Abstract
The present study was designed to assess the dynamic patterns of pro-inflammatory cytokines, including IFN-γ, TNF-α, IL-4, IL-6, acute phase protein (α1-acid-glycoprotein, AGP), and an inflammation associated factor (adenosine deaminase; ADA) following experimental caprine coccidiosis. Ten kids aging from 2 to 4 months were infected orally with 5×104 sporulated oocysts and 10 animals served as controls. Blood samples were collected in both groups before infection and at days 3, 7, 14, 21, 28, and 35 post-infection (PI), and the levels of above-mentioned factors were measured. IFN-γ, TNF-α, IL-4, IL-6, AGP, and ADA activities were significantly higher in infected animals from day 7 PI (P<0.05). In conclusion, the circulatory levels of most systemic inflammatory markers, including pro-inflammatory cytokines (IFN-γ, TNF-α, IL-4, IL-6), AGP, and ADA increased significantly starting from day 3 to day 7 PI in caprine coccidiosis.
-
Key words: Eimeria caprina, Eimeria ninakohlyakimovae, Eimeria arloingi, systemic innate immunity, inflammatory factor, caprine coccidiosis
INTRODUCTION
Caprine coccidiosis is one of the most economically important diseases of the goat production industry [
1]. The disease has a worldwide distribution and is a common cause of diarrhea in young kids [
2]. Severe coccidiosis damages the intestinal mucosa resulting in malabsorption and weight loss [
3]. Stress factors, such as weaning, inclement weather, dietary changes, traveling, and over-crowding play important roles in caprine coccidiosis [
4]. Typically, more than 1 species of Eimeria are isolated from epidemics in goat herds. However,
E. ninakohlyakimovae and
E. arloingi are the predominant species in outbreaks reported from many countries in the world, including Iran [
5,
6]. The life cycle of coccidian parasites involves both intracellular and extracellular stages provoking complex cellular and humoral immune responses [
2,
7].
Eimeria produces parasite-specific antibodies in both the circulation and mucosal secretions but humoral immunity plays only a minor role in protection against the disease. Rather, recent evidence implicates cell-mediated immunity as the major factor conferring resistance to coccidiosis [
7].
It is now widely accepted that the adequate development of protective cell-mediated immune response is crucially dependent on effective triggering by full activation of innate immunity components [
8]. Therefore, more precise understanding of innate immune mechanisms and dynamic patterns can be used to guide the protective immunity. In this regard, the present study was designed to assess the dynamic patterns of the most important markers of innate immune responses, including pro-inflammatory cytokines (IFN-γ, TNF-α, IL-4, IL-6), acute phase protein (α1-acid-glycoprotein; AGP), and ADA following experimental caprine coccidiosis.
MATERIALS AND METHODS
Collection and preparation of sporulated oocysts
The current study was carried out in Fars province, in the south of Iran, where caprine coccidiosis with different
Eimeria species is prevalent [
5]. Kids from distinct herds referred to Veterinary Clinic of Shiraz University and that died from suspected clinical signs, mainly light green-diarrhea, were subjected to necropsy diagnosis. To obtain the oocysts, the intestinal contents were collected and checked using flotation technique by sucrose-saturated solution (specific gravity, 1.3). Samples from each
Eimeria-positive feces (approximately 5 g of each positive animal) were filtered through different sieves and centrifuged at 250 g for 10 min. Filtered material was placed into a shallow layer of 2.5% (w/v) aqueous potassium dichromate (K
2Cr
2O
7) solution. The samples containing unsporulated oocysts were allowed to sporulate in petri dishes in wet chamber at approximately 26–28°C and aerated daily for about 1 month until it could be confirmed that more than 90% of oocysts were sporulated. Then, the contents of all petri dishes were mixed to form the main stock and stored at 4°C until use.
The sporulated oocysts were counted per 1.0 ml of stock solution using the hemocytometer method as described earlier [
9]. The species identification of oocysts was performed based on biological and morphological characteristics (size, shape, color, shape index, presence or absence of micropyle and cap, presence or absence of residual, polar, and stieda bodies) under×400 magnification, as was done in a previous study [
10]. The stock solution comprised of a proportion of 3
Eimeria species, including
Eimeria caprina (57%),
Eimeria ninakohlyakimovae (28%), and
Eimeria arloingi (15%), as the most pathogenic species.
