Abstract
The application of Giemsa technique to stain compressed diaphragm samples obtained from rodents experimentally infected with Trichinella spiralis is described. Diaphragm samples from rats heavily infected with 20 muscle larvae per gram of body weight (20 ML/gbw) were cut into several pieces and stained with Giemsa; on the other hand, whole diaphragms from slightly infected mice (1 ML/gbw) were also stained with Giemsa. Besides, muscle samples were also stained with Giemsa. Observation at 10 × magnification revealed that both ML and nurse cells (NC) look as bluish structures clearly contrasting with the pinkish color of the non-infected muscle fibers. NC in the diaphragms of mice could be easily observed at naked eye as blue points contrasting with the pink surrounding areas formed by the non-infected muscle fibers. Among NC observed in the diaphragms of rats infected with 20 ML/gbw, 4.4% was multiple infection. These findings were confirmed in sectioned and hematoxylin-eosin stained specimens. This data could be usefulness for a rapid diagnosis of trichinellosis in post-mortem mammals without magnification procedures.
-
Key words: Trichinella spiralis, nurse cell, muscle larvae, Giemsa stain
Nowadays, there are several procedures to search for
Trichinella in muscle samples, including direct methods, such as trichinoscopy or muscle compression. The major use of these methods has been for post-mortem detection. Since the method represents facilities in performance and economy, it is routinely used to investigate the presence of
Trichinella muscle larvae (ML), although its diagnostic sensitivity values depends on the experience of the operator or mistakes in the sample examination procedure (
Vignau et al., 1997). In order to improve the sensitivity of direct diagnosis of several other parasitic diseases, contrast stains are usually used. The staining properties of the ML as well as the nurse cell (NC), has been widely described from histological sections stained with haematoxylin-eosin (H-E) technique (
Matsuo et al., 2000;
Boonmars et al., 2004). Descriptions point out that both NC amorphous material and several ML internal structures are blue-stained, suggesting a basophilic nature in these structures, while the surrounding non-infected muscle cells are pinkly stained, suggesting its acidic nature. Since the Giemsa stain method offers similar contrasting coloration as H-E with the advantage that it can be employed in non-truly histological preparations, the aim of this work was to investigate if the Giemsa technique could be used to stain diaphragm samples obtained from
Trichinella spiralis experimentally infected rodents.
Five male Wistar rats (6-week-old, 250g, maintained in our animal facilities following the National Research Council Guide for the Care and Use of Laboratory Animals) were orally infected with 20 ± 0.5 ML per gram of body weight (ML/gbw, approximately 5,000 ML per rat, equivalent to a massive infection) of the MSUS/ME/92/CM-92 T. spiralis strain. Six weeks later, animals were sacrificed and diaphragms dissected out; besides, muscle samples of thigh from an additional infected rat were collected. The 4 diaphragms and the muscle samples were cut into 3-4 mm pieces and compressed between 2 glass slides, then submitted to Giemsa staining. For stain, compressed sample pieces were separated from glass slides, fixed with FAA fixative solution (v/v formaldehyde-acetic-alcohol, 10 : 40 : 50) during 4 hr at room temperature and transferred to a Petri dish containing 50% ethyl alcohol. Samples were immersed in 10 mL of Giemsa solution diluted 1 : 6 in 0.01 M, pH 7.2 phosphate buffer solution during 45 min at room temperature with slow constant stirring. Afterwards, samples were individually transferred to acidic alcohol (0.02 N HCl in 50% ethyl alcohol) solution during about 45 sec, and then dehydrated with graded alcohol series (30%, 50%, 70% and 100%) lasting 2-3 min in each solution and applying gentle stirring. During this step, samples were also de-stained, a visual inspection was carried out for each sample. Samples were then incubated (5 min each) in a mixture (v/v) of absolute ethyl alcohol and xylene, and finally in absolute xylene. After then, permanent slides were prepared as usual. Microscopic observations were carried out at 10 × and 40 × magnifications. Total NC and ML were counted. The remaining rat diaphragm with larvae was fixed with 10% formaldehyde solution during 24 hr. Afterwards, sample was dehydrated, paraffin embedded, and 3 µm thin slides were cut with microtome, stained with H-E, dehydrated and mounted as usual. Since preliminary results of Giemsa stain, promptly suggest the helpfulness of the technique for a rapid direct diagnosis without magnification procedures, 5 male CD1 mice were infected orally with approximately 1 ML/gbw (g of body weight), equivalent to a light infection. Thus, the combination of a handy piece of muscle and a low parasite load could establish the usefulness of the technique. After 6 weeks of infection, diaphragms were compressed as a whole, fixed, stained and examined by naked eyes.
