Abstract
The genus Spirometra belongs to the family Diphyllobothriidae and order Pseudophyllidea, and includes intestinal parasites of cats and dogs. In this study, a plerocercoid labeled as Spirometra mansonoides from the USA was examined for species identification and phylogenetic analysis using 2 complete mitochondrial genes, cytochrome c oxidase I (cox1) and NADH dehydrogenase subunit 3 (nad3). The cox1 sequences (1,566 bp) of the plerocercoid specimen (USA) showed 99.2% similarity to the reference sequences of the plerocercoid of Korean Spirometra decipiens (GenBank no. KJ599679), and 99.1% similarity in regard to nad3 (346 bp). Phylogenetic tree topologies generated using 4 analytical methods were identical and showed high confidence levels with bootstrap values of 1.00, 100%, 100%, and 100% for Bayesian inference (BI), maximum-likelihood (ML), neighbor-joining (NJ), and maximum parsimony (MP) methods, respectively. Representatives of Diphyllobothrium and Spirometra species formed a monophyletic group, and the sister-genera status between these species was well supported. Trapezoic proglottids in the posterior 1/5 region of an adult worm obtained from an experimentally infected cat were morphologically examined. The outer uterine loop of the uterus coiling characteristically consisted of 2 complete turns. The results clearly indicated that the examined Spirometra specimen from the USA matched to S. decipiens very well, and indicated possible presence of the life cycle of this species in this region.
-
Key words: Spirometra decipiens, sparganum, molecular detection, cox1, nad3, USA
INTRODUCTION
The genus
Spirometra belongs to the family Diphyllobothriidae and order Pseudophyllidea, and includes intestinal parasites of cats and dogs. Humans can be infected by procercoid or plerocercoid larvae of the genus
Spirometra. The procercoids develop in
Cyclops spp. and the plerocercoids develop in reptiles or amphibians, and can cause sparganosis in humans. The genus
Diphyllobothrium was divided taxonomically into 2 subgenera
Diphyllobothrium and
Spirometra by Faust et al. [
1], but later this genus was established as an independent genus by Mueller [
2]. The genus
Spirometra has been reviewed using the morphological characteristics of spirometrid species (under the name
Diphyllobothrium), including spirometrids
S. erinaceieuropaei (Rudolphi, 1819),
S. decipiens (Diesing, 1850),
S. ranarum (Gastaldi, 1854),
S. mansoni (Cobbold, 1882),
S. houghtoni (Faust et al., 1929), and
S. okumurai (Faust et al., 1929) by Faust et al. [
1]. In 1935, Mueller [
2] described the new species
S. mansonoides and indicated that it could be distinguished from
S. mansoni distributed in the Asian region based on morphological characteristics.
The taxonomy of the genus
Spirometra has long been controversial. In 1929, Faust, Campbell and Kellogg [
1] studied on the identification of 6
Spirometra species. In 1952, Wardle and McLeod [
4] recognized 14
Spirometra species and divided them into 2 separate groups. In 1959, Yamaguti [
5] insisted that all species in the genus
Spirometra were synonyms of
S. erinaceieuropaei. However, in 1999, Kamo [
6] acknowledged
S. erinaceieuropaei,
S. pretoriensis (Baer, 1924),
S. theileri (Baer, 1924), and
S. mansonoides (Mueller, 1935) as valid species [
6].
Among these species,
S. mansonoides has been known as the only
Spirometra species in North America that has a C-shaped outer loop of the uterus with its anterior limb constricted in the midline to form a lateral expulsion chamber [
2]. Iwata [
7], in 1972, argued that the morphological difference in uterine shape relative to the mature proglottids of
S. erinaceieuropaei is due to differences in developmental stages but not specific characters. Later, a molecular difference was observed between
S. erinaceieuropaei and
S. mansonoides using PCR-RFLP and
cox1 sequence analysis [
8]. More recently, human sparganosis was identified by morphological and genetic analyses in Korea, and the complete mitochondrial genomes of
S. erinaceieuropaei and
S. decipiens isolated from Korea have been recorded and compared [
9,
10]. It has been shown that mtDNA sequences are a useful molecular marker for identification of
Spirometra species and phylogenetic analysis in order to obtain accurate resolution of taxonomic relationships [
10].