Twenty Iranian crossbred kids (Capra aegagrus hircus) aged 2–3 months were purchased from a non-infected herd. All animals were subjected to potentiated-sulfonamide (PANTRISUL, Pantex Holland BV, Holand, The Netherlands) antimicrobial therapy for 5 consecutive days and maintained uninfected for 14 days before starting the study and were subjected to routine parasitological tests to ensure lack of infections. The animals were kept on a ration composed of alfalfa hay and standard commercial concentrate with a ratio of 60 to 40, respectively, with total dry matter intake of 3–5% of bodyweight. Animals were divided into 2 age- and sex-matched groups and kept in completely isolated rooms under controlled conditions. Ten kids were infected orally with 5×104 sporulated oocysts (E. caprina 28,500, E. ninakohlyakimovae 14,000, and E. arloingi 7,500) in 200 ml distilled water per animal. Before inoculation, the oocyst suspension was freed of potassium dichromate by multiple washings and centrifugations at 750 g for 5 min. The other 10 kids received 200 ml distilled water and remained uninfected as control. The fecal samples were examined daily for the presence of oocysts to confirm the infection of infected group and to confirm the absence of the infection in control animals. The experiment was performed under the approval of the state committee on animal ethics, Shiraz University, Shiraz, Iran (IACUC no: 4687/63). Also, the recommendations of European Council Directive (86/609/EC) of November 24, 1986, regarding the protection of animals used for experimental purposes were considered.
Blood sampling
Blood samples were taken at days 0 (before inoculation), 3, 7, 14, 21, 28, and 35 post infection (PI). Samples were collected from the jugular vein into sterile tubes containing sodium citrate for plasma isolation (5 ml) and into sterile plain tubes for serum isolation (5 ml). Whole blood samples in sodium citrate were centrifuged at 700 g for 15 min, and the plasma portions were collected. Serum samples were prepared by centrifugation of clotted blood samples at 2,000 g for 15 min. Serum and plasma samples were distributed in 500 μl aliquots and kept at −20°C until further use. The samples with hemolysis were discarded.
Measurements of IFN-γ, TNF-α, IL-4, IL-6, AGP, and ADA
Serum levels of IFN-γ, TNF-α, IL-4 and IL-6 were measured by a quantitative sandwich enzyme immunoassay using commercial goat-specific kits (Shanghai Crystal Day Biotech, Shanghai, China). The analytical sensitivities of the tests in serum have been determined as 1.95 pg/ml for IFN-γ, 3.9 pg/ml for TNF-α and IL-4, and 0.39 pg/ml for IL-6 by the manufacturer. Serum levels of AGP were determined by a commercial goat-specific kit (Shanghai Crystal Day Biotech) using ELISA and the assay sensitivity was reported to be 0.5 ng/ml. ADA was assessed by an enzymatic-calorimetric assay kit (Diazyme Laboratories, Gregg Court, California, USA). This assay is based on the enzymatic deamination of adenosine to inosine which is converted to hypoxanthine by purine nucleoside phosphorylase (PNP). Hypoxanthine is then converted to uric acid and hydrogen peroxide (H2O2) by xanthine oxidase (XOD). The generated quinone dye was monitored spectrophotometrically and expressed as U/L of serum.
Statistical analysis
Two-way repeated measurement ANOVA test was used to compare values within sampling time points and between study groups. Mauchly’s test of sphericity and Levene’s test of equality of error variances were performed, and data transformation was carried out in case of violations of test assumptions. P-values less than 0.05 were considered significant. All statistical analyses were carried out using SPSS software (version 22).
RESULTS
All infected animals developed the clinical signs of the disease from day 19 PI starting with dehydration, anorexia, and depression followed by semi-liquid diarrhea in some animals and profuse diarrhea in others. The fecal shedding of oocysts was observed from day 14 PI in 4 goats and from day 17 PI in the other 6 goats. The peak fecal shedding occurred between days 17–25 PI with a mean value of 21×104 oocysts per gram of feces.
Statistical analyses were performed on transformed data (log in base 10) in case of violations of test assumptions. Measure levels were compared between sampling days during the course of the disease and between infected and control animals at individual sampling days. Also, the interaction between time (day PI) and study groups (infected or control) was evaluated, and in case of statistically significant interaction between 2 factors on the dependent variable, simple main effects were reported.
