary files (
Supplementray Fig. S3;
Supplementary Table S1). The gray-white nodules were dominantly prevalent in Cs and Cs+NDMA+DC groups (42.9% and 21.4%, respectively). Highest mean intensity (1.7) of graywhite nodules was observed in Cs+NDMA+DC group and highest average volume (3.9±3.5 mm
3) was observed in Cs group (
Supplementary Table S2). No nodule was detected in NDMA, Cs+NDMA, DC, or uninfected control groups. Mechanical approach to recover the infected adult Cs worm was able to collect only 1 adult worm from a mouse of Cs+DC group suggests spontaneous expulsion of worms during the study period.
Histopathological examination revealed bile duct dilatation and proliferation of ductal epithelial cells in all
C. sinensis infected groups (Cs, Cs+DC, Cs+NDMA, and Cs+NDMA+DC). Cs (b) group showed typical hyper-proliferative bile ducts due to
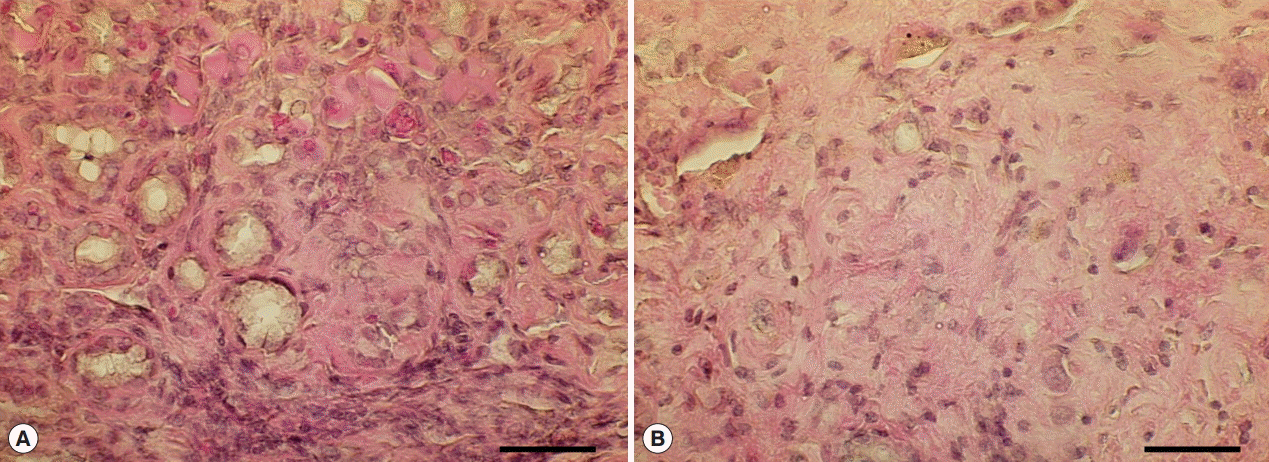
C. sinensis infection. In Cs+NDMA+DC group, fibrotic zones were observed (
Fig. 3B-h) and higher magnifications demonstrated hyperplastic changes and extensive fibrosis in these areas (
Fig. 5;
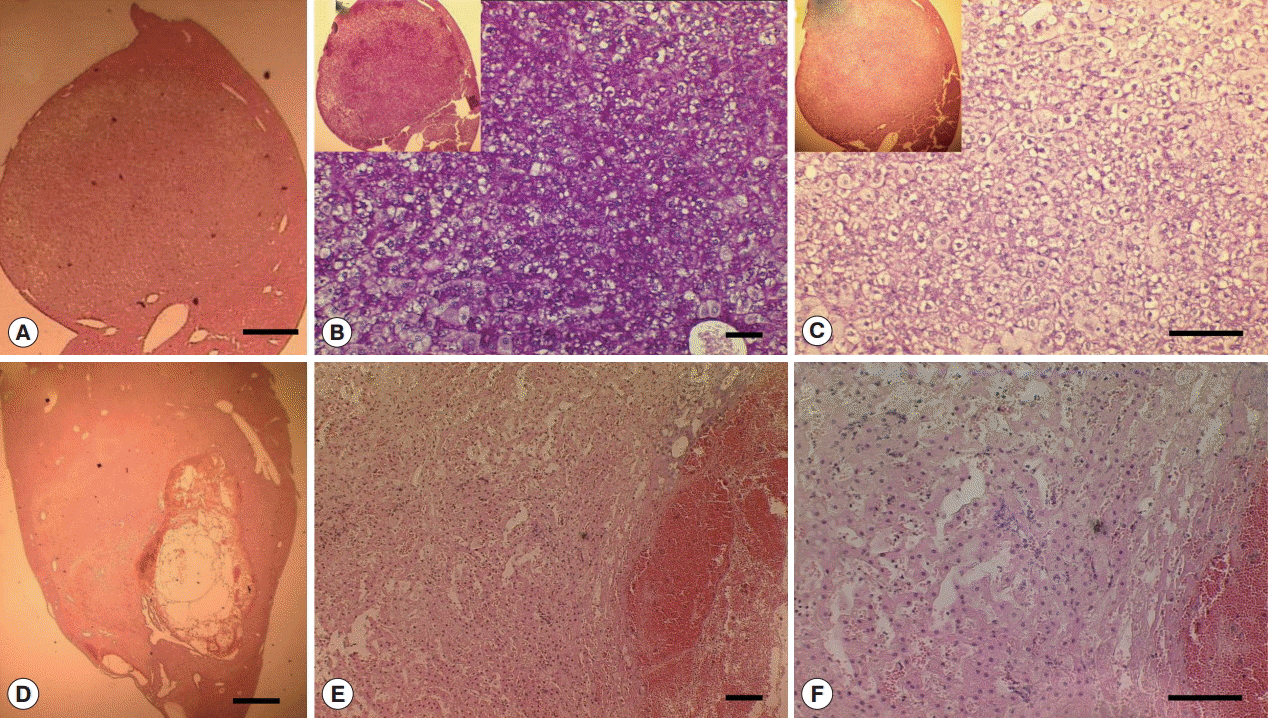
Supplementary Fig. S4), however, no CCA developed. The large mass forming lesions observed in Cs group confirmed as glycogen rich clear cell hepatocellular adenoma surrounded by eosin stained healthy hepatocytes. The mass contained clear cell hepatocytes rich in glycogen as revealed by PAS/DPAS staining (
Fig. 6). The other mass forming lesions observed in Cs+DC group diagnosed as peliosis resulting from dilated sinusoid (
Fig. 6). The center of this cystic lesion marked with coagulative necrosis without any inflammatory cells (
Fig. 3B-f; indicated with asterisk). Blood filled cavities also evident from histopathology (
Fig. 3B-f; arrow).
DISCUSSION
The present study aimed to establish a CCA mice model, but no C3H/He mice developed characteristic CCA changes in their liver. Utmost liver pathology was hyperplastic changes with extensive fibrosis only observed in the Cs+NDMA+DC group. Unexpectedly current finding indicates that
C. sinensis might be related with hepatocellular adenoma or peliosis like pathological lesions in mice. The body weight of mice reduced significantly when challenged with 2 or more agents as observed in Cs+NDMA, Cs+DC, NDMA+DC, and Cs+NDMA+DC groups with an increase in liver weight percentage to body weight. Loss of body weight was measured as an assessment of disease progression. Weight loss in human and animals can be related with the carcinogenic progression [
4,
11,
12]. Here, among the 8 groups, more than 10% weight loss was observed in the Cs+DC (10.8%) and Cs+NDMA+DC (10.5%) groups. The Cs+NDMA (7.6%) and NDMA+DC (9.7%) groups also showed significant weight loss. The present study also observed moderate hepatomegaly but not splenomegaly in the mice challenged with 2 more carcinogenic agents at sub-carcinogenic levels. Hepatomegaly with the infection of the liver fluke was reported in previous studies [