In this study, we identified a Spirometra species of USA origin (plerocercoid) by molecular analysis using 2 complete mitochondrial genes, cytochrome c oxidase I (cox1) and NADH dehydrogenase subunit 3 (nad3) as well as by morphological observations of an adult tapeworm experimentally obtained from a cat fed the plerocercoid.
MATERIALS AND METHODS
Specimens
The parasite materials, a plerocercoid and an adult
Spirometra worm (USA-derived) used in this study, were donated by the Parasite Resource Bank of Korea (specimen no. PRBA333), which was deposited by one of the authors, Dr. Woon-Mok Sohn of the Department of Parasitology and Tropical Medicine, Institute of Health Sciences, Gyeongsang National University College of Medicine, Jinju, Korea. The specimen history began from eggs of
S. mansonoides originating from Dr. C. K. Phares of the Department of Biochemistry and Molecular Biology, University of Nebraska, USA [
8]. The eggs were incubated in the laboratory to establish an experimental life cycle using cyclops, tadpoles and frogs, and a cat in Korea (Sohn WM. Personal communication). Plerocercoids were obtained from experimental tadpoles and frogs. The plerocercoids were used to infect a cat. After 3 weeks of infection, an adult tapeworm was recovered from the intestine of the cat. The adult tapeworm was compressed and fixed in alcohol-formalin-acetic acid (AFA) for carmine staining. Some of the proglottids preserved in 70% ethanol were sent to the Department of Parasitology, Chungbuk National University School of Medicine, Cheongju, Korea for morphological characterization and molecular analysis (
Fig. 1). The vaginal opening, uterus, uterine pore, cirrus, genital pore, testes, and vitellaria of mature and gravid proglottids were compared to those of Faust et al. [
1].
Total genomic DNA was extracted from a single proglottid using a DNeasy tissue kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. The entire cox1 and nad3 genes were amplified by PCR. Primers were designed from the complete sequences of S. erinaceieuropaei (KJ599680) and S. decipiens (KJ599679) mitochondrial genomes and used for amplifying the full cox1 gene. The PCR primers were used to amplify the cox1 region (spcox1f: 5ʹ-GTA TTG AAG GAA TTA GTT AGG TTA-3ʹ and spcox1r: 5ʹ-CAA CCC AAT TAA ATT AAG TTC CAC-3ʹ) and nad3 region (spnad3f: 5ʹ-GTG TGT TTT TGC ACT GTG-3ʹ and spnad3r: 5ʹ-ATT GAC AAT AGA TTA TTA GCA-3ʹ) in order to amplify and sequence the cox1 and nad3 genes, respectively. PCR was performed in 50 μl reaction mixture with 0.01 μg/μl genomic DNA, 10× PCR buffer (20 mM Mg+), a 10 mM dNTP mixture, 10 pmols of each primer, and 2.5 U/μl Taq DNA polymerase (High Fidelity PCR system, Roche, Mannheim, Germany). PCRs were performed in a GeneAmp PCR system 9700 (Applied Biosystems, Langen, Germany) as follows: 1 cycle of initial denaturation at 94˚C for 3 min, 30 cycles at 94˚C for 1 min, 50˚C for 1 min, 72˚C for 2 min, and extension at 72˚C for 10 min. This resulted in cox1 (1,566 bp) and nad3 (346 bp) DNA fragments, which were isolated on a 1.0% agarose gel, excised under long-wave UV light, and extracted using a QIAquick PCR purification kit (Qiagen). Cyclic sequencing was performed using a Big-Dye terminator sequencing kit (version 3.2, Applied Bio systems, Foster City, California, USA). The reaction products were directly sequenced using a DNA sequencer (3739XL model, Applied Biosystems).