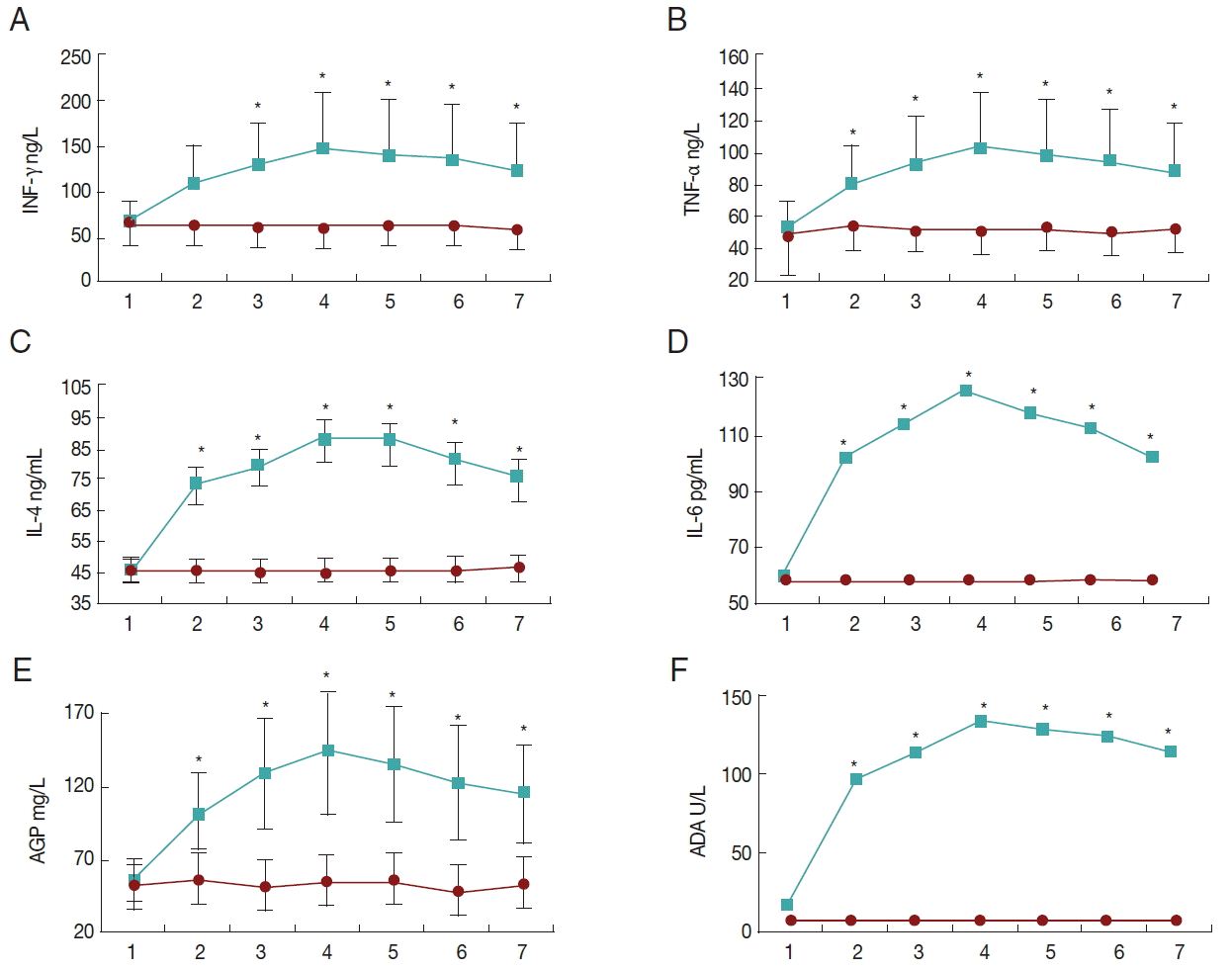
There were statistically significant effects of sampling time and study groups on serum IFN-γ levels (
P<0.05). Also, the interaction between sampling time and study groups was significant (
P<0.05). The IFN-γ levels were not significantly different during the study course in the control group. However, in the infected group it increased significantly at day 14 PI and remained elevated to the end of the study. IFN-γ was not significantly different between infected and control groups at days 0 and 3 PI, while it was higher in the infected group from day 7 PI to the end (
Fig. 1A).
There were statistically significant effects of sampling time and study groups on serum TNF-α levels (
P<0.05). Also, the interaction between sampling time and study groups was significant (
P<0.05). TNF-α levels were not significantly different during the study course in the control group. However, in the infected group it increased significantly at day 7 PI and remained elevated till the end of the study. TNF-α was not significantly different between infected and control groups at day 0 PI, while it was higher in the infected group from day 3 PI to the end (
Fig. 1B).
There were statistically significant effects of sampling time and study groups on serum IL-4 and IL-6 levels (
P<0.05). Also, the interaction between sampling time and study groups was significant (
P<0.05). IL-4 and IL-6 levels were not significantly different during the study course in the control group. However, in the infected group they increased significantly at day 3 PI and reached their maximum values at day 14 PI, and then they decreased at day 35 PI. IL-4 and IL-6 were not significantly different between infected and control groups at day 0 PI, while they were higher in the infected group from day 3 PI to the end (
Fig. 1C, D).
