Abstract
There is renewed interest in natural products as a starting point for discovery of drugs for schistosomiasis. Recent studies have shown that phytol reveals interesting in vivo and in vitro antischistosomal properties against Schistosoma mansoni adult worms. Here, we report the in vitro antischistosomal activity of phytol against Schistosoma haematobium juvenile and adult worms and alterations on the tegumental surface of the worms by means of scanning electron microscopy. The assay, which was carried out with 6 concentrations (25, 50, 75, 100, 125, and 150 μg/ml) of phytol, has shown a promising activity in a dose and time-dependent manner. There was a significant decline in the motility of the worms and a mortality rate of 100% was found at 48 hr after they had been exposed to phytol in the concentration of 150 μg/ml. Male worms were more susceptible. On the ultrastructural level, phytol also induced tegumental peeling, disintegration of tubercles and spines in addition to morphological disfiguring of the oral and ventral suckers. This report provides the first evidence that phytol is able to kill S. haematobium of different ages, and emphasizes that it is a promising natural product that could be used for development of a new schistosomicidal agent.
-
Key words: Schistosoma haematobium, juvenile, adult, phytol, in vitro, schistosomicidal activity
INTRODUCTION
Schistosomiasis remains a truly neglected tropical disease caused by blood flukes of the genus
Schistosoma, with the 3 species
S. mansoni,
S. haematobium, and
S. japonicum responsible for the majority of human infections [
1]. Recent reports of the World Health Organization suggested that more than 249 million people have been infected in 78 countries where the disease is endemic, located in sub-Saharan Africa, the Middle East, the Caribbean, and South America resulting in approximately 200,000 deaths annually [
2]. The close link with poverty, geographical isolation, underappreciated global burden, stigmatization, lack of a political voice of those affected and the aforementioned absence of an established global funding mechanism are some of the factors that explain the general neglect of schistosomiasis [
3-
5].
In the absence of an effective vaccine, the treatment and control of schistosomiasis virtually relies on a single drug, praziquantel (PZQ), which has been used in mass drug administration programs since the 1970s [
6]. The pressing need to develop new antischistosomal compounds has been emphasized [
7] due to the emergence of praziquantel resistant strains in clinical practice [
8]. In addition, an important defect of praziquantel schistosomicidal properties is that it is much less efficient against young developing stages of
S. haematobium [
9]. This might impact the cure rate of the patients treated with praziquantel or reinfection of individuals after treatment [
10,
11]. Underlying reasons are the scarce resources available for schistosomiasis and other neglected tropical diseases and the high failure rates of compounds during preclinical and clinical testing [
12]. Therefore, the development of new anti-
Schistosoma drugs are being pursued until an effective anti-
Schistosoma vaccine can be delivered.
There is a promising trend of using natural compounds derived from plant extracts as drugs against
Schistosoma, being safe and with fewer medical side effects [
13-
15]. It is believed that natural products have the advantage of offering access to the development of novel compounds of schistosomicidal agents due to their well-documented, good coverage of the space relative to large synthetic compounds [
16]. Phytol is the product of chlorophyll metabolism in plants. It is an acyclic diterpene alcohol that can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1 [
17], which are important for many functions of the human body. The use of phytol in the human body is indispensable, it is essential in activating enzymes that have a positive effect on the production of insulin. It can also be effective in decrease of blood cholesterol levels. In medicine, phytol possesses antioxidant, antinociceptive [
18], anti-inflammatory, anti-allergic effects [
19], and excellent immunostimulant effects [
20]. Phytol has also been shown to inhibit the growth of
Staphylococcus aureus [
21], and to block the teratogenic effects of retinol [
22]. de Moraes et al. [
23] reported for the first time the in vitro and in vivo schistosomicidal activity of phytol against
S. mansoni.
So, this study research question was: Does phytol have similar antischistosomal prosperities on S. haematobium too? Particularly in view of the absence of academic work accessing the in vitro or in vivo degree of phytol efficacy on this species up till now, this study investigated the in vitro antischistosomicidal properties of phytol in different concentrations against both juvenile (immature) and adult (mature) S. haematobium stages using light and scanning electron microscopic (SEM) observations.