Phylogenetic analyses
The DNA sequences were assembled and aligned using Geneious 6.1.5 (Biomatter, Auckland, New Zealand) and identified by BLAST searches and compared with the sequences of
S. erinaceieuropaei and
S. decipiens in the GenBank database. Phylogenetic analysis was performed using PAUP 4.0 [
11]. Phylogenetic trees were constructed using the complete
cox1 sequences (1,566 bp) of 5 taxa of Diphyllobothriidae as represented by
S. erinaceieuropaei (KJ599680),
S. decipiens (KJ599679), Sparganum proliferum (AB015753),
Diphyllobothrium latum (NC_008945), and
D. nihonkaiense (NC_ 009463). The phylogenetic relationships were evaluated using Bayesian inference (BI), maximum-likelihood (ML), neighbor-joining (NJ), and maximum parsimony (MP) criteria. ML analyses of
cox1 and
nad3 used the TVM + I + G model chosen according to the Modeltest [
12]. BI analyses were used in MrBayes 3.2 [
13], running 2 independent MC
3 runs of 4 chains each, for 10 million generations.
RESULTS
Sequence similarity
The
cox1 sequences (1,566 bp) of the USA-derived
Spirometra species (PRBA333) showed 99.2% similarity to the reference sequences of Korean-derived
S. decipiens (GenBank no. KJ599679) and 89.6% similarity with reference sequences of the Korean-derived
S. erinaceieuropaei (GenBank no. KJ599680). However, the similarity with the other 3 species was only 89.1% (Sparganum proliferum), 84.5% (
D. nihonkaiense), and 83.5% (
D. latum). The genetic similarity of
nad3 sequences (346 bp) between the
Spirometra species (USA origin) and the reference sequence was 99.1% (
S. decipiens), 89.7% (
S. erinaceieuropaei), 89.1% (Sparganum proliferum), 84.1% (
D. nihonkaiense), and 83.1% (
D. latum) (
Table 1).
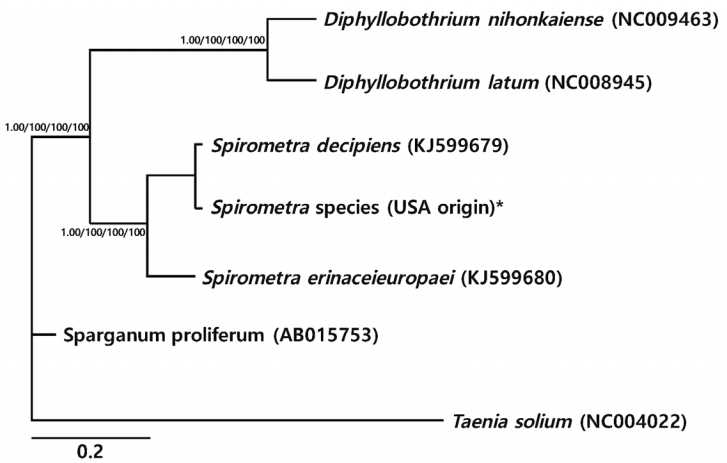
Phylogenetic analyses of
Spirometra species were performed using the 4 methods (BI, ML, NJ, and MP) based on mitochondrial
cox1 and
nad3 sequences of
S. decipiens,
S. erinaceieuropaei, Sparganum proliferum,
D. nihonkaeiense, and
D. latum. The
cox1 sequences (1,566 bp) showed 151 polymorphic sites, with 10 sites of non-synonymous substitutions, while
nad3 sequences (346 bp) presented 45 polymorphic sites, with 8 sites of non-synonymous substitu tions between the
Spirometra species (USA origin) and
S. erinaceieuropaei (no. KJ599680). Phylogenetic tree topologies generated using the 4 analytic methods were identical and showed high confidence levels (bootstrap values of 1.00, 100%, 100%, 100%, and 100% for BI, ML, NJ, and MP, respectively) for the 2 major branches representing each of the 3 species. Representatives of
Diphyllobothrium and
Spirometra species formed a monophyletic group, and sister genera were confirmed (
Fig. 1).