There were statistically significant effects of sampling time and study groups on serum ADA activity (
P<0.05). Also, the interaction between sampling time and study groups was significant (
P<0.05). Serum ADA activities were not significantly different during the study course in the control group. However, in the infected group they increased significantly at day 3 PI and reached its maximum levels at day 14 PI, and then it decreased at day 35 PI. Serum ADA activity was not significantly different between infected and control groups at day 0 PI, while it was higher in the infected group from day 3 PI to the end (
Fig. 1E).
There were statistically significant effects of sampling time and study groups on serum AGP levels (
P<0.05). Also, the interaction between sampling time and study groups was significant (
P<0.05). AGP levels were not significantly different during the study course in the control group. However, in the infected group it increased significantly at day 7 PI and remained elevated to the end of the study. AGP was not significantly different between infected and control groups at day 0 PI, while it was higher in the infected group from day 3 PI to the end (
Fig. 1F).
DISCUSSION
The current study was designed to assess the changing patterns of important systemic markers of innate immunity and inflammatory associated factors in experimental caprine coccidiosis. Effective induction of innate immune responses is crucial in development of cell-mediated adaptive immunity which is considered more protective in coccidiosis [
8,
11]. Those vaccine adjuvants that could induce the same pattern and magnitude of innate immune responses as occur in the real disease challenges are considered to be potentially more effective in formation of cell-mediated adaptive immunity and consequently the effective prevention of the disease. Following microbial invasion and tissue injury, pro-inflammatory cytokines are released, and the vascular endothelium and inflammatory cells are activated. Further activation of innate immune mechanisms and formation of specific acquired immunity are dependent on cytokines generated by activated cells [
8]. The importance of several cytokines in coccidiosis has been well-studied [
12]. Among these, IFN-γ and TNF-α are particularly important [
13]. Results from several studies have confirmed the role of IFN-γ in resistance to coccidiosis [
13]. In the current study, IFN-γ was increased significantly in the infected group from day 14 PI and remained elevated to the end of the study.
Hashemnia et al. [
14] reported an earlier significant increase of IFN-γ (about 3 times) from day 7 PI in newborn kids inoculated with oocysts of
E. arloingi. Due to the significant and constant increase in the level of IFN-γ in several studies, the authors propose that it may be considered as a useful marker for immunizing goats against coccidiosis; however, it remains to be established. TNF-α is secreted primarily by activated macrophages, NK cells, and Th1 lymphocytes [
15]. It exerts both protective and pathological effects in response to infection [
15]. TNF-α acts synergistically with IFN-γ and plays an anti-parasitic role in a number of protozoan infections in both humans and animals [
13]. In the current research, the TNF-α level was increased significantly at day 7 PI in the infected group and remained elevated to the end of the study. Similarly, results from Hashemnia et al. [
14] indicated a significant increase (about 2.4 times) at day 7 PI.
IL-4 plays a key regulatory role in humoral and adaptive immunity. It is essential for B cell differentiation, proliferation, and production of antibodies [
16]. Furthermore, it is suggested that IL-4 may be involved in the pathogenesis of caprine coccidiosis [
17]. In our study, IL-4 levels were increased significantly at day 3 PI and reached maximum values at day 14 PI, and then decreased at day 35 PI. IL-6 is the principal regulator of most acute phase proteins and is produced during immune responses to parasitic infections [
18]. In the current study, IL-6 levels followed the same changes as IL-4. The changing patterns of IL-4 and IL-6 had not been studied in caprine coccidiosis before.
The acute phase response (APR) is a core part of the early-defense or innate immune system [
18]. Acute phase proteins are mainly synthesized by hepatocytes as part of APR. The acute phase proteins are grouped as either positive acute phase proteins or negative acute phase proteins [
18]. AGP is an acute-phase protein and a marker for systemic inflammation [
19]. AGP is used as a success marker of various field coccidiosis control programs in commercial broiler chicken; so the levels of AGP have been significantly lower in treatment groups (either in ovo vaccination or use of antimicrobials) compared with controls [
20]. The changing pattern of AGP in caprine coccidiosis had not been studied yet. In our study, we showed that the serum AGP concentration was increased significantly at day 7 PI and remained elevated to the end of the study.
ADA is an enzyme produced by the monocytic-macrophage system following invasions by intracellular microorganisms [
21]. It has recently been considered as a mediator of inflammation and immune responses [
22]. The serum ADA activity had not been measured in caprine coccidiosis. We showed that the serum ADA activity was increased in the infected group at day 3 PI and reached its maximum levels at day 14 PI, and then it decreased from day 35 PI.
