Abstract
This study investigated the development characteristics of Dermacentor everestianus under laboratory conditions. The time taken for D. everestianus to complete the whole life cycle was 110.2 days on average, and the average developmental durations of larvae and nymphs were 17.1 days and 29.5 days, respectively. The summation of the prefeeding, feeding, and preoviposition periods of females was 17.8 days, and the oviposition and egg incubation lasted for 18.1 days and 27.7 days, respectively. A highly positive correlation was observed between the weight of engorged female and the number of egg mass laid (r=0.947). The reproductive efficiency index and the reproductive fitness index were 7.1 and 6.1, respectively.
-
Key words: Dermacentor everestianus, ixodid tick, life cycle, laboratory, development
INTRODUCTION
Ticks are globally distributed bloodsucking ectoparasites, which are notorious as vectors in maintaining enzootic cycles of numerous pathogens [
1]. Among the approximately 900 tick species worldwide [
2], the tick
Dermacentor everestianus Hirst, 1926 is mainly distributed in Northwestern China and Nepal [
3], and recognized as an important vector of
Anaplasma ovis,
Francisella tularensis, and
Rickettsia raoultii-like bacteria in Qinghai-Tibet Plateau of China, including Qinghai, Tibet, and Gansu province [
4]. Additionally, this tick species even occurs above 4,000 meters in altitude in Tibet Plateau [
5], where is featured by extremely hostile environments, including poor oxygen, drought conditions, and low temperatures [
6].
As a 3-host tick, the immature
D. everestianus usually attacks lagomorphs and rodents, whereas the adults mainly parasitize on hares, domestic sheep, and yaks, which cause great losses in livestock production [
5]. Though the tick
D. everestianus was regarded a junior synonym of
D. abaensis and
D. birulai [
3], detailed knowledge on their biology and life cycle has not been thoroughly studied. Therefore, this paper investigated the development characteristics of
D. everestianus under laboratory conditions, which will be helpful for further exploration of its ecological adaptation to the hostile environments in Qinghai-Tibet Plateau, China.
MATERIALS AND METHODS
Collection and rearing of the ticks
D. everestianus adult ticks were collected by flag-dragging in the field in Damxung County (90°45′ to 91°31′E, 29°31′ to 31°04′N, 4353 m in altitude), north Lhasa City, Tibet Autonomous Region, China. Ticks were placed into cloth bags attached on ears of domestic rabbits, and were checked daily for feeding or engorgement. During non-feeding periods, they were incubated in cotton-plugged glass tubes in laboratory incubator, i.e., 22±1°C, 75±5% relative humidity (RH), and a 16 hr light and 8 hr darkness photoperiod. The rabbits were maintained at 20–25°C with 50% RH and exposed to natural daylight cycles, and each rabbit was used only for a single infestation.
Biology of the immature and adult D. everestianus
To evaluate the prefeeding period (no. of days from emergence to attachment) of immature and adult
D. everestianus, 100 newly emerged larvae and nymphs and 50 newly molted females were put on hosts, and checked daily for attachment. Three weeks after emergence, 300 immature ticks and 50 females and males were weighed and fed on rabbits, and the feeding periods (no. of days from attachment to detachment) were determined. After engorgement, immature ticks were collected, weighed, and then placed into separate tubes for molting in a laboratory incubator. Premolting periods of the larvae and nymphs were recorded from their detachment to ecdysis according to Labruna et al. [
7].
After engorgement, detached females were immediately collected and weighed individually, then they were put into separate tubes for oviposition in the laboratory incubator. The preoviposition periods (no. of days from detachment to the beginning of oviposition) and oviposition periods (no. of days from the beginning to the end of oviposition) of females were recorded. Once oviposition started, the daily deposited eggs were collected, weighed, and placed into separate glass tubes. A thousand eggs were used for observing the incubation period (no. of days from oviposition to the first larva hatchment), and percent of hatchment was calculated. Additionally, the reproductive efficiency index (REI) (amount of eggs/weight of engorged female) [
8] and the reproductive fitness index (RFI) (amount of eggs incubated to larvae/weight of engorged female) [
9] were also determined as described by Chen et al. [
10].