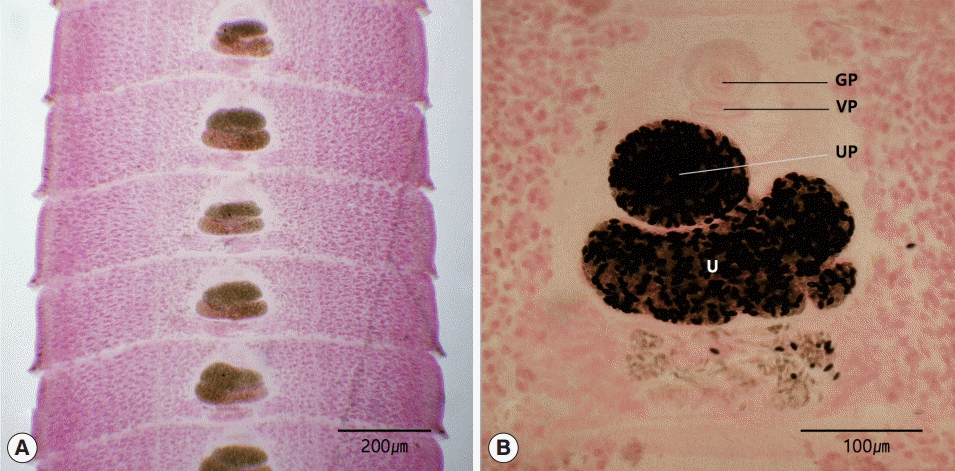
The length of the strobila was 41 cm. The scolex was spatulate and had a diameter of 0.2 mm and the length of 1.5 mm. The neck was behind the scolex and measured 1.0 cm. The genital primordium measured 2.0-2.5 cm. The mature proglottids with eggs were first visible almost 10 cm downward from the neck. The width and length of gravid proglottids were 0.5-0.9 mm and 0.1-0.2 mm, respectively. The genital pore was situated ventrally on the midline in the anterior 1/5 of the segment. The uterine pores were on the midline behind the anterior margin of the terminal ball, 70-90 μm. The uterus consisted of 2 loops. The dumbbell-shaped ovary was connected to the uterus and situated near the posterior margin of the segment (
Fig. 2).
DISCUSSION
In the present study, morphological observations were conducted and the 2 mitochondrial cox1 and nad3 genes of the Spirometra species (USA origin) were sequenced and analyzed. The sequence homology of more than 99% for the cox1 and nad3 genes was within the congeneric range (less than 2%) detected between S. decipiens and the USA-derived Spirometra specimen. These results support the view that the Spirometra specimen (labeled as S. mansonoides) isolated from the USA and examined in this study should be classified as S. decipiens. As far as the literature is concerned, this case is considered the first report of S. decipiens detected in North America based on morphological and genetic identification.
The only
Spirometra species that has been reported in North America was
S. mansonoides, which was based on an adult tapeworm obtained from cats in New York State, and from spargana found in snakes (
Natrix) from Florida by Mueller in 1935 [
3]. The notable features of this worm were the C-shaped outer loop of the uterus with its anterior limb constricted in the midline to form a lateral expulsion chamber, which is a characteristic feature [
3].
S. decipiens was established by Diesing in 1850 [
1] as
Dibothrium decipiens based on an adult worm from an unidentified catlike animal in Brazil. The first alleged rediscovery of this worm was made by Chandler in 1925 [
14] from a domestic cat and a clouded leopard (
Felis nebulosa) in the Calcutta Zoological Gardens.
S. decipiens was then recorded from a naturally infected wild cat, a leopard, and a dog in Peking and Amoy in China, and was described in detail with morphologic characteristics by Faust, Campbell and Kellogg in 1929 [
1]. The geographical distribution of
S. decipiens includes Brazil, China, and Korea [
1,
9]. It seems probable that
S. decipiens spread from South to North America through their customary host, i.e., dogs and cats.
A number of species of
Spirometra described on the basis of morphological features of the adult worms are of uncertain taxonomic status because of considerable variability in their features. Yamaguti in 1959 [
5] and Iwata in 1972 [
7] conducted morphological investigations and insisted that
S. mansonoides is a synonym of
S. erinaceieuropaei and that these 2
Spirometra species should be considered the same species. However,
S. erinaceieuropaei can be clearly distinguished from
S. decipiens morphologically by its spirally coiled uterus. The uterus of
S. erinaceieuropaei consists of 5-7 complete turns, whereas that of
S. decipiens consists of 4-4½ coils [
1]. The major distinguishing features between
S. mansonoides and other
Spirometra species are the C-shaped outer loop of the uterus and the 2-turn uterine coils [
2].