Conclusively, the present study revealed a precise picture of both important innate immunity and systemic inflammatory markers during caprine coccidiosis. The circulatory levels of most systemic inflammatory markers, including pro-inflammatory cytokines (INF-γ, TNF-α, IL-4, IL-6), acute phase protein (AGP), and ADA increased significantly starting from day 3 to day 7 after infection in caprine coccidiosis. It seems that vaccines that induce the same patterns of systemic inflammatory responses may be potentially more effective in protective cell mediated immunity against caprine coccidiosis.
Notes
-
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank the Research Council of Shiraz University and School of Veterinary Medicine, Shiraz University, Iran for financial and technical support of this study (grant no.71-GR-VT-5). Furthermore, we would like to thank F. Nejati and M. Rajabloo for their technical assistance.
Fig. 1The changing patterns of systemic inflammatory markers (A, IFN-γ; B, TNF-α; C, IL-4; D, IL-6; E, AGP; F, ADA) following experimental clinical caprine coccidiosis with mixed Eimeria species. Asterisks show statistically significant differences between control and treatment groups (P<0.05). Data are means±SD. The upper and lower lines are from treatment and control groups, respectively, and the horizontal axes show the sampling time points (days 0, 3, 7, 14, 21, 28, and 35 PI).

References
- 1. Chartier C, Paraud C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res 2012;103:84-92.
- 2. Foreyt WJ. Coccidiosis and cryptosporidiosis in sheep and goats. Vet Clin North Am Food Anim Pract 1990;6:655-670.
- 3. Hashemnia M, Khodakaram-Tafti A, Razavi SM, Nazifi S. Experimental caprine coccidiosis caused by Eimeria arloingi: morphopathologic and electron microscopic studies. Vet Res Commun 2012;36:47-55.
- 4. Cox FE. Control of coccidiosis: lessons from other sporozoa. Int J Parasitol 1998;28:165-179.
- 5. Razavi SM, Hassanvand A. A survey on prevalence of different Eimeria species in goats in Shiraz suburbs. J Vet Res 2006;61:373-376.
- 6. Tafti AK, Mansourian M. Pathologic lesions of naturally occurring coccidiosis in sheep and goats. Comp Clin Pathol 2008;17:87-91.
- 7. Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol 2000;24:303-324.
- 8. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015;16:343-353.
- 9. Holdsworth PA, Conway DP, McKenzie ME, Dayton AD, Chapman HD, Mathis GF, Skinner JT, Mundt HC, Williams RB. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol 2004;121:189-212.
- 10. Jalila A, Dorny P, Sani R, Salim NB, Vercruysse J. Coccidial infections of goats in Selangor, Peninsular Malaysia. Vet Parasitol 1998;74:165-172.
- 11. Frölich S, Entzeroth R, Wallach M. Comparison of protective immune responses to apicomplexan parasites. J Parasitol Res 2011;Article ID 852591. 1-11.
- 12. Shivaramaiah C, Barta JR, Hernandez-Velasco X, Téllez G, Hargis B. Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet Med 2014;5:23-34.
- 13. Ovington KS, Alleva LM, Kerr EA. Cytokines and immunological control of Eimeria spp. Int J Parasitol 1995;25:1331-1351.
- 14. Hashemnia M, Khodakaram-Tafti A, Razavi SM, Nazifi S. Changing patterns of acute phase proteins and inflammatory mediators in experimental caprine coccidiosis. Korean J Parasitol 2011;49:213-219.
- 15. Lillehoj H. Role of T lymphocytes and cytokines in coccidiosis. Int J Parasitol 1998;28:1071-1081.
- 16. Hofman FM, Brock M, Taylor CR, Lyons B. IL-4 regulates differentiation and proliferation of human precursor B cells. J Immunol 1988;141:1185-1190.
- 17. Ibarra-Velarde F, Alcala-Canto Y. Downregulation of the goat β-defensin-2 gene by IL-4 in caprine intestinal epithelial cells infected with Eimeria spp. Parasitol Res 2007;101:613-618.
- 18. Baumann H, Gauldie J. The acute phase response. Immunol Today 1994;15:74-80.
- 19. Tothova C, Nagy O, Seidel H, Kovac G. Acute phase proteins and their use in the diagnosis of diseases in ruminants: a review. Vet Med Czech 2014;59:163-180.
- 20. Lee KW, Lillehoj HS, Jang SI, Lee SH. Effects of various field coccidiosis control programs on host innate and adaptive immunity in commercial broiler chickens. Korean J Poult Sci 2012;39:17-25.
- 21. Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest 2006;116:1835-1837.
- 22. Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 2004;25:33-39.