4,
13,
14]. The hepatomegaly must have been an outcome of inflammation triggered by
C. sinensis worm and/or chemical irritations caused by NDMA, and DC. The spleen weight was not correlated with liver weight which suggested rather mild immunoreactions. Though significant increase in the spleen length was noticed in the NDMA and DC groups, it was not in agreement with weights of the spleen, liver, or body. Other unknown reasons might be involved for such the change.
NDMA is a potent carcinogen [
15] and has been reported for 50% mortality of mice (LD50) at 20 mg/kg intraperitoneal injection [
16]. An earlier study showed that about 2 mg/kg/day dose of NDMA to C3H/He mice for 32 days causes promutagenic DNA lesion (O
6-methylguanine) [
17]. In a study, CBA mice were subjected to a single dose of NDMA (5 mg/kg) and kept the animals for 26 weeks but any apparent hepatocellular tumor was not observed [
18]. In a liver fibrosis study, ICR strains of mice were subjected to 8 mg/kg dose of NDMA for 15 days at regular intervals (totaling 120 mg/kg) [
19]. To keep the NDMA dose in sub-carcinogenic level we used 5 mg/kg dose thrice and kept the mice for a longer duration. We observed no dose-induced toxicity in the liver of NDMA groups by gross or histopathological observations. Both NDMA dose (totaling 15 mg/kg) and frequency in this study was rather less compared to that of most previous studies. Therefore, the present finding suggests that the C3H/He mice should receive higher dose of NDMA to be established as CCA mice model. Otherwise, any kind of genetic manipulation may be recommended simultaneously.
DC in most genotoxicity studies showed negative results [
20], however, an 18-month long study demonstrated hepatocellular adenomas and carcinomas in mice with highly elevated incidence in females when allowed to consume 0.15% DC containing diet [
21]. In another study, the same dose for 13 weeks was not carcinogenic for B6C3F1
gpt delta mice, which was produced by breeding C57BL/6
gpt delta and non-transgenic C3H mice [
9]. The study observed predominant
gpt GC:TA transversion mutations in the DC treated females probably due to 8-OHdG formation. In the present study with equivalent dose (1,500 ppm) of DC, we did not observe adenoma or carcinoma in the DC treated groups, which might be due to the fact that male mice are relatively resistant to DC-induced carcinogenicity [
9]. There were chances that the mice consumed less amount of DC. Moreover, the water solubility and other physical properties may have influenced the effect of DC.