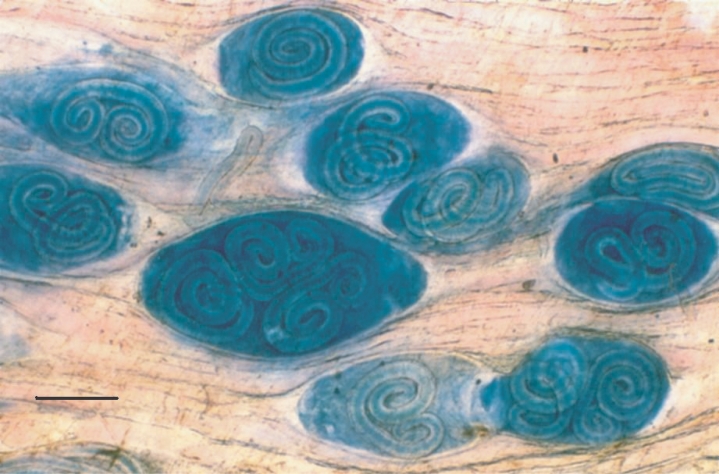
After Giemsa staining, the ML and NC itself were observed under the microscope as blue structures surrounded by non-infected muscle cells, which appeared with a pink coloration; similar contrast was observed in both diaphragm pieces (
Fig. 1) and muscle samples. During the staining standardization process, changes in some technical steps deal with a number of critical observations. Firstly, different fixation times (range; 18-24 hr) do not modified the intensity or quality of coloration. Secondly, no contrasted colored structures were obtained when neutral or alkaline alcohol was used, or when acidification was performed before staining step, and the colorant excess is not removed during the dehydration process. Thirdly, the de-coloration time must not be exceeding the stated values, since the contrast is lost. Thus, acidification of tissue samples are imperative and must be carried out immediately after the staining step and during no more than 45 sec. During the microscopical examination of rat diaphragms (20 ML/gbw), either one or more ML were observed inside of a single NC (
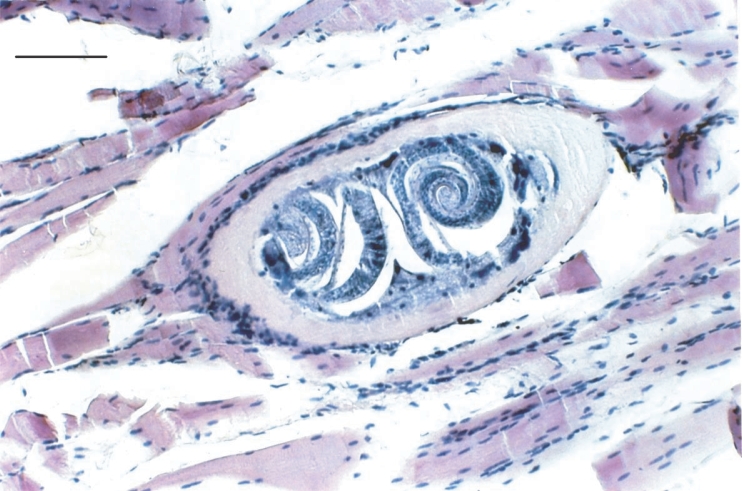
Fig. 1). The possibility that this observation could be a technical artefact due to a superposition of more than 2 NC after compression of muscle samples was discarded, since the presence of multiple ML in a single NC was clearly identified in histological slides (
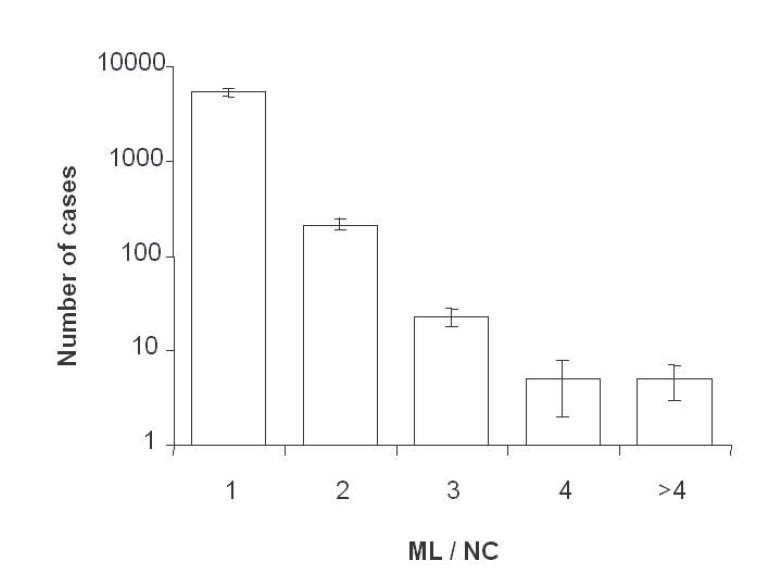
Fig. 2). A frequency of 4.4% of NC contained more than 2 ML (2, 3, 4 or more), but no NC without ML were detected (
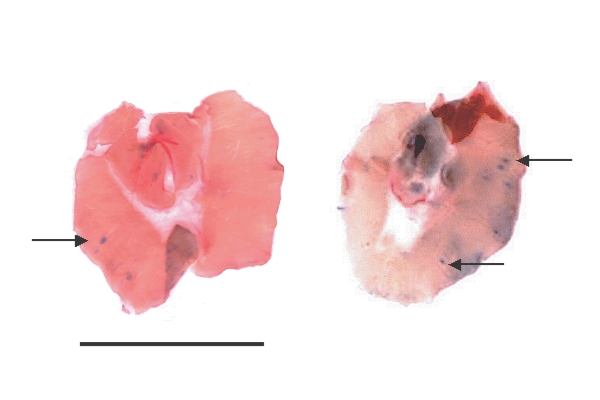
Fig. 3). The contrasting staining characteristics of the observed colour between parasite structures and its surrounding environment, promptly suggested the possibility that parasite could be detected without the use of microscope; thus, the complete diaphragms of the lightly infected mice were macroscopically observed. Few blue points contrasting with pink muscle tissues were clearly observed (