RESULTS
Life cycle of D. everestianus
Under laboratory conditions, a mean of 110.2 days (range 87–136 days) were required for the tick
D. everestianus to complete the whole life cycle from unfed adults to next generation adults. The average developmental durations of larvae and nymphs were 17.1 days (range 14–19 days) and 29.5 days (range 25–34 dyas), respectively. The summation of the prefeeding, feeding, and preovipositon periods of females was 17.8 days, and the oviposition and egg incubation lasted for 18.1 days (range 12–27 dyas) and 27.7 days (range 24–31 days), respectively. The durations of the various developmental stages are shown in
Table 1.
After ecdysis, the larvae, nymphs, and adults required an average of 2.5 days (range 2–3 days), 3.2 days (range 2–4 days), and 5.3 days (range 4–7 days) to acquire the ability to attach on hosts for feeding, respectively. When feeding began, it took an average of 2.4 days and 4.1 days for larvae and nymphs to engorge. The premolting periods of larvae and nymphs averaged 12.2 days (range 11–13 days) and 22.2 days (range 20–25 days), respectively. Copulation is necessary for rapid engorgement of females, and after engorgement, the average weights of females increased by about 41.2 folds, whereas no obvious difference was observed on weight changes of males (1.2-fold). For larvae and nymphs, the weight ratio of engorged to unfed was 10.2 and 27.5, respectively. The average body weights of the larvae, nymphs, and adults before and after feeding are listed in
Table 2.
The egg mass laid by the tick
D. everestianus ranged from 1,364 to 2,951 per female, and percent of hatchment reached 85.9%. The REI and RFI was 7.1 and 6.1, respectively (
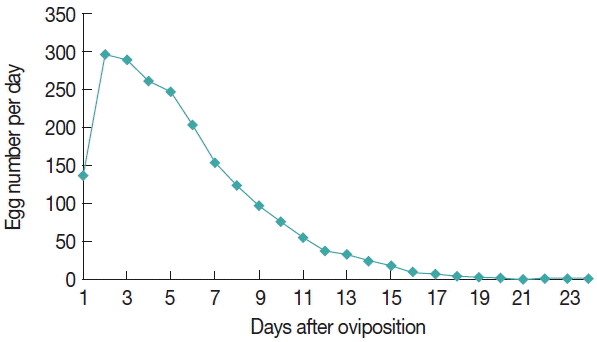
Table 3). The egg amount was relatively low at the onset of oviposition, and peaked on the second day, thereafter the egg number gradually declined. The daily oviposition of
D. everestianus is presented in
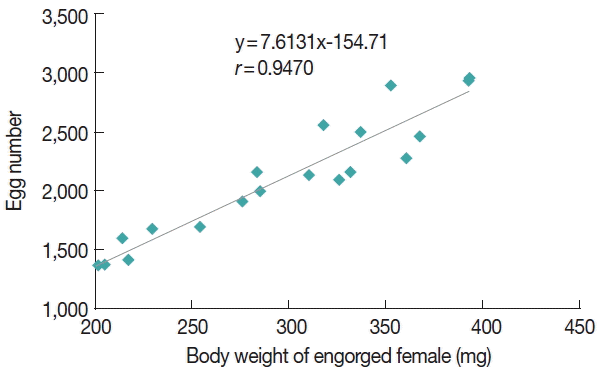
Fig. 1. Linear regression analysis revealed a highly positive correlation between the weight of the engorged female and the number of egg mass laid (
r=0.947) (
Fig. 2).
DISCUSSION
The tick
D. everestianus is endemic in Qinghai-Tibet Plateau of China, and can transmit diseases between wild animals and livestock [
5]. However, the biology and development characteristics of this tick species has not been extensively studied yet, which has hampered the understanding of its vector potential and ecological adaptation to hostile environment. Therefore, the current study investigated the biological characteristics of all developmental stages of
D. everestianus, using rabbits as the host under laboratory conditions (22±1°C, 75±5% RH, and a 16 hr light and 8 hr darkness photoperiod).
The duration of the life cycle of
D. everestianus averaged 110.2 days (ranged 87–136 days) under laboratory conditions, which was shorter than that of
D. variabilis (176–191 days) and
D. occidentalis (180–195 days) incubated at 22–24°C and 90% RH [
11], though they both used rabbits as hosts. When compared to the life cycle of
D. silvarum (87.5 days in average, range 74–102 days) under 27±1°C and 70% RH [
12], the tick
D. everestianus required a longer duration. Hence, the differences of the development duration may still attribute to the complex interplay of ambient temperature and relative humidity [
13,
14].