The present study observed MFLs and nodules particularly in the Cs group or in the Cs with one or both carcinogenic chemicals (NDMA, DC) groups. The mass lesions suggested a certain role of
C. sinensis worms to produce the pathology. Though hepatocellular adenoma is a pathological event in DC treated mice [
21], we observed it in a mouse of Cs group. The hepatocellular adenoma is a rare unexpected finding for
C. sinensis infection. Among various types, it falls under the category of glycogen storage disease (glycogen rich clear cell hepatocellular adenoma). Moreover, the Cs+DC group showed peliosis which was also a noble instance for both
C. sinensis and DC treatment. In the formation of peliosis, sinusoidal dilation created extensive pressure on surrounding blood vessel causing such ischemic damage. No CCA was evident from the Cs+ NDMA+DC group; however, it showed highest mean intensity and highest average volume of nodules. Other histopathological findings such as bile duct dilatation, hyperplasia was common in all
C. sinensis infected groups but progressed to extensive fibrosis in Cs+NDMA+DC group. The histopathological findings suggest that the worms made the histological changes although most of the mice did not have the worms in their liver. Most of the inoculated worms invaded into the intrahepatic bile duct but could not survive and discharged within a few weeks after infection [
6]. On the other hand, chemical treated groups (NDMA or DC alone) did not show any detectable changes revealed by histopathology.
Fibrosis is a wound healing response to chronic liver inflammation. Activated hepatic stellate cells (HSCs) or hepatic progenitor cells play a key role for the development of liver fibrosis.
C. sinensis excretory-secretory products might activate those cells resulting expression of collagen I, a-SMA and MMP2 [
22]. A recent study indicates that
C. sinensis secreted calmodulin (CsCaM) can induce mild to moderate fibrosis when injected in rats [
23]. The attenuating effect also observed by
C. sinensis molecule, lysophospholipase A (CsLysoPLA) in in-vitro [
22]. The chronic inflammatory response due to the presence of liver fluke in the biliary tree is well noted. Moreover, mechanical damage by worms can induce wound healing response of the host tissue.
Host susceptibility of C3H/He mice against
C. sinensis was relatively higher than that of other mouse strains but overall mice susceptibility is low when compared to hamsters or rats [
3]. Failure to detect adult worms except one suggested unfavorable immunological response in
C. sinensis infected C3H/He mice against the parasite. During the study period, to assess the progression of CCA, histopathological examination of representative mice at regular intervals should be considered and the doses of NDMA and DC are better optimized.
Our study model using C3H/He mice challenged with C. sinensis, NDMA, and DC produces hepatocellular adenoma, hyperplastic changes, peliosis, and extensive fibrosis but not CCA. The C3H/He mouse is not a good experimental model of C. sinensis related CCA.
Notes
-
We have no conflict of interest related to this study.
The present study was supported by a research grant from Seoul National University Hospital (21-2005-0330).
Supplementary Material
Table S2.
The prevalence, intensity and volume of gray-white nodules in different groups of mice
Supplementary Fig. S1.
Kaplan-Meier survival analysis for the NDMA treated C3H/He mice. Mice were treated with 3 mg/kg, 5 mg/kg, 7.5 mg/kg or left untreated and observed up to 20 weeks for their survival. The survival curves were compared using the log-rank test (P<0.01).
Supplementary Fig. S2.
Monthly body weights of C3H/He mice. Mice were divided into 8 different groups, including uninfected control. Data presented as mean±SEM. *P<0.05 and †P<0.01 at 6th month of infection.
Supplementary Fig. S3.
Gray-white nodule in different groups of mice. (A) Cs group. (B) Cs+DC group. (C) NDMA+DC group. (D) Cs+NDMA+DC group mice. Most of the nodular lesions showed by the mice infected with C. sinensis (scale bar=10 mm).
Supplementary Fig. S4.
Different pathological lesions from a Cs+NDMA+DC group mice. (A) Hyper proliferative bile duct. (B) Inflammatory cells accumulation around hyperplastic zone. (C) Hyper proliferative bile duct with in fibrous tissue. Scale bar=50 μm.
Fig. 1.Experimental scheme for the development cholangiocarcinoma (CCA) in C3H/He mice.