MATERIALS AND METHODS
The present study was carried out at the Schistosome Biological Supply Center (SBSC), Malacology Laboratory, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Animals and parasites
Syrian golden hamsters (
Mesocricetus auratus), 100-120 g each, were purchased from SBSC, kept under environmentally-controlled conditions temperature 25˚C, humidity 70%, 12-hr light and 12-hr dark cycle and acclimatized for 1 week before infection.
S. haematobium (Egyptian strain) cercariae obtained from SBSC were used to infect the hamsters by abdominal skin exposure. The cercariae were shed from infected
Bulinus truncatus snails and used within 1 hr of shedding. Schistosomes were removed from portal and mesenteric veins of animals after 50 days for juvenile and 90 days for adult according to a previous report [
24], sexed, and counted as described by Xiao et al. [
25].
Phytol (10 g as 97% mixture of isomers) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA) and PZQ tablets (Distocide®) were purchased from EIPICO (Cairo, Egypt). Drugs were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) to obtain stock solutions of 4 mg/ml.
Reagents for cell culture and parasite preparation
RPMI-1640 culture medium with 13.3 µM (molar) phenol red and 2.05 mM L-glutamine and fetal calf serum (FCS) were obtained from GIBCO (OOOO, Germany). FCS was heat-in-activated at 56˚C for 30 min before use. Penicillin G (benzylpenicillin), streptomycin sulfate, heparin sodium salt, L-arginine, and D-glucose were obtained from Sigma (OOOO, Germany).
Drug application
Phytol was used to obtain final concentrations of 25 to 150 µg/ml (25, 50, 75, 100, 125, and 150 µg/ml; equivalent to 84.3, 168.6, 252.9, 337.2, 421.5, and 505.8 µM) in culture plates with a final volume of 3 ml (taking into consideration the constant culture media volume in each well) and applied to 12-well plates filled with a final volume of 3 ml RPMI culture medium. Six worms were cultured per cavity at 37˚C and 5% CO2 immediately after perfusion of animals to ensure vitality. Negative control wells contained adults incubated with 0.5% DMSO plus culture media while positive control wells contained worms incubated with 1 µg/ml PZQ plus the culture media. All experiments were performed in quadruplicates and read at different time points; 24, 48, and 72 hr.
Assessment of drug effect
After schistosomes were exposed to phytol for 24, 48, and 72 hr, parasite survival was evaluated by examination under a dissecting microscope. Parasite death was defined as no motor activity during a 2-min observation.
For SEM studies
Samples of S. haematobium juvenile and adult worms were fixed in glutaraldehyde buffer solution (25%) overnight at 4˚C, and worms were then washed out of the fixative by keeping them overnight at 4˚C in PBS and then post-fixed in 1% OsO4 for 1 hr. Samples were washed and dehydrated in ascending grades of alcohol (30%, 40%, and 50%) each for 15 min. Worms were then kept in 70% alcohol until the time of examination. Before examination, they were washed twice for 30 min in 80% and 90% alcohol, respectively. The last wash was for 1 hr in 95% alcohol, after which worms were mounted on stainless steel holders and put in a drier for about 30 min and then subjected to a sputter coat of gold, the different parts of worms were examined using Jeol JEM-1200 SEM, with a camera fitted to it. Areas in the worms that showed specific changes were examined and photographed; they were mainly suckers and tubercles on the tegument.
Ethical statement
This study was approved by the Scientific Research Ethical Committee, Faculty of Medicine, Benha University, Egypt. The experimental animal studies were conducted in accordance with the ethical guidelines approved by the Ethical Committee of the Federal Legislation and National Institutes of Health Guidelines in the USA and were approved by the Medical Ethical Committee of Theodor Bilharz Research Institute (TBRI) in Egypt (FWA no. 000010609).