Fig. 4). The observation at 4 × magnification confirmed that all of them were NC containing only one ML.
As herein described, Giemsa stain procedure can be applied to muscle samples to contrast the presence of
Trichinella. The NC, containing the ML, could be easily visualized as bluish-stained oval structures, while non-infected muscle cells were pinkly stained; same contrast has been observed in histological sections stained with H-E (
Boonmars et al., 2004;
Matsuo et al., 2000). Such color contrast can be obtained, when samples have an acidic treatment just after the staining step, otherwise, the colorant excess is not removed during the dehydration process, and therefore, the contrast is lost. At present, regarding multiple larvae in one NC, a simple relationship of one ML in one NC is widely accepted (
Despommier, 1998). However, we found 4.4% of NC containing inside more than 2 ML in heavy infections (20 ML/gbw), but a normal rate (1 : 1 ML/NC) in lightly infected animals (1 ML/gbw). Since a previous finding reported by Perez and Luengo (
1969), where 9 ML inside of a single NC were detected in a muscle biopsy from a massive infected pig, it is suggested that this phenomenon could be related to the intensity of infection, i. e., more than one new borne larvae invades the myocyte at the same or nearly time. To our knowledge, there is no information regarding the possible fusion of 2 or more NC during the infection process (
Despommier, 1998). The multiple invasion hypothesis mentioned before seems to be more acceptable, suggesting that this observation is more than a simple laboratory finding, but a real and non-studied biological phenomenon. Further studies are needed to investigate if the type of host is related with the occurrence of multiple larvae and how Giemsa stain could improve the diagnostic sensitivity.
