Abstract
The ability of nematodes to manipulate the immune system of their host towards a Th2 and T regulatory responses has been proposed to suppress the inflammatory response. Clinical trials have proposed a useful effect of helminth infections on improvement of inflammatory disorders. In this study, we investigated the immunomodulatory effect of Syphacia obvelata infection to induce intestinal tolerance in C57BL/6 mice. Mice were infected through the cagemates with self-infected BALB/c mice. Four weeks post-infection, expression levels of IFN-γ, TNF-α, IL-17, and IL-10 were assessed in the supernatant of mesenteric lymph node (MLN) culture. Foxp3+Treg were measured in MLN cells by flow cytometry. In the S. obvelata-infected group, the percentage of Tregs (5.2±0.4) was significantly higher than the control (3.6±0.5) (P<0.05). The levels of IL-10 (55.3±2.2 vs 35.2±3.2), IL-17 (52.9±3.8 vs 41±1.8), IFN-γ (44.8±4.8 vs 22.3±2.3) and TNF-α (71.1±5.8 vs 60.1±3.3) were significantly increased in infected mice compared to the control group (P<0.05). The above results showed the potential effects of S. obvelata to induce intestinal tolerance. Therefore, it seems that S. obvelata may increase the immunological suppressive function in the intestinal tract.
-
Key words: Syphacia obvelata, T regulatory cell, Foxp3, C57BL/6, inflammatory disorder, intestinal tolerance
It has been known that nematodes develop immunomodulatory strategies to escape from the hosts’ immune attack, including interference with antigen processing by cells of the innate immune system or evasion of humoral and cellular immunity by antigenic variation [
1]. Intestinal nematodes have ability to induce Th2 immune responses which have been shown to be important for control of the infections [
2]. Nematode infections enhance the induction of dendritic cells (DCs) supporting the result of CD4
+CD25
+Foxp3
+ T cells (Tregs) populations and production of anti-inflammatory IL-10 in the intestine and gut-draining lymph nodes [
3]. It has been found that Tregs to be involved in the parasites immune escape and to prevent the protective immunity to helminths [
4]. In this regard, some of the nematodes, including
Trichuris spp. [
5],
Heligmosomoides polygyrus [
6], and filarial worms have been investigated and have shown significant anti-inflammatory effects in experimental studies. There is an essential need for more research to identify the most suitable helminths for this purpose.
The most important criteria for the introduction of other nematodes is recognition and availability of them. The nematodes
Syphacia spp. are pinworm nematode parasites infecting laboratory animals in high abundance. These nematodes belong to the order Oxyurina (
Syphacia obvelata and
Syphacia muris) and are colonized in large and small intestines of mice and rats. Identification of
Syphacia spp. is based on examination of male worms for the position of the mamelons. Female
S. obvelata and
S. muris differs only in the location of the vulva that is being anterior in
S. obvelata than that in
S. muris [
7].
S. obvelata lives in the caecum and anterior colon of the host, where the female matures within 11–15 days. Gravid females deposit an average of 350 eggs in the perianal region, which develop into infective third stage larvae within 20–24 hr [
8]. It has been well documented that
S. obvelata infections can have efficacy on the results of immunological experiments and interventions [
9,
10]. Remarkably, the preexisting infections of mice with
S. obvelata [
9] can reduce the incidence and severity [
9,
10] of experimental rheumatoid arthritis (RA).
Regarding the prevalence of species, BALB/c mice have shown a higher sensibility to
S. obvelata infection compared with the C57BL/6 strain [
11]. However, there are documents that mouse strains influence susceptibility to some diseases; C57BL/6 strain is more susceptible to express many inflammatory conditions compared to another strain [
12].
Analysis of S. obvelata mouse model has the potential to manifest activated immunological responses by the host to encounter infection with helminth parasites. This could be considered as a useful tool for preventive studies and therapeutic approach in medicine, and also for expanding the novel therapies to treat the autoimmune and inflammatory disorders. The main aim of this study was to investigate the immunological patterns of S. obvelata infection in C57BL/6 mice.
Parasite-free C57BL/6 male mice at 6- to 8-week-old and weighing 20–24 g were purchased from the animal laboratory core facility of Royan Institute of Iran. Before starting the experience, to ensure absence of parasite infections in mice, formalin-ether concentration and scotch tape anal swab tests were conducted. The mice were matched by age and weight with a control group. All mice were allowed to adapt for 7 days before the experiments according to the described protocol previously [
13]. The mice were kept under conventional conditions, housed in mice cages with 12-hr light–dark cycle in standard animal cages and fed with standard commercial diet, as well as plastic bottle water ad libitum. C57BL/6 mice were infected through the cagemates with self-infected BALB/c mice. The scotch tape test revealed the presence of
S. obvelata eggs in C57BL/6 mice from the 14 days on. The recognition of eggs and female
S. obvelata was based on criteria to helminths of mice [
7].