Statistical analysis
Statistical analysis was performed using microstate statistical software. Effects of the substances were analyzed with respect to the viability status at 24, 48, and 72 hr time points. Z-test of 2 proportions from a single group was used as the test of significance to compare the proportion of dead worms with that of live in the treated group. A P-value <0.05 or less was considered to be statistically significant.
RESULTS
Phytol-induced antischistosomal effects on juvenile (immature) worms
After being incubated with phytol at 25 µg/ml concentration, juvenile
S. haematobium worms showed normal motor activity and viability throughout the incubation. During this period, all male worms attached to the well wall with their ventral sucker and revealed natural peristalsis of the worm body. At 50 µg/ml phytol concentration, only
S. haematobium males showed reduced motor activity with abnormal body attitude and died after 72 hr of incubation, while females remained viable throughout the incubation period at that concentration, and began to be affected only after 48 hr exposure to 75 µg/ml phytol concentration, and the body become elongated. This concentration caused death of 100% of male adult parasites within 48 hr. A 100% female death rate was observed at 125 and 150 µg/ml phytol concentration (
Table 1).
In negative control groups (worms were maintained in phytol-free RPMI 1640 medium containing only 0.5% DMSO for 72 hr), there was no change in the motor activity and their appearance was similar to those maintained in the same medium without DMSO (DMSO-free RPMI 1640 medium). While worms incubated in medium containing 1 µg/ml concentration of PZQ, there was complete loss of motor activity in all worms and death of all parasites within 24 hr was observed (total death rate was 100%).
Phytol-induced tegumental damages in juvenile S. haematobium worms
The effect of phytol on both male and female schistosomes started at 50 and 75 µg/ml after 72 hr concentrations in the form of mild to moderate tegumental alterations. Severely damaged worms were seen at higher concentrations (100, 125, and 150 µg/ml) after 72 hr (
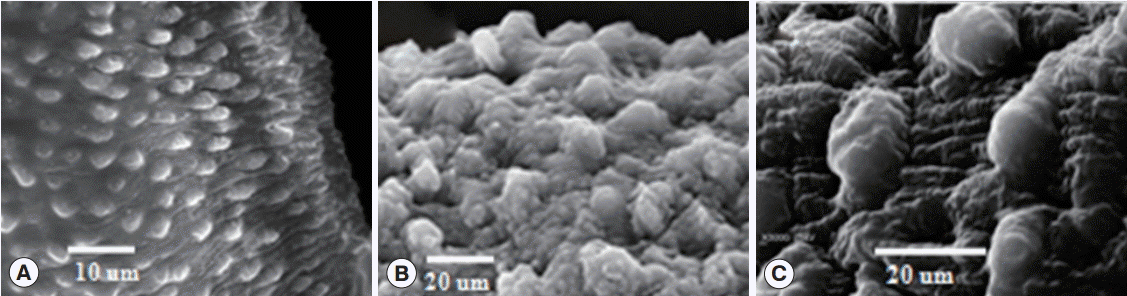
Fig. 1). Morphological alterations on the surface of male schistosomes were in the form of worm deformity and flattened shrunken suckers. The tegument was swollen in some parts and flattened in other parts with loss of tubercles, shrinking, and furrowing (
Fig. 1B,
C) and with swelling and deformity in the dorsal tegumental surface which appeared swollen and ballooned with edematous interpapillary ridges. Schistosomes from negative control groups showed an intact tegument (
Fig. 1A).
Reduced motility was observed with lower phytol concentrations, and in the early incubation stages the motility was in the form of reduced sluggish movement particularly in male worms. Phytol effect was dose and time-dependent as these changes became progressive with increased dose and time associated with complete loss of sucking capacity of male worms, deformity, followed by separation of the female worms from the gynecophoral canal of the paired male worm. Phytol’s lethal effect on adult male worms was observed initially at 50 µg/ml concentration after 48 hr (33.3%) and increased to 66.6% after 72 hr of incubation (
Table 2). The death rate was progressively increased to reach 50% and 100% at 48 hr and 72 hr at 75 µg/ml concentration, respectively. A 100% death of male schistosomes was observed after 48 hr at the subsequent concentrations (100, 125, and 150 µg/ml). The initial effect on adult female schistosomes was observed at 75 µg/ml with 66.7% death rate after 72 hr. This effect was progressively increased with a total 100% death rate after 72 hr at 100 and 125 µg/ml concentrations. At 150 µg/ml concentration, the total female death was observed after 48 hr incubation.