Notes
-
This work was partially supported by CONACyT, Mexico, Grant 34654-M.
ACKNOWLEDGMENTS
We are grateful to S. Zamora-Carrillo and N. Álvarez for excellent laboratory assistance and Dr. R. Tapia-Romero for critical reading of the manuscript, Biól. B. Salceda for English review. Rats and mice were provided from our animal house, I. Valle and M. Piñón for their excellent animal care.
References
Fig. 1Compressed diaphragm tissue of an experimentally infected rat stained with Giemsa, showing 1, 2 or 3 Trichinella spiralis muscle larvae inside a single nurse cell. Scale bar = 0.2 mm.

Fig. 2Histological section stained with hematoxylin-eosin technique of one nurse cell containing 2 muscle larvae. Diaphragm sample was obtained from one heavily experimentally infected rat. Scale bar is 0.1 mm.

Fig. 3Frequency of nurse cells containing one or more T. spiralis muscle larvae in diaphragms from 4 experimentally infected rats, each with 5,000 larvae. Vertical bars are SDs.

Fig. 4Two representative diaphragms of T. spiralis-infected mice stained with Giemsa technique. Animals were experimentally infected with approximately 1 ML/gbw, equivalent to a light infection. Photographs were shot without magnification. Arrows show nurse cells containing one-muscle larvae. Scale bar is 1 cm.

Citations
Citations to this article as recorded by

- Kinetics of Eosinophils during Development of the Cellular Infiltrate Surrounding the Nurse Cell of Trichinella spiralis in Experimentally Infected Mice
Vicente Vega-Sánchez, Fabián-Ricardo Gómez-De-Anda, Georgina Calderón-Domínguez, Mary-Carmen-del-Sol Ramírez-y-Ramírez, Nydia-E. Reyes-Rodríguez, Andrea-P. Zepeda-Velázquez, Raquel Tapia-Romero, Jorge-Luis de-la-Rosa-Arana
Pathogens.2021; 10(11): 1382. CrossRef - Immune response to aTrichinella spiralisinfection in house mice from lines selectively bred for high voluntary wheel running
Elizabeth M. Dlugosz, Heidi Schutz, Thomas H. Meek, Wendy Acosta, Cynthia J. Downs, Edward G. Platzer, Mark A. Chappell, Theodore Garland
Journal of Experimental Biology.2013;[Epub] CrossRef - Comparative effects of levamisole, Staphylococcus, and Freund's adjuvant on rat immunization with excretory and secretory antigens of Trichinella spiralis muscle larvae
Jorge-Luis de-la-Rosa-Arana, Rafael Campos-Rodríguez, Víctor Rivera-Aguilar, Alejandro Escobar-Gutiérrez, Ángel Miliar-García, Norma-Elena Herrera-González, Rosa-Adriana Jarillo-Luna
Parasitology Research.2012; 111(4): 1599. CrossRef - Influence of different processing procedures on the reproductive capacity of Trichinella spiralis in pork meat
M. S. Medina-Lerena, A. Ramirez-Álvarez, M. Kühne, A. Gómez-Priego, J.-L. de-la-Rosa
Tropical Animal Health and Production.2009; 41(4): 437. CrossRef