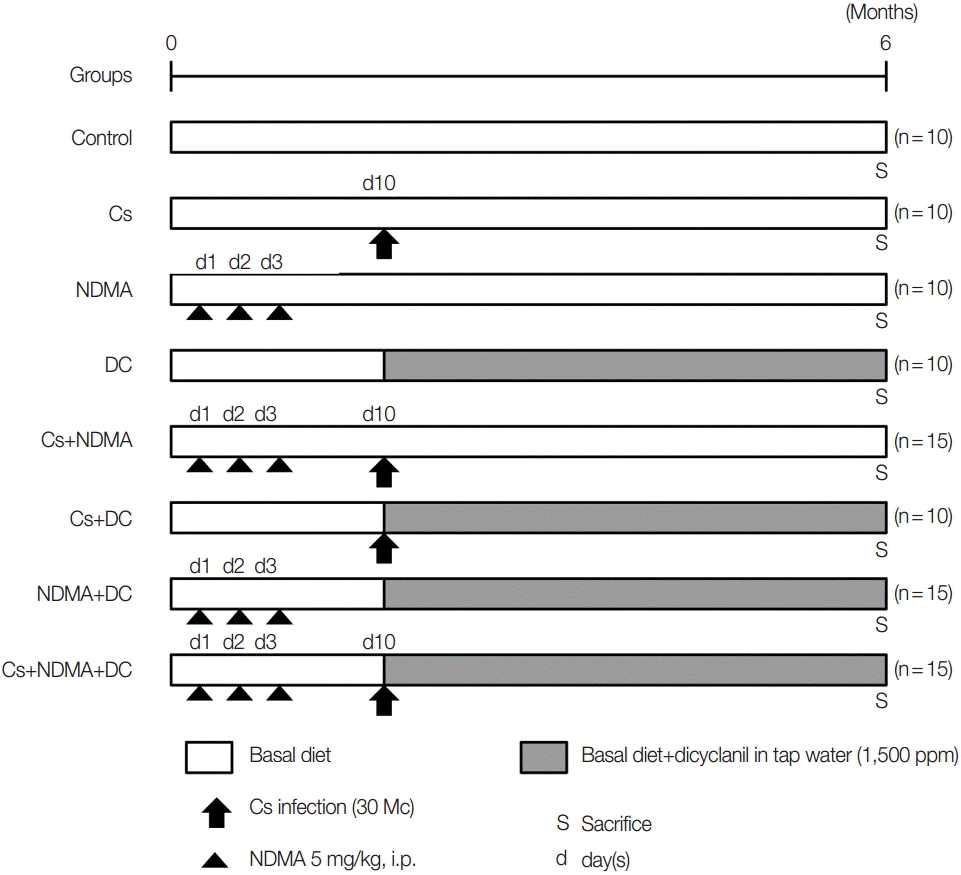
Fig. 2.Gross findings in different groups of mice at necropsy. (A) Body weight. (B) Liver weight. (C) Liver weight percentage to body weight. (D) Spleen weight. (E) Spleen weight percentage to body weight. (F) Length of spleen. Bar diagrams are mean±SEM. *P<0.05 and †P<0.01.
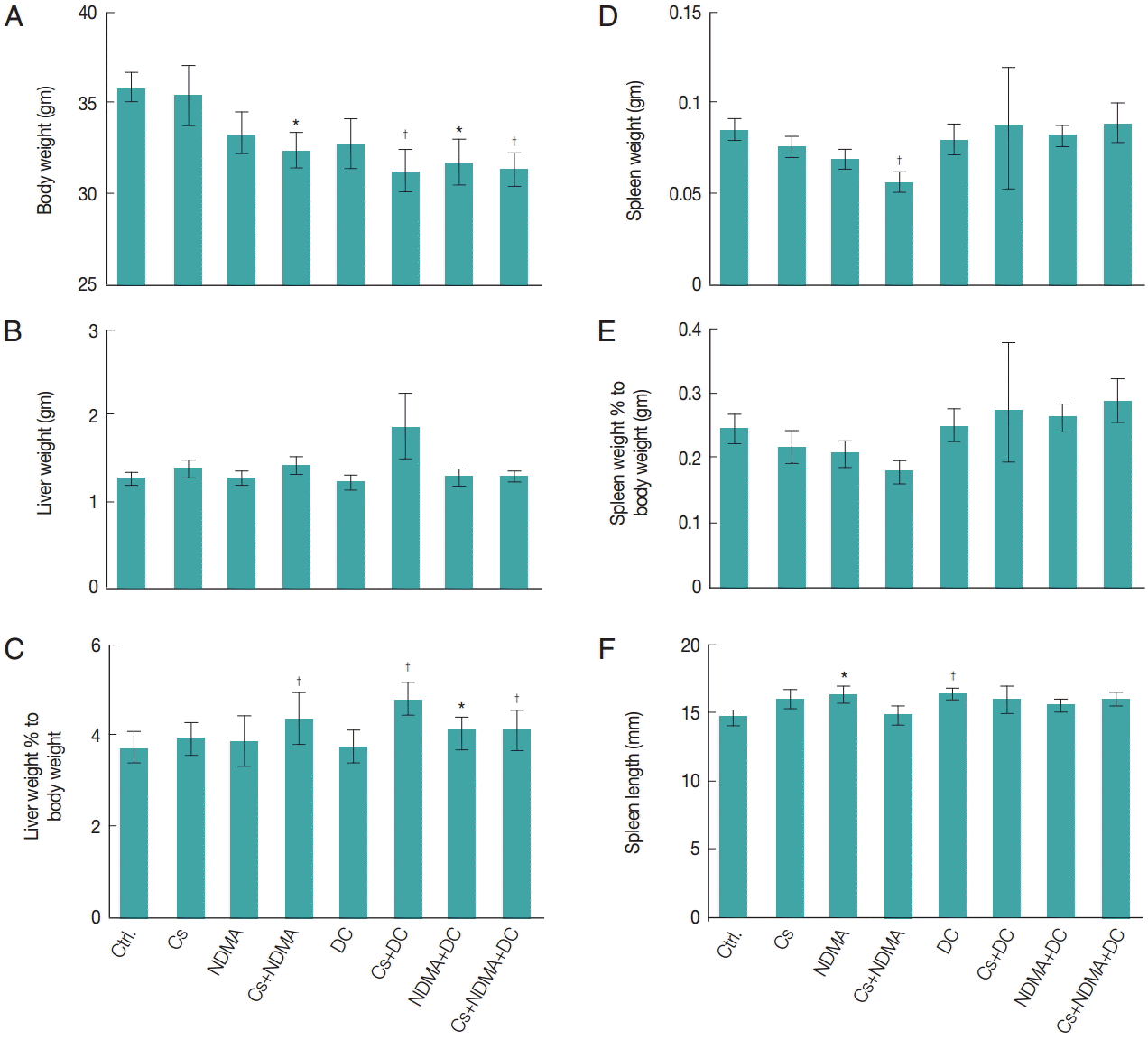
Fig. 3.Gross photos and histopathology of mice liver from different groups. (A) Gross appearance of liver showing normal appearance among most