S. obvelata gravid females were collected from the caecum and colon of experimentally infected C57BL/6 mice and destroyed mechanically to harvest the eggs. Their eggs were sufficiently embryonated to continue developing into the infectious phase under appropriate artificial conditions. These conditions consisted of a 4–6 hr incubation period in a 0.9% saline solution at 37°C [
8].
Twenty parasite-free C57BL/6 male mice were divided into 2 groups: control and infection groups. Each of 10 helminth-free C57BL/6 mice (infection group) were inoculated orally with 500 infective S. obvelata eggs via a gavage needle. Mice were sacrificed 4 weeks post-S. obvelata infection. The mesenteric lymph nodes (MLNs) were removed aseptically and homogenized in glass tissue grinders with RPMI medium, and then suspensions were pressed through a 70-μm nylon cell strainer (Falcon, BD Labware, Franklin Lakes, New Jersey, USA) to remove temperature and discard the supernatant. The cells were resuspended in 5 ml complete tissue culture medium (RPMI containing 10% FCS, 2 mM L-glutamine, 100 μg of streptomycin/ml, and 100 U penicillin/ml). Cells (1×106) without using anti-CD3 were cultured in 24-well plates and incubated in a CO2 incubator at 37°C and 5% CO2. Supernatants were collected 12 hr later and stored at −80°C until assayed for cytokine production. The levels of tumor necrosis factor-α (TNF-α), IFN-γ, IL-10, and IL-17 in cell culture supernatants were measured using commercial ELISA kits (R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer’s introduction. In brief, mouse TNF-α, IL-17, IFN-γ, and IL-10 were detected by biotinylated monoclonal antibodies, which were evidenced by avidin-conjugated horseradish peroxidase followed by incubation with tetramethylbenzidine (TMB) substrate. The minimum significant values of these assays were: 31.3 pg/ml for TNF-α, IFN-γ, and IL-10 and 15.6 pg/ml for IL-17. OD values at 450 nm were recorded using Anthos ELISA reader.
To evaluate the effects of S. obvelata infection on the percentage of CD4+CD25+ Foxp3+Tregs, mice in control and infected groups were sacrificed by cervical dislocation 4 weeks post- S. obvelata infection. Flow cytometry was performed with cells from MLN. Cells were stained using mouse regulatory T cell staining kit (Miltenyi Biotech, Bergisch-Gladbach, Germany) and analyzed on FACSCalibur (BD Biosciences, Mountain View, California, USA) with CellQuest software. The following conjugated antibodies were incubated with lymphocyte populations: fluorescein-isothiocyanate (FITC)-conjugated anti-mouse CD4, phycoerythrin (PE) -conjugated anti-mouse CD25, and allophycocyanin (APC)-conjugated anti-mouse forkhead box P3 (Foxp3). Data were analyzed using Flowing Software_2_5_1.
All the analyses were performed by using Graphpad Prism v6 and SPSS 16 software. Calculation of means±SD was performed and the Student’s t-test was used for determination of significant differences between 2 groups. P-values<0.05 were considered significant. After prepatent period of S. obvelata in experimentally infected mice, the new eggs were observed by scotch tape test in the perianal area. After 4 weeks (end of the study period) and before the start of analyses, mice were sacrificed; a lot of alive and active worms were observed in their caeca. Also, a large number of eggs were seen in the perianal region. Observation of worms and eggs indicated the active presence of S. obvelata during the study.