In this study, the Spirometra specimen examined exhibited 2-2½ uterine coils and the following distinguishing features: the uterine pore lies in the midline, a conspicuous sphincter with a ventral position under the bulge of the uterine terminal ball, a crescent shaped and elliptical genital pore, and the muscular and elongated shape of cirrus. These morphological characteristics of the male and female reproductive organs of the Spirometra specimen (USA origin) examined in this study are consistent with those of S. decipiens regarding the shape of the uterus, the vaginal opening, and the cirrus. The use of molecular approaches for genetic analysis of Spirometra spp. obtained from a wide range of hosts and geographical locations should enable specific identification. Genetic analysis will provide diagnostic benefits such as determining the number of genotypes related to human infection, and clarifying which intermediate host is related to human sparganosis.
This study has shown that specific identification of Spirometra species and assays utilizing cox1 and nad3 genetic markers could be used effectively to clarify epidemiological questions and determine whether different transmission patterns exist. Our results clearly indicated that features of the USA-derived Spirometra specimen examined coincided well with those of S. decipiens, which suggests the possible distribution of this species in North America.
Notes
-
We have no conflict of interest related to this work.
This work was supported by the National Research Foundation of Korea (2014R1A1A2004933). Parasite materials used in this study were provided by the Parasite Resource Bank of Korea National Research Resource Center (2010-0003456), the Republic of Korea.
Fig. 1.Phylogenetic relationships of Spirometra species based on cox1 and nad3 gene sequences. Numbers above the branches represent bootstrap values for Bayesian inference (BI), maximum likelihood (ML), neighbor joining (NJ), and maximum parsimony (MP) methods, respectively. *Currently known as S. mansonoides.
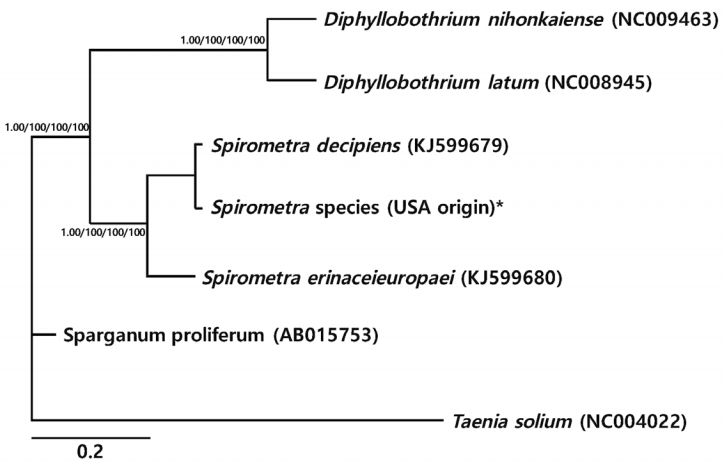
Fig. 2.Gravid proglottids of Spirometra decipiens (USA origin). Whole mounted specimens of proglottids showing the uterus (U), genital pore (GP), vaginal pore (VP), and uterine pore (UP) (H-E stain). (A) ×12. (B) ×40.