of the groups (scale bar=5 mm). (B) Representative H-E staining of liver section showing typical biliary triad in most of the mice groups (scale bar=200 μm). a, control; b, Cs; c, NDMA; d, DC; e, Cs+NDMA; f, Cs+DC; g, NDMA+DC; h, Cs+NDMA+DC.
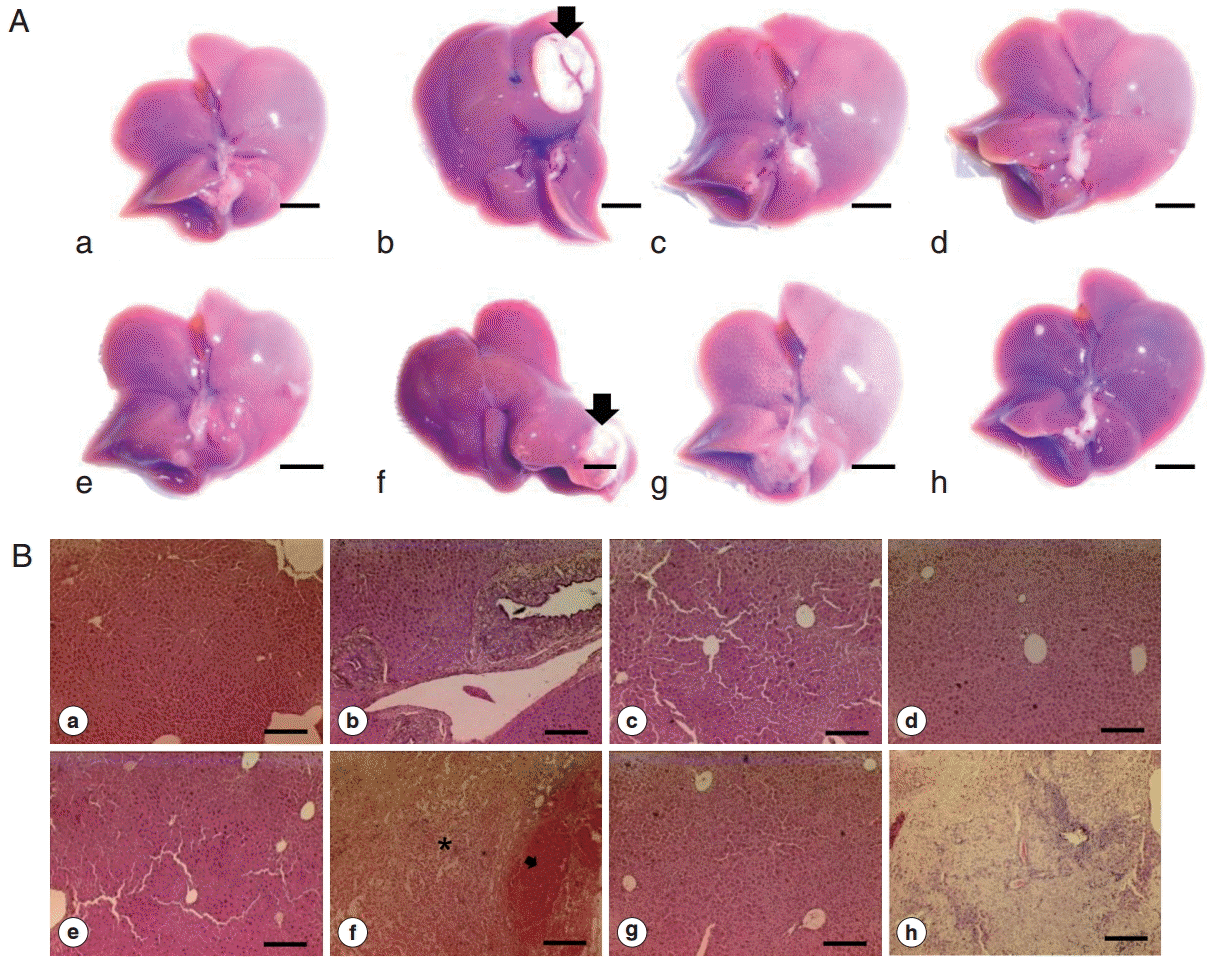
Fig. 4.Gross and histopathological section of abscess from Cs group liver. (A) Gross enlarged view of abscess (scale bar=5 mm). (B-D) Representative H-E staining of abscess (B-C: scale bar=100 μm; D: scale bar=50 μm).