The body weights of engorged females (294.8 mg on average, range 204.6–444.1 mg) and nymphal (12.7 mg on average, range 3.4–19.8 mg)
D. everestianus were much lower than that of
D. silvarum (461.4 mg in female, 64.7 mg in nymph). Before and after feeding, the weight ratio of female
D. everestianus from unfed to engorgement was approximately 41.2-fold, which was lower than 76.1 folds in
D. silvarum, whereas the 27.5 folds weight increase in nymphs were much higher than that observed in
D. silvarum (11.3 folds), and no difference was observed in larval weight changes between these 2 species [
12].
Linear regression analysis revealed a highly positive correlation between the weight of the engorged female
D. everestianus and the number of the egg mass laid (
r=0.947). The amount of eggs laid was low at the onset of oviposition, and reached the peak on the second day; thereafter, the egg amount was gradually decreased until the end of oviposition. The unimodel oviposition pattern was also observed in other tick species, including
D. silvarum,
Haemaphysalis doenitz [
15], and
Hyalomma asiaticum [
16]. The egg incubation required an average of 27.7 days, which was much longer than that observed in
D. silvarum (15.3 days on average, range 13–16 days) [
12], but shorter than 32.1 days (range 25–40 days) observed in
Haemaphysalis tibetensis under field conditions [
16]. Premolting period of nymphs required 22.2 days under laboratory conditions, which was longer than 14.6 days (range 13–15 days) in
D. silvarum [
12], but much shorter than 52.7 days (range 41–55 days) in
H. tibetensis which showed overlapping distribution [
17].
Overall, the laboratory colony of D. everestianus was established, which paved the way for further exploration of its vector potential and allowed us to study its ecological adaptation to hostile environments in Qinghai-Tibet Plateau, China.
Notes
-
CONFLICT OF INTEREST
We declare that we have no conflict of interest related to this work.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (nos. 31272372, 31400342), the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20131303130001), the Natural Science Foundation of Hebei province (no. C2015205124), and Natural Science Research Programs of the Educational Department of Hebei Province (no. BJ2016032). The visit of Zhijun Yu to the Carleton University was generously supported by China Scholarship Council and Hebei Normal University.
Fig. 1Mean daily oviposition of engorged females of Dermacentor everestianus (based on 20 females) under laboratory conditions.

Fig. 2Relationship between the weight of engorged females of Dermacentor everestianus and number of eggs laid (based on 20 females) under laboratory conditions.

Table 1The duration of various developmental stages of Dermacentor everestianus under laboratory conditions
Table 1
|
Developmental stages |
Period |
No. |
Duration (day) |
|
|
Range |
Mean±SEM |
|
Egg |
Incubation |
1,000 |
24–31 |
27.7±1.9 |
|
|
Larva |
Prefeeding |
100 |
2–3 |
2.5±0.5 |
|
Feeding |
100 |
1–3 |
2.4±0.6 |
|
Premoulting |
300 |
11–13 |
12.2±0.7 |
|
|
Nymph |
Prefeeding |
100 |
2–4 |
3.2±0.7 |
|
Feeding |
100 |
3–5 |
4.1±0.7 |
|
Premoulting |
300 |
20–25 |
22.2±1.6 |
|
|
Female |
Prefeeding |
50 |
4–7 |
5.3±0.9 |
|
Feeding |
50 |
3–8 |
5.7±1.2 |
|
Preoviposition |
20 |
5–10 |
6.8±1.2 |
|
Oviposition |
20 |
12–27 |
18.1±3.6 |
|
|
Life cycle |
- |
20 |
87–136 |
110.2±13.6 |
Table 2Changes of the body weights of larvae, nymphs, and adults of Dermacentor everestianus before and after feeding
Table 2
|
Developmental stages |
No. |
Unfed (mg) (Mean±SEM) |
Engorged (mg) (Mean±SEM) |
Weight ratio (engorged/unfed) |
|
Larva |
100 |
0.058±0.00 |
0.594±0.00 |
10.2 |
|
Nymph |
100 |
0.46±0.04 |
12.65±3.30 |
27.5 |
|
Female (♀) |
50 |
7.15±0.74 |
294.80±63.85 |
41.2 |
|
Male (♂) |
50 |
6.28±1.07 |
7.72±0.48 |
1.2 |
Table 3The characteristics of oviposition of Dermacentor everestianus under laboratory conditions
Table 3
|
Parameters |
No. |
Range |
Mean±SEM |
|
Weight of engorged female (mg/♀) |
50 |
204.6–444.1 |
294.8±63.9 |
|
Egg mass laid (No./♀) |
20 |
1,364–2,951 |
2,126.0±623.9 |
|
Hatchment rate (%) |
1,000 |
78.4–94.6 |
85.9±4.5 |
|
REI |
20 |
6.32–8.21 |
7.1±0.12 |
|
RFI |
20 |
5.26–7.26 |
6.1±0.13 |
References
- 1. Sonenshine DE, Roe RM. Biology of Ticks. 2nd ed. New York, USA. Oxford; 2013.