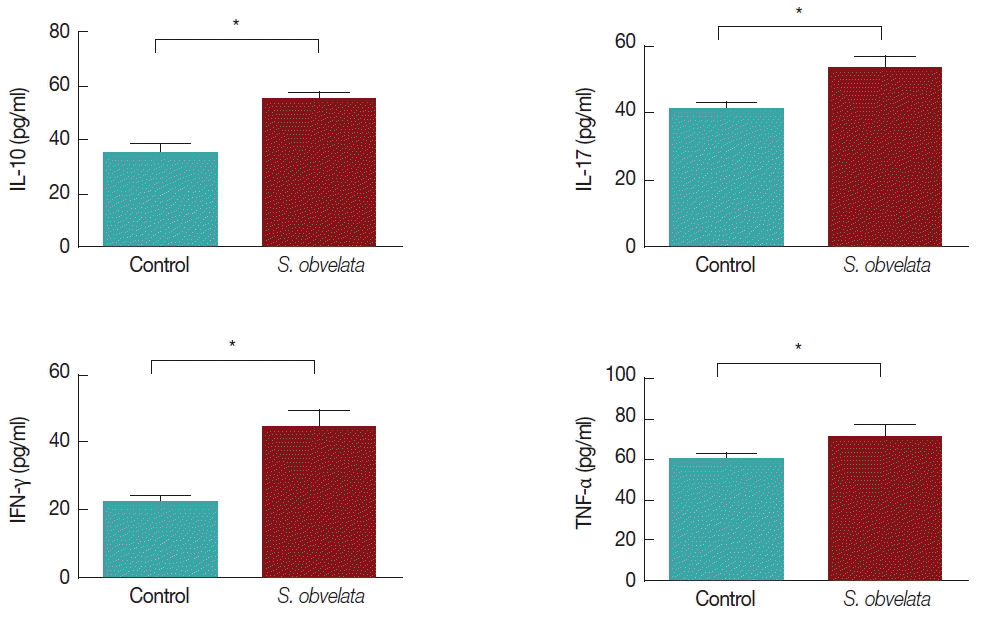
Fig. 1 displays production of cytokines by MLN cells from control and infected group. As shown in
Fig. 1A, MLN cells from infected mice released more IL-10 (55.3±2.2) than the control group (35.2±3.2). The level of IL-17 was increased all over the study in infected animals (52.9±3.8) compared to controls (41±1.8) (
Fig. 1B). Production of IFN-γ in infected group (44.8±4.8) was significantly increased compared to control animals (22.3±2.3), during the course of the study (
Fig. 1C). TNF-α production in infected mice (71.1±5.8) was significantly increased compared to the control animals (60.1±3.3) (