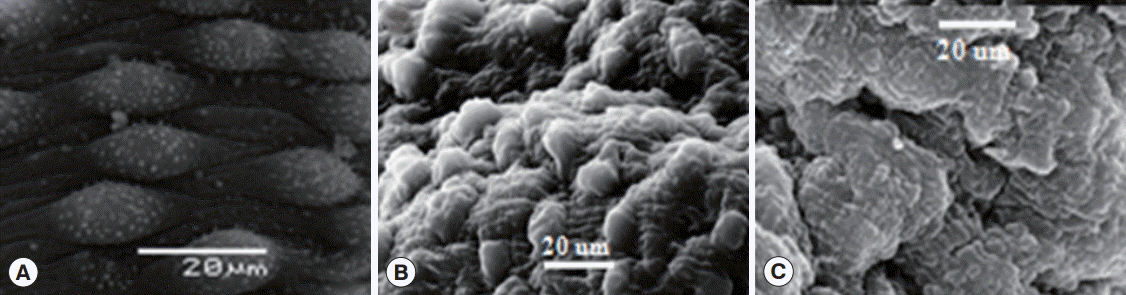
As with juvenile worms, both male and female adults were also found to be susceptible to phytol, as shown by SEM of the exposed worms (
Fig. 2). Mild to moderate tegumental alterations were observed in the worms exposed to phytol at a concentration of 50 and 75 µg/ml after 72 hr, whereas severely damaged worms were seen at higher concentrations (100, 125, and 150 µg/ml) after the same time period (at 72 hr). Morphological alterations on the surface of male schistosomes were detected with phytol at concentrations of 50 to 100 µg/ml, in the form of worm deformity. The tegument was swollen in some parts and flattened in other parts with loss of tubercles and edema of intertubercular spaces. This change progressively increased with higher concentrations to complete disruption of the tegument with the dorsal tegumental surface of adult treated worms showing massive deformity of tubercles with edematous intertubercular spaces (
Fig. 2B,
C). Schistosomes from negative control groups showed intact tegument (
Fig. 2A).
DISCUSSION
Schistosomiasis control relies on a single drug. Praziquantel is effective mainly against the adult but not the larval stages. In addition, it has many shortcomings [
26-
28]. In recent years, a great interest in natural products for the treatment of a number of diseases, including schistosomiasis, has been growing. Many researches are screening the possible antischistosomal activity of many plant originated substances [
15,
23,
29-
32]. Of them, phytol, which is a diterpene, is a member of the group of branched-chain unsaturated alcohols [
33].
In vitro and in vivo promising antischistosomal properties of phytol against adult
S. mansoni have been reported [
23]. However, no study with phytol against
S. haematobium has been performed. To further deepen our understanding of the activity of this natural compound against schistosomes, we investigated, for the first time, the schistosomicidal effect of phytol on
S. haematobium of different stages (juvenile and adult stages) as well as the drug induced tegumental alterations at various time points.
In this study, phytol was proved to have a promising antischistosomal property against both juvenile (immature) and adult (mature) stages of S. haematobium. Phytol effects were time and dose-dependent, and the highest efficacy was observed at 150 µg/ml concentration throughout a relatively shorter period of incubation (48 hr), with affection of both sexes. However, the effect was more pronounced against male worms. Furthermore, we observed that the schistosomes exposed to phytol showed motility changes in the form of slow contractions and the parasites’ death. Dissecting microscopic investigations demonstrated highly wide-scaled effects of phytol on S. haematobium worm viability and a forcible effect on their morphology.