- 2. Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol 2012;28:437-446.
- 3. Apanaskevich DA, Duan W, Apanaskevich MA, Filippova NA, Chen J. Redescription of Dermacentor everestianus Hirst (Acari: Ixodidae), a parasite of mammals in mountains of China and Nepal with synonymization of D. abaensis Teng and D. birulai Olenev. J Parasitol 2014;100:268-278.
- 4. Yu ZJ, Wang H, Wang TH, Sun WY, Yang XL, Liu JZ. Tick-borne pathogens and the vector potential of ticks in China. Parasit Vectors 2015;8:24.
- 5. Teng KF, Jiang ZJ. Economic Insect Fauna of China. Fasc. 39, Acari: Ixodidae. Beijing, China. Science 1991;(in Chinese).
- 6. Dai J, Wang Y, Zhang L, Tang YL, Luo XS, An HL, Fang CX. Hymenobacter tibetensis sp. nov., a UV-resistant bacterium isolated from Qinghai-Tibet plateau. Syst Appl Microbiol 2009;32:543-548.
- 7. Labruna MB, Souza SL, Menezes AC, Horta MC, Pinter A, Gennari SM. Life-cycle and host specificity of Amblyomma tigrinum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 2002;26:115-125.
- 8. Drummond RO, Whetstone TM. Oviposition of the Gulf Coast tick. J Econ Entomol 1970;63:1547-1551.
- 9. Chilton NB. An index to assess the reproductive fitness of female ticks. Int J Parasitol 1992;22:109-111.
- 10. Chen Z, Li YQ, Liu ZJ, Yang JF, Yin H. The life cycle of Hyalomma rufipes (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 2012;56:85-92.
- 11. Troughton DR, Levin ML. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J Med Entomol 2007;44:732-740.
- 12. Liu JZ, Liu ZN, Zhang Y, Yang XL, Gao ZH. Biology of Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 2005;36:131-138.
- 13. Chilton NB, Andrews RH, Bull CM. Influence of temperature and relative humidity on the moulting success of Amblyomma limbatum and Aponomma hydrosauri (Acari: Ixodidae) larvae and nymphs. Int J Parasitol 2000;30:973-979.
- 14. Debárbora VN, Mangold AJ, Oscherov EB, Guglielmone AA, Nava S. Study of the life cycle of Amblyomma dubitatum (Acari: Ixodidae) based on field and laboratory data. Exp Appl Acarol 2014;63:93-105.
- 15. Chen XJ, Yu ZJ, Guo LD, Li LX, Meng H, Wang D, Liu RJ, Liu JZ. Life cycle of Haemaphysalis doenitzi (Acari: Ixodidae) under laboratory conditions and its phylogeny based on mitochondrial 16S rDNA. Exp Appl Acarol 2012;56:143-150.
- 16. Chen Z, Yu ZJ, Yang XL, Zheng HY, Liu JZ. The life cycle of Hyalomma asiaticum kozlovi Olenev, 1931 (Acari: Ixodidae) under laboratory conditions. Vet Parasitol 2009;160:134-137.
- 17. Liu M, Li T, Yu ZJ, Gao XH, Zuo CW, Wang RR, Li NX, Wang H, Liu JZ. Characterization of the life cycle of the tick Haemaphysalis tibetensis under field conditions in Qinghai-Tibet plateau. Exp Appl Acarol 2016;69:107-115.