Fig. 1D). Since Foxp3 is a specific marker for CD4
+ CD25
+ Tregs [
14], we determined the percentage of CD4
+ CD25
+Foxp3
+ T cells as that of CD4
+CD25
+ Tregs. As shown in
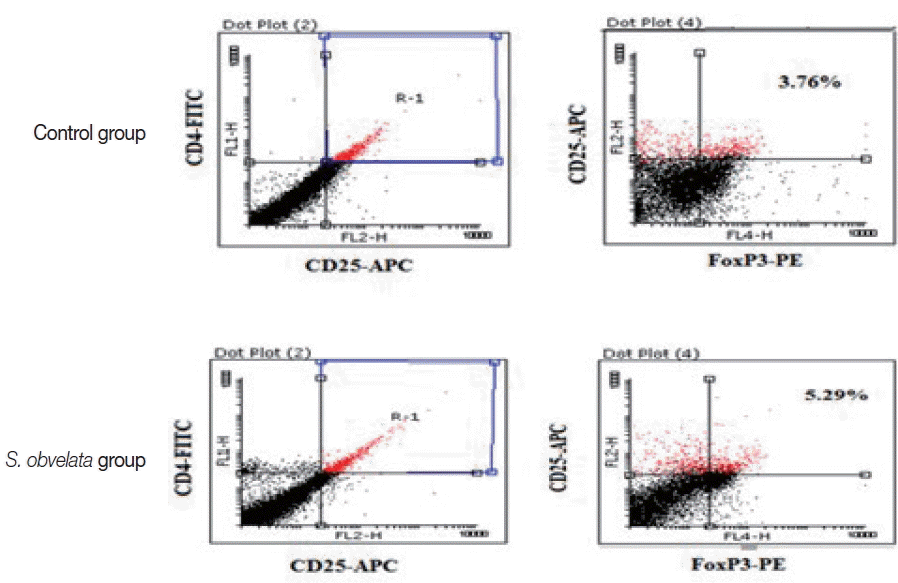
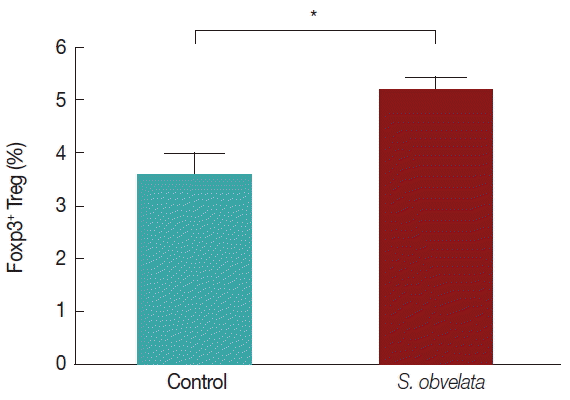
Figs. 2 and
3, the percentages of CD4
+CD25
+Foxp3
+Tregs in mice infected with
S. obvelata (5.2±0.3) were significantly higher than those in the control group (3.6±0.5).
Helminth infections are associated with immunomodulatory sophisticated mechanisms that affect all facets of the host immune response to ensure their persistence within the host [
15]. The protective immune responses against many helminth parasites have been characterized by 2 key features; a mainly Th2 immune response [
16] and downregulation of the immune response as described by elevated levels of IL-10, TGF-β, and frequency of regulatory T cells. Th2 cytokines (IL-4 and IL-10) may play an important role in reducing the severity of the disease and allowing parasite survival [
17]. MLN and lamina propria T cells make more IL-10 and TGF-β after early
H. polygyrus infection [
18]. Several different helminth species promote IL-10 secretion.
CD4
+CD25
+ Treg cells were increased in the MLNs during helminth infections and may promote the survival of helminths. They block protective immunity in mouse models of filariasis [
19]. Some species of Treg cells expressing the transcription factor Foxp3 are numerous and more active in helminth-infected individuals but decreased after anthelmintic therapy [
15]. Foxp3
+Treg cells play very important role in suppression of immune responses during helminth infection, both by preventing immunity in order to survive parasites in host and by controlling harmful immunopathology [
20]. The expansion and activation of Foxp3
+Treg cells occur within the first week of both filarial and intestinal nematode [
21] infections. Also after
H. polygyrus infection increases the number of Foxp3
+ Treg cells in the MLNs [
22] and intestinal lamina propria.
Filariasis patients with pathological symptoms (onchocerciasis/river blindness) and lymphatic filariasis (elephantiasis) show reduced Treg cell levels compared with those in unresponsive asymptomatic carriers [
23]. This evidence supported the argument that the Treg cells maintain tolerance and prevent pathology in these infections. The effects of Treg cells in worm-infected mice also mirror those in human subjects. Treg cells are instrumental in debilitation of autoimmune disease in mice infected with schistosomes and gastrointestinal nematodes [
24]. The increased and expansion of these Treg cells during helminth infections may be responsible for the suppression of autoimmune diseases, which has been noted in several studies [
25]. Many reports have shown that increased Treg cell activities and immunosuppressive cytokine productions were observed in prevalent soil-transmitted nematode infections [
26].
Experimental murine models of intestinal helminth infections are highly informative in providing an understanding of the factors contributing to resistance or susceptibility to autoimmune and idiopathic inflammatory diseases in both humans and animals [
27]. Before doing any immunology research, it should be noted that some strains of mice models tend to produce unilateral cytokine profiles. C57BL/6 and BALB/c mice are common Th1 and Th2-type mouse strains, respectively [
28]. C57BL/6 strain is more susceptible to express many inflammatory conditions compared to another strain [
12].
The oxyurid nematode
S. obvelata is a global outbreak and do not cause specific disease in humans.
S. obvelata is easy to be administered, produces in large numbers and stable in storage and transportation.
S. obvelata is easily eradicated by anthelmintic drugs, such as piperazine, thiabendazole, mebendazole, or ivermectin [
29].
We determined S. obvelata-induced host immune responses which showed evidence for protective IL-10 and Foxp3+ Treg cells being essential for survival of helminths in the host. Our data indicated that the percentage of FoxP3+ Treg was increased significantly in infected C57BL/6 mice compared to control mice, it indicated that Foxp3+ Treg cells may be involved in the modulation during S. obvelata exposure.
We concluded that the nematode
S. obvelata and its products have potential and agreed criteria [
30] to be suitable for use in helminth therapy. These are our preliminary data and further studies are needed.
Notes
-
ETHICAL APPROVAL
This study received the approval from the Shahid Beheshti University of Medical Science Ethical Committee.
-
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
ACKNOWLEDGMENT
The authors would like to thank Dr. Zohreh Lasjerdi, for his assistance during the preparation of this manuscript.
Fig. 1Different cytokine levels in cell culture of mesenteric lymph node (MLN) cells.

Fig. 2FACS analysis demonstrating the percentage of CD4+CD25+Foxp3+ Tregs population in MLN cells from a representative sample of each group.

Fig. 3Comparison of the average Foxp3+ T regulatory cell count in the 2 studied groups.