These findings were broadly in line with that obtained by de Moraes et al. [
23] who investigated, for the first time, in vivo and in vitro schistosomicidal efficacy of phytol on adult
S. mansoni worms and recorded reduction of the motor activity of worms causing their death. However, they recorded that female worms were more affected than males which was contrary to the results observed in the present study, which detected a more pronounced effect on male worms, whether in juvenile or adult stages.
Comparable results regarding antischistosomal properties were obtained by previous in vitro trials conducted by other researchers, using compounds isolated from other plant species (e.g., Piplartina) isolated from
Piper tuberculatum, artemether, and 8-hydroxyquinoline derivatives [
28,
30,
34]. They have demonstrated activities against schistosomula and juvenile worms of
S. mansoni. However, the mechanisms of action have not been elucidated.
In relation to PZQ assays (1 µg/ml), all the worms, males and females, were contracted with no movement whatsoever. Earlier studies by Pica-Mattoccia and Cioli [
35] inform us about the PZQ effects on the worms, causing contractions whenever the parasite is exposed to concentrations 0.1 and 1 µg/ml. However, it is known that PZQ causes a quick calcium influx followed by contraction, paralysis, and tegumental destruction. Nevertheless, that process was not completely clarified [
36]. Praziquantel caused severe muscle contractions and the worms became partially curved or ‘swirled.’ In contrast, phytol caused worm paralysis but no muscle contraction; however, the exact mechanisms underlining this effect are still unclear.
Schistosoma tegument is involved in a variety of functions that are important for the worm, such as nutrient absorption, and it presents proteins that are responsible for maintenance of the host immune responses or damage repair. Moreover, the tegument is the interface between the parasite and the host environment. Therefore, the tegument represents an important target for many drug actions [
37,
38]. Alterations in the surface topography of schistosome worms were used by several investigators for evaluation of antischistosomal drug activities in vitro and in vivo [
39-
41]. SEM allows detailed observation of the morphology of the tegument making it possible to interpret its functionality [
42]. In the present study, by means of SEM analysis, it was observed that phytol has induced extensive tegumental damages in both immature and mature stages of
S. haematobium following in vitro incubation, which was dose and time-dependent, i.e., it was intensified progressively as the incubation period and phytol concentration increased. Abnormal body attitude, flattened spines, shrinking, corrugations, unfolding and widening of the gynecophoral canal, and sloughing and disintegration of the tegument were observed on the tegument of examined worms in the present investigation. Comparable results were obtained by previous studies using other antischistosomal natural compounds, such as dermaseptin [
43], epiisopiloturine [
41], (+) limonene epoxide [
32], and phytol [
23]. The exact mechanisms phytol exerts in its in vitro schistosomicidal effect is still unclear. However, an evident correlation between worm viability and tegumental alterations was observed.
In conclusion, the present findings proved that phytol has promising schistosomicidal properties on immature and adult S. haematobium worms manifested by extensive morphological, tegumental alterations, and a direct killing effect which are both concentration and time-related, thus opening a new horizon for effective treatment of prepatent human S. haematobium infection and guarding against the health burden of mature infection. However, more studies are needed to elucidate the mechanisms of action and to address phytol suitable dosage regimen in treating human infection through clinical trials. Decreasing or postponing the imminent threat of resistant S. haematobium strains resulting from sole dependence on praziquantel in the treatment of human schistosomiasis might be more achievable than interested academics have so far taught.
Notes
-
The authors declare that there is no conflict of interest.
Fig. 1.SEM investigation of juvenile Schistosoma haematobium male worm after in vitro incubation with phytol. (A) Negative control (RPMI 1640 medium) after 120 hr showing intact tubercles on the dorsal part of the tegument (T). (B) A worm treated with 100 µg/ml phytol after 72 hr; dorsal tegumental surface shows deformity of tubercles with edematous intertubercular spaces. (C) A worm treated with 150 µg/ml phytol after 72 hr, showing swelling and deformity in the dorsal tegumental surface, which appeared to be swollen and ballooned with edematous interpapillary ridges.