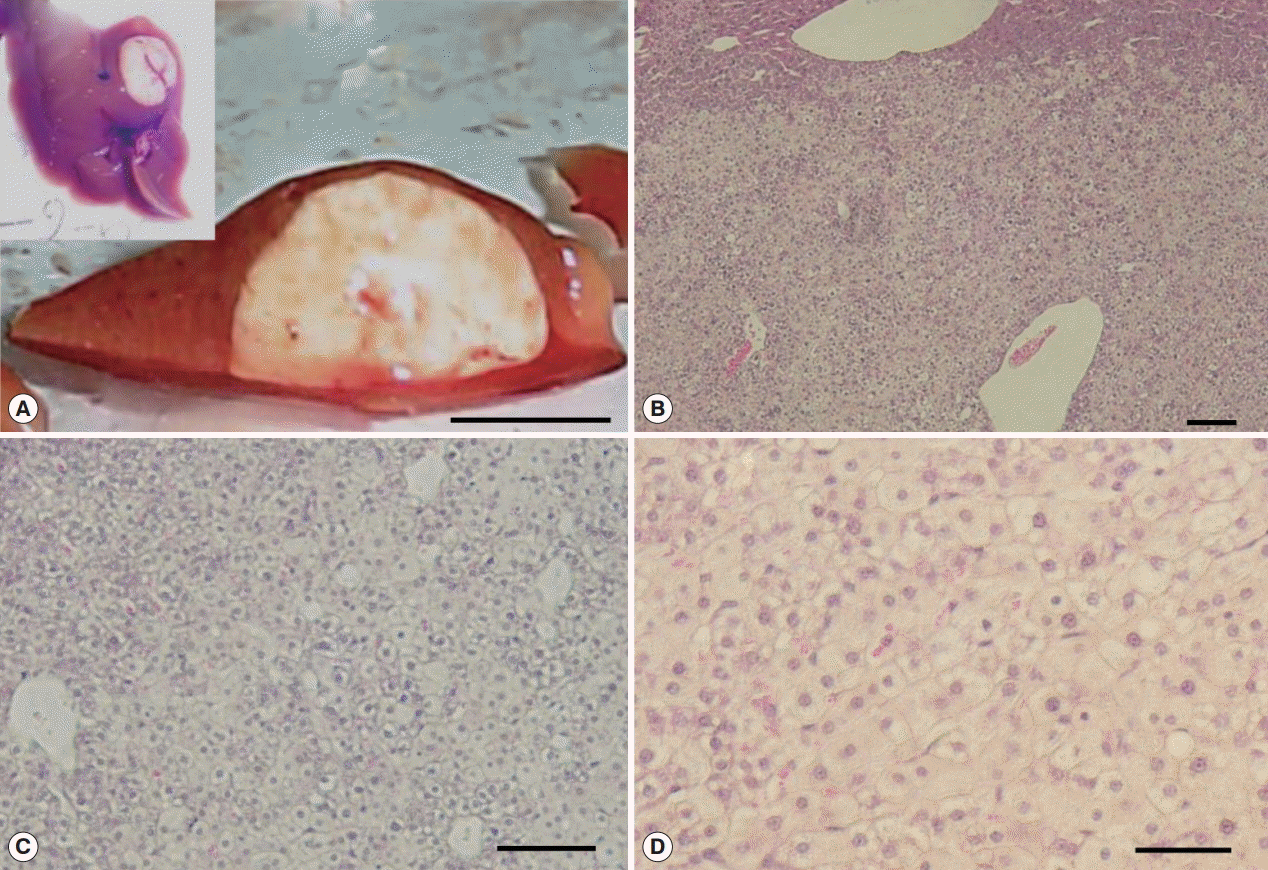
Fig. 5.Hyperplastic and fibrotic changes in Cs+NDMA+DC group mice. (A) H-E staining of liver section showing hyper-proliferative bile ducts surrounded by a fibrotic zone. (B) A portion of extensive fibrosis (scale bar=50 μm).