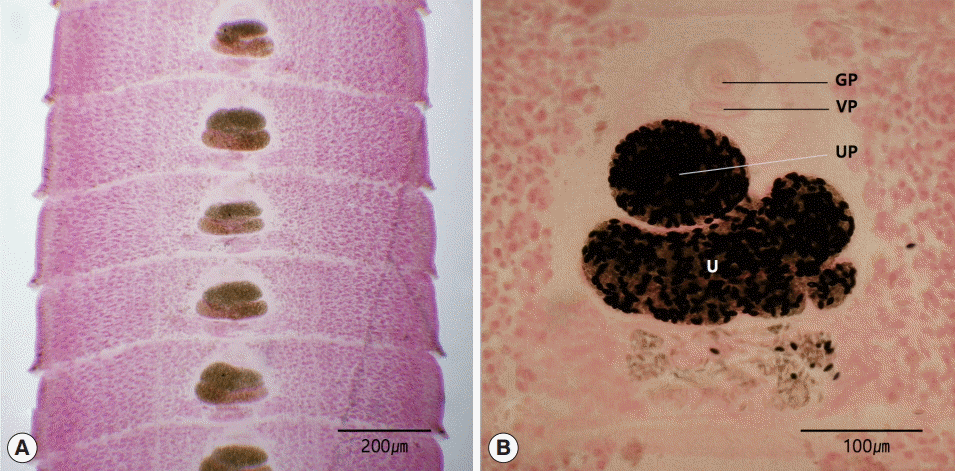
Table 1.Percentage pairwise sequence homologies of mitochondrial
cox1 and
nad3 genes between a
Spirometra sp.
a of Nebraska and various
Spirometra species,
Diphyllobothrium latum, and
D. nihonkaiense
Table 1.
|
Species genes |
S. decipiens
|
S. erinaceieuropaei
|
Sparganum proliferum
|
D. nihonkaiense
|
D. latum
|
|
cox1
|
nad3
|
cox1
|
nad3
|
cox1
|
nad3
|
cox1
|
nad3
|
cox1
|
nad3
|
|
Spirometra sp. (Nebraska, USA) |
99.2 |
99.1 |
89.6 |
89.7 |
89.1 |
89.1 |
84.5 |
84.1 |
83.5 |
83.1 |
References
- 1. Faust EC, Campbell HE, Kellogg CR. Morphological and biological studies on the species of Diphyllobothrium in China. Am J Hyg 1929;9:560-583.
- 2. Mueller JF. A repartition of the genus Diphyllobothrium. J Parasitol 1937;23:308-310.
- 3. Mueller JF. New host records for Diphyllobothrium mansonoides Mueller 1935. J Parasitol 1937;23:313-315.
- 4. Wardle RA, McLeod JA. The Zoology of Tapeworms. Minneapolis, USA. University of Minnesota Press; 1952, pp 559-615.
- 5. Yamaguti S. Systema helminthum. Vol. II. The cestodes of vertebrates. New York, USA. Interscience Publishers; 1959, pp 358-361.
- 6. Kamo H. Guide to identification of diphyllobothriid cestodes. Gendai Kikaku; Tokyo, Japan. 1999, pp 1-146 (in Japanese).
- 7. Iwata S. Experimental and morphological studies of Manson's tapeworm, Diphyllobothrium erinacei, Rudolphi. Special reference with its scientific name and relationship with Sparganum proliferum, Ijima. Progress Med Parasitol Jpn 1972;4:536-590.
- 8. Lee SU, Huh S, Phares CK. Genetic comparison between Spirometra erinacei and S. mansonoides using PCR-RFLP analyses. Korean J Parasitol 1997;35:277-282.
- 9. Jeon HK, Park HS, Lee DM, Choe SJ, Kim KH, Huh S, Sohn WM, Chai JY, Eom KS. Human infections with Spirometra decipiens plerocercoids identified by morphologic and genetic analyses in Korea. Korean J Parasitol 2015;53:299-305.
- 10. Eom KS, Park HS, Lee DM, Choe SJ, Kim KH, Jeon HK. Mitochondrial genome sequences of Spirometra erinaceieuropaei and S. decipiens (Cestoidea: Diphyllobothriidae). Korean J Parasitol 2015;53:455-463.
- 11. Swofford DL. Paup*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, Massachusettes, USA. Sinauer Associates; 2003.
- 12. Lanfear R, Calcott B, Hos YW, Guindon S. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 2012;29:1695-1701.
- 13. Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes v3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 2012;61:539-542.
- 14. Chandler AC. The helminthic parasites of cats in Calcutta and the relation of cats to human helminthic infections. Indian J Med Res 1925;13:213-227.