Fig. 2.SEM investigation of adult Schistosoma haematobium male worm after in vitro incubation with phytol. (A) Negative control (RPMI 1640 medium) after 120 hr showing intact tubercles on the dorsal part of the tegument (T). (B) A worm treated with 50 µg/ml of phytol after 72 hr, showing edema and deformity of the dorsal tegumental surface with irregular intertubercular spaces and collapse of tubercles in some parts and swelling in other parts. (C) A worm treated with 100 µg/ml phytol after 72 hr; dorsal tegumental surface shows massive deformity of tubercles with edematous intertubercular spaces.

Table 1.Effect of different phytol concentrations on immature Schistosoma haematobium
Table 1.
|
Groups |
|
No. of tested worms
|
No. (%) of dead worms
|
P1 |
Total death rate (%) |
P2 |
|
♂ |
♀ |
♂ |
♀ |
|
Control (negative) |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
72 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
Control (positive) (Praziquantel 1 µg/ml) |
24 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
--- |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
--- |
|
25 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
72 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
50 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
72 hr |
6 |
6 |
6 (100.0) |
0 (0.0) |
< 0.001a
|
50.0 |
1 |
|
75 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
---- |
0.0 |
----- |
|
48 hr |
6 |
6 |
6 (100.0) |
0 (0.0) |
< 0.001a
|
50.0 |
1 |
|
72 hr |
6 |
6 |
6 (100.0) |
4 (66.7) |
0.12 |
83.3 |
< 0.001a
|
|
100 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
5 (83.3) |
3 (50.0) |
0.22 |
66.7 |
0.22 |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
---- |
|
125 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
---- |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
---- |
|
150 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
---- |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
---- |
Table 2.Effect of different phytol concentrations on adult Schistosoma haematobium
Table 2.
|
Groups |
|
No. of tested worms
|
No. (%) of dead worms
|
P1 |
Total death rate (%) |
P2 |
|
♂ |
♀ |
♂ |
♀ |
|
Control (negative) |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
72 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
Control (positive) (Praziquantel 1 µg/ml) |
24 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
--- |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
--- |
100.0 |
--- |
|
25 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
48 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
72 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
--- |
0.0 |
--- |
|
50 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
---- |
0.0 |
----- |
|
48 hr |
6 |
6 |
2 (33.3) |
0 (0.0) |
0.12 |
16.7 |
< 0.001*
|
|
72 hr |
6 |
6 |
4 (66.7) |
0 (0.0) |
0.014*
|
33.3 |
0.22 |
|
75 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
---- |
0.0 |
----- |
|
48 hr |
6 |
6 |
3 (50.0) |
0 (0.0) |
0.044*
|
25.0 |
0.044*
|
|
72 hr |
6 |
6 |
6 (100.0) |
4 (66.7) |
0.12 |
83.3 |
< 0.001a
|
|
100 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
---- |
0.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
0 (0.0) |
< 0.001a
|
50.0 |
1.0 |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
---- |
100.0 |
---- |
|
125 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
---- |
0.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
0 (0.0) |
< 0.001a
|
50.0 |
1.0 |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
---- |
100.0 |
---- |
|
150 µg/ml |
24 hr |
6 |
6 |
0 (0.0) |
0 (0.0) |
---- |
0.0 |
--- |
|
48 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
---- |
100.0 |
---- |
|
72 hr |
6 |
6 |
6 (100.0) |
6 (100.0) |
---- |
100.0 |
---- |
References
- 1. Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol 2014;36:347-357.
- 2. WHO. Schistosomiasis. Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs115/en (accessed on 10 December 2014)
- 3. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 2009;373:1570-1575.
- 4. Gray DJ, McManus DP, Li YS, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis 2010;10:733-736.
- 5. Payne L, Fitchett JR. Bringing neglected tropical diseases into the spot light. Trends Parasitol 2010;26:421-423.
- 6. Gönnert R, Andrews P. Praziquantel, a new broad spectrum antischistosomal agent. Z Parazitenkunde 1977;52:129-150.