References
- 1. Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol 2002;3:1041-1047.
- 2. Urban JF Jr, Madden KB, Svetić A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev 1992;127:205-220.
- 3. Li Z, Liu G, Chen Y, Liu Y, Liu B, Su Z. The phenotype and function of naturally existing regulatory dendritic cells in nematode-infected mice. Int J Parasitol 2011;41:1129-1137.
- 4. Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol 2004;4:841-855.
- 5. Klementowicz JE, Travis MA, Grencis RK. Trichuris muris: a model of gastrointestinal parasite infection. Semin Immunopathol 2012;34:815-828.
- 6. Maizels RM, Hewitson JP, Murray J, Harcus YM, Dayer B, Filbey KJ, Grainger JR, McSorley HJ, Reynolds LA, Smith KA. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol 2012;132:76-89.
- 7. Abdel-Gaber R. Syphacia obvelata (Nematode, Oxyuridae) infecting laboratory mice Mus musculus (Rodentia, Muridae): phylogeny and host-parasite relationship. Parasitol Res 2016;115:975-985.
- 8. Chan KF. Life cycle studies on the nematode Syphacia obvelata. Am J Hyg 1952;56:14-21.
- 9. Pearson DJ, Taylor G. The Influence of the nematode Syphacia oblevata on adjuvant arthritis in the rat. Immunology 1975;29:391-396.
- 10. Shi M, Wang A, Prescott D, Waterhouse CC, Zhang S, McDougall JJ, Sharkey KA, McKay DM. Infection with an intestinal helminth parasite reduces Freund’s complete adjuvant-induced monoarthritis in mice. Arthritis Rheum 2011;63:434-444.
- 11. Goncalves L, Pinto RM, Vicente JJ, Noronha D, Gomes DC. Helminth parasites of conventionally maintained laboratory mice. II. Inbred strains with an adaptation of the anal swab technique. Mem Inst Oswaldo Cruz 1998;93:121-126.
- 12. De Vooght V, Vanoirbeek JA, Luyts K, Haenen S, Nemery B, Hoet PH. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PLoS One 2010;5:e12581.
- 13. Taghipour N, Molaei M, Mosaffa N, Rostami-Nejad M, Asadzadeh Aghdaei H, Anissian A, Azimzadeh P, Zali MR. An experimental model of colitis induced by dextran sulfate sodium from acute progresses to chronicity in C57BL/6: correlation between conditions of mice and the environment. Gastroenterol Hepatol Bed Bench 2016;9:45-52.
- 14. Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 2009;30:626-635.
- 15. Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016;138:666-675.
- 16. Pulendran B, Artis D. New paradigms in type 2 immunity. Science 2012;337:431-435.
- 17. Hoffmann KF, James SL, Cheever AW, Wynn TA. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol 1999;163:927-938.
- 18. Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun 2007;75:4655-4563.
- 19. Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol 2005;174:4924-4933.
- 20. Redpath SA, van der Werf N, MacDonald AS, Maizels RM, Taylor MD. Schistosoma mansoni larvae do not expand or activate Foxp3+ regulatory T cells during their migratory phase. Infect Immun 2015;83:3881-3889.
- 21. Blankenhaus B, Klemm U, Eschbach ML, Sparwasser T, Huehn J, Kühl AA, Loddenkemper C, Jacobs T, Breloer M. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol 2011;186:4295-4305.
- 22. Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med 2010;207:2331-2341.
- 23. Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, Karthik C, Kumaraswami V, Nutman TB. Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis 2009;3:e420.
- 24. Layland LE, Straubinger K, Ritter M, Loffredo-Verde E, Garn H, Sparwasser T, Prazeres da Costa C. Schistosoma mansoni-mediated suppression of allergic airway inflammation requires patency and Foxp3+ Treg cells. PLoS Negl Trop Dis 2013;7:e2379.
- 25. Wiria AE, Djuardi Y, Supali T, Sartono E, Yazdanbakhsh M. Helminth infection in populations undergoing epidemiological transition: a friend or foe? Semin Immunopathol 2012;34:889-901.
- 26. Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, Alcantara-Neves NM. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun 2010;78:3160-3167.
- 27. Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol 2014;7:753-762.
- 28. Breser ML, Lino AC, Motrich RD, Godoy GJ, Demengeot J, Rivero VE. Regulatory T cells control strain specific resistance to experimental autoimmune prostatitis. Sci Rep 2016;6:33097.
- 29. Pritchett KR, Johnston NA. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 2002;41:36-46.
- 30. Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol 2007;37:457-464.