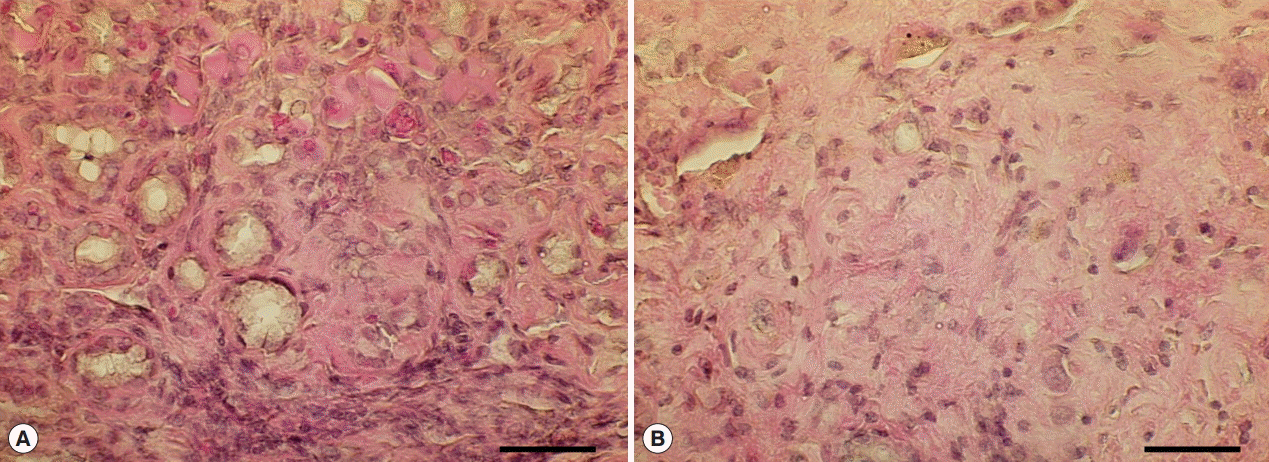
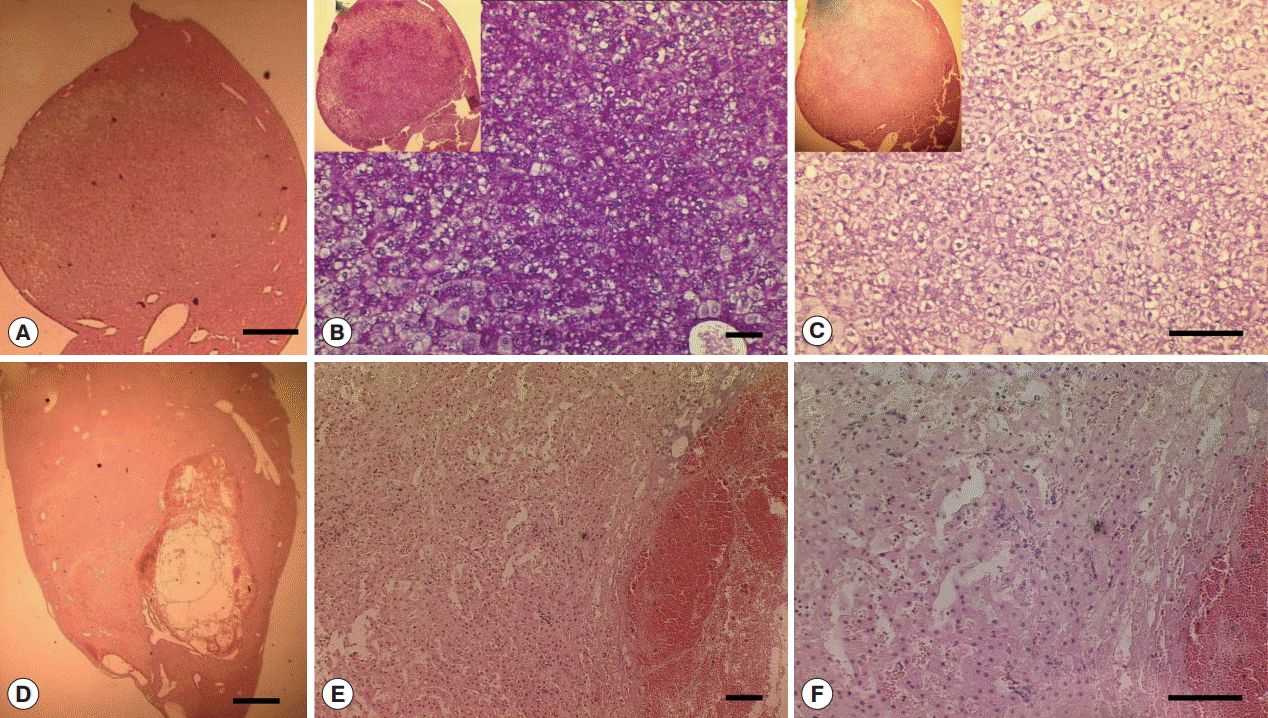
Fig. 6.Glycogen rich clear cells in hepatocellular adenoma of a mouse in Cs group and peliosis (cystic lesion) in a Cs+DC group mouse. (A) H-E staining of adenoma mass (scale bar=400 μm). (B) Periodic acid Schiff staining (scale bar=100 μm). (C) Diastase periodic acid Schiff staining (scale bar=50 μm). (D) H-E staining of cystic lesions (scale bar=1 mm). (E) Pooled erythrocytes lined by hepatocytes (scale bar=100 μm). (F) Enlarged view showed no accumulation of inflammatory cells (scale bar=100 μm).