- 7. Caffrey CR. Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol 2007;11:433-439.
- 8. Alonso D, Muñoz J, Gascón J, Valls ME, Corachan M. Short report: failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am J Trop Med Hyg 2006;74:342-344.
- 9. Botros S, Pica-Mattoccia L, William S, El-Lakkani N, Cioli D. Effect of praziquantel on the immature stages of Schistosoma haematobium. Int J Parasitol 2005;35:1453-1457.
- 10. N'Goran EK, Gnaka HN, Tanner M, Utzinger J. Efficacy and side-effects of two praziquantel treatments against Schistosoma haematobium infection, among schoolchildren from Côte d'Ivoire. Ann Trop Med Parasitol 2003;97:37-51.
- 11. Grandiére-Pérez L, Ansart S, Paris L, Faussart A, Jaureguiberry S, Grivois JP, Klement E, Bricaire F, Danis M, Caumes E. Efficacy of praziquantel during the incubation and invasive phase of Schistosoma haematobium schistosomiasis in 18 travelers. Am J Trop Med Hyg 2006;74:814-818.
- 12. Hopkins AL, Witty MJ, Nwaka S. Mission possible. Nature 2007;449:166-169.
- 13. Molgaard P, Nielsen SB, Rasmussen DE, Drummond RB, Makaza N, Andreassen J. Anthelminthic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol 2001;74:257-264.
- 14. Kayser O, Kiderlen AF, Croft SL. Natural products as antiparasitic drugs. Parasitol Res 2003;90:S55-S62.
- 15. Parreira NA, Magalhães LG, Morais DR, Caixeta SC, de Sousa JP, Bastos JK, Cunha WR, Silva ML, Nanayakkara NP, Rodrigues V, da Silva Filho AA. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem Biodivers 2010;7:993-1001.
- 16. Harvey AL, Clark RL, Mackay SP, Johnston BF. Current strategies for drug discovery through natural products. Expert Opin Drug Discov 2010;5:559-568.
- 17. Daines AM, Payne RJ, Humphries ME, Abell AD. The synthesis of naturally occurring Vitamin K and Vitamin K analogues. Curr Organ Chem 2003;7:1625-1634.
- 18. Santos CCMP, Salvadori MS, Mota VG, Costa LM, Almeida AACO, de Oliveira GAL, Costa JP, de Sousa DP, de Freita RM, de Almeida RN. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci J 2013;Article ID 949452.
- 19. Ryu KR, Choi JY, Chung S, Kim DH. Anti-scratching behavioral effect of the essential oil and phytol isolated from Artemisia princeps Pamp. in mice. Planta Med 2011;77:22-26.
- 20. Lim SY, Meyer M, Kjonaas RA, Ghosh SK. Phytol-based novel adjuvants in vaccine formulation: 1. assessment of safety and efficacy during stimulation of humoral and cell-mediated immune responses. J Immune Based Ther Vaccines 2006;4:6.
- 21. Inoue Y, Hada T, Shiraishi A, Hirose K, Hamashima H, Kobayashi S. Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Antimicrob Agents Chemother 2005;49:1770-1774.
- 22. Arnhold T, Elmazar MMA, Nau H. Prevention of vitamin A teratogenesis by phytol or phytanic acid results from reduced metabolism of retinol to the teratogenic metabolite, all-trans-retinoic acid. Toxicol Sci 2002;66:274-282.
- 23. de Moraes J, de Oliveira RN, Costa JP, Junior ALG, de Sousa DP, Freitas RM, Allegretti SM, Pinto PL. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl Trop Dis 2014;8:e2617.
- 24. Duvall RH, Dewitt WB. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg 1967;16:483-486.
- 25. Xiao SH, Keiser J, Chollet J, Utzinger J, Dong Y, Endriss Y, Vennerstrom JL, Tanner M. The in vitro and in vivo activities of synthetic trioxolanes on major human schistosome species. Antimicrob Agents Chemother 2007;51:1440-1445.
- 26. Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. Mefloquine - an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis 2009;3:e350.
- 27. Beckmann S, Leutner S, Gouignard N, Dissous C, Grevelding CG. Protein kinases as potential targets for novel anti-schistosomal strategies. Curr Pharm Des 2012;18:3579-3594.
- 28. El-Lakkany NM, Seif El-Din SH. Haemin enhances the in vivo efficacy of artemether against juvenile and adult Schistosoma mansoni in mice. Parasitol Res 2013;112:2005-2015.
- 29. Pereira AC, Magalhães LG, Gonçalves UO, Luz PP, Moraes AC, Rodrigues V, da Matta Guedes PM, da Silva Filho AA, Cunha WR, Bastos JK, Nanayakkara NP, de Silva ML. Schistosomicidal and trypanocidal structure-activity relationships for (±)-licarin A and its (-)- and (+)- enantiomers. Phytochemistry 2011;72:1424-1430.
- 30. Moraes J. Antischistosomal natural compounds: present challenges for new drug screens. In Rodriguez-Morales AJ ed, Current Topics in Tropical Medicine. Intech; Rijeka. 2012, pp 333-358.
- 31. Moraes J, Nascimento C, Lopes PO, Nakano E, Yamaguchi LF, Kato MJ, Kawano T. Schistosoma mansoni: in vitro schistosomicidal activity of piplartine. Exp Parasitol 2011;127:357-364.
- 32. Moraes Jd, Almeida AA, Brito MR, Marques TH, Lima TC, Sousa DP, Nakano E, Mendonça RZ, Freitas RM. Anthelmintic activity of the natural compound (+)-limonene epoxide against Schistosoma mansoni. Planta Med 2013;79:253-258.
- 33. McGinty D, Letizia CS, Api AM. Fragrance material review on phytol. Food Chem Toxicol 2010;48:S59-S63.
- 34. Allam G, Eweas AF, Abuelsaad ASA. In vivo schistosomicidal activity of three novels 8-hydroxyquinoline derivatives against adult and immature worms of Schistosoma mansoni. Parasitol Res 2013;112:3137-3149.
- 35. Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol 2004;34:527-533.
- 36. Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 2008;21:659-667.
- 37. Hoffmann KF, Strand M. Molecular identification of a Schistosoma mansoni tegumental protein with similarity to cytoplasmic dynein light chains. J Biol Chem 1996;271:26117-26123.
- 38. Xiao S, Shen B, Utzinger J, Chollet J, Tanner M. Ultrastructural alterations in adult Schistosoma mansoni caused by artemether. Mem Inst Oswaldo Cruz 2002;97:717-724.
- 39. de Oliveira RN, Rehder VLG, Santos Oliveira AS, Júnior ÍM, de Carvalho JE, de Ruiz ALT, Jeraldo VIS, Linhares AX, Allegretti SM. Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp Parasitol 2012;132:135-143.
- 40. de Oliveira RN, Rehder VLG, Santos Oliveira AS, Jeraldo V de LS, Linhares AX, Allegretti SM. Anthelmintic activity in vitro and in vivo of Baccharis trimera (Less) DC against immature and adult worms of Schistosoma mansoni. Exp Parasitol 2014;139:63-72.
- 41. Veras LM, Guimaraẽs MA, Campelo YD, Vieira MM, Nascimento C, Lima DF, Vasconcelos L, Nakano E, Kuckelhaus SS, Batista MC, Leite JR, Moraes J. Activity of epiisopiloturine against Schistosoma mansoni. Curr Med Chem 2012;19:2051-2058.
- 42. Senft AW, Gibler WB. Schistosoma mansoni tegumental appendages: scanning microscopy following thiocarbohydrazide-osmium preparation. Am J Trop Med Hyg 1977;26:1169-1177.
- 43. de Moraes J, Nascimento C, Miura LM, Leite JR, Nakano E. Evaluation of the in vitro activity of dermaseptin 01, a cationic antimicrobial peptide, against Schistosoma mansoni. Chem Biodivers 2011;8:548-558.