References
- 1. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens-Part B: biological agents. Lancet Oncol 2009;10:321-322.
- 2. Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, Won YJ, Park S, Park SJ, Hong ST. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci 2010;25:1011-1016.
- 3. Uddin MH, Li S, Bae YM, Choi MH, Hong ST. In vitro maintenance of Clonorchis sinensis adult worms. Korean J Parasitol 2012;50:309-315.
- 4. Uddin MH, Choi MH, Kim WH, Jang JJ, Hong ST. Involvement of PSMD10, CDK4, and tumor suppressors in development of intrahepatic cholangiocarcinoma of syrian golden hamsters induced by Clonorchis sinensis and N-nitrosodimethylamine. PLoS Negl Trop Dis 2015;9:e0004008.
- 5. Yongvanit P, Pinlaor S, Loilome W. Risk biomarkers for assessment and chemoprevention of liver fluke-associated cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014;21:309-315.
- 6. Uddin MH, Li S, Bae YM, Choi MH, Hong ST. Strain variation in the susceptibility and immune response to Clonorchis sinensis infection in mice. Parasitol Int 2012;61:118-123.
- 7. Lee JH, Yang HM, Bak UB, Rim HJ. Promoting role of Clonorchis sinensis infection on induction of cholangiocarcinoma during two-step carcinogenesis. Korean J Parasitol 1994;32:13-18.
- 8. Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, Kawanishi S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev 2013;2013:387014.
- 9. Umemura T, Kuroiwa Y, Tasaki M, Okamura T, Ishii Y, Kodama Y, Nohmi T, Mitsumori K, Nishikawa A, Hirose M. Detection of oxidative DNA damage, cell proliferation and in vivo mutagenicity induced by dicyclanil, a non-genotoxic carcinogen, using gpt delta mice. Mutat Res 2007;633:46-54.
- 10. Jensen MM, Jorgensen JT, Binderup T, Kjaer A. Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med Imaging 2008;8:16.
- 11. Nourissat A, Bairati I, Samson E, Fortin A, Gelinas M, Nabid A, Brochet F, Tetu B, Meyer F. Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer. Cancer 2010;116:2275-2283.
- 12. Chen SP, Peng LN, Lin MH, Lai HY, Hwang SJ, Chen LK. Evaluating probability of cancer among older people with unexplained, unintentional weight loss. Arch Gerontol Geriatr 2010;50(suppl 1):S27-S29.
- 13. Kaewpitoon N, Kaewpitoon SJ, Pengsaa P. Opisthorchiasis in Thailand: review and current status. World J Gastroenterol 2008;14:2297-2302.
- 14. Lee JH, Rim HJ, Bak UB. Effect of Clonorchis sinensis infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian golden hamsters. Korean J Parasitol 1993;31:21-30.
- 15. Zeilmaker MJ, Bakker MI, Schothorst R, Slob W. Risk assessment of N-nitrosodimethylamine formed endogenously after fish-with-vegetable meals. Toxicol Sci 2010;116:323-335.
- 16. WHO. N-nitrosodimethylamine in drinking-water: Background document for development of WHO Guidelines for drinking-water quality. Geneva, Switzerland. World Health Organization; 2006.
- 17. Lindamood C 3rd, Bedell MA, Billings KC, Swenberg JA. Alkylation and de novo synthesis of liver cell DNA from C3H mice during continuous dimethylnitrosamine exposure. Cancer Res 1982;42:4153-4157.
- 18. Uehara T, Kashida Y, Watanabe T, Yasuhara K, Onodera H, Hirose M, Mitsumori K. Susceptibility of liver proliferative lesions in heterozygous p53 deficient CBA mice to various carcinogens. J Vet Med Sci 2002;64:551-556.
- 19. Hu QW, Liu GT. Effects of bicyclol on dimethylnitrosamine-induced liver fibrosis in mice and its mechanism of action. Life Sci 2006;79:606-612.
- 20. Moto M, Sasaki YF, Okamura M, Fujita M, Kashida Y, Machida N, Mitsumori K. Absence of in vivo genotoxicity and liver initiation activity of dicyclanil. J Toxicol Sci 2003;28:173-179.
- 21. Moto M, Okamura M, Muguruma M, Ito T, Jin ML, Kashida Y, Mitsumori K. Gene expression analysis on the dicyclanil-induced hepatocellular tumors in mice. Toxicol Pathol 2006;34:744-751.
- 22. Zhou L, Shang M, Shi M, Zhao L, Lin Z, Chen T, Wu Y, Tang Z, Sun H, Yu J, Huang Y, Yu X. Clonorchis sinensis lysophospholipase inhibits TGF-beta1-induced expression of pro-fibrogenic genes through attenuating the activations of Smad3, JNK2, and ERK1/2 in hepatic stellate cell line LX-2. Parasitol Res 2016;115:643-650.
- 23. Zheng M, Hu K, Liu W, Yu X. Characterization of a Clonorchis sinensis antigen, calmodulin, and its relationship with liver fibrosis. Nan Fang Yi Ke Da Xue Xue Bao 2015;35:659-664. (in Chinese).