Abstract
Although Vietnam has a high risk of fishborne zoonotic trematode (FZT) infections for humans, little information exists on the epidemiology of these infections in the country's fish. Because of the importance of cultured catfish and snakehead production in An Giang province, a major production area in the Mekong Delta of Vietnam, a survey for FZTs was carried out in randomly selected fish farms between June 2005 and March 2006. For comparison, wild fish from the same area were also surveyed. A total of 852 cultured fish from 4 districts were collected and examined by pepsin digestion to determine their FZT infection status. In Tra catfish, the prevalence of all types of metacercariae was 2.6%, of which the prevalence of Haplorchis pumilio was 0.7%. The overall prevalence of metacercariae in wild fish was 30.6%, of which 10.3% harbored zoonotic species: H. pumilio (2.8%) and Procerovum sp. (5.6%). The prevalence of Opisthorchis metacercariae, which were diagnosed as O. viverrini, was 1.9%. No metacercariae were found in cultured snakehead fish, although wild-caught snakehead fish had a FZT prevalence of 10.3%: 5.1% were O. viverrini; 2.6% H. pumilio; and 2.6% were Procerovum sp. These are the first reports of H. pumilio, Procerovum sp., and O. viverrini metacercariae in Vietnamese fish. These results indicate that consumption of improperly prepared fish represents a significant risk of acquiring FZTs in this south Vietnam region.
-
Key words: Haplorchis, Procerovum, Opisthtorchis, zoonoses, parasite, trematode, liver fluke, intestinal fluke, metacercaria, aquaculture, wild fish, Vietnam
INTRODUCTION
Fish-borne zoonotic liver and intestinal trematodes are recognized as an important group of human pathogens (
WHO, 1995,
2004;
Chai et al., 2005a;
Keiser and Utzinger, 2005). The number of people currently infected with FZTs was recently estimated by the World Health Organization (WHO) to exceed 18 million, with the number of people at risk worldwide estimated at more than half a billion (
WHO, 2004). These zoonotic parasites are especially prevalent in South East Asia, where recent data suggest that there are about 1.5 million people in Korea, 6 million people in China, and over 5 million in Thailand infected with liver flukes, either
Clonorchis sinensis or
Opisthorchis viverrini (
Chai et al., 2005a). Further, more than 50 species of foodborne intestinal flukes belonging to the Heterophyidae and Echinostomatidae have been reported from China (
Yu and Xu, 2005), Korea (
Chai, 2005), Thailand (
Waikagul and Radomyos, 2005), and Laos (
Chai et al., 2005b).
Current information on the prevalence and species diversity of FZTs in Vietnam suggests that these parasites are important public health problems in the country, although epidemiological and health impact information is quite limited (reviewed by
De et al., 2003).
C. sinensis has been reported from 9 Northern provinces of Vietnam with human infection rates ranging from 0.2% in Thai Binh province to 26% in Nam Dinh province.
O. viverrini is reported from 3 southern provinces with infection rates ranging from 0.3% (Da Nang province) to more than 10% (Phu Yen province) (reviewed by
De et al., 2003). However, there are few reports of intestinal flukes, which are highly prevalent elsewhere in Southeast Asia (
De et al., 2003). This may be due, at least in part, to the difficulty in distinguishing between the eggs of liver and intestinal flukes in human fecal surveys (
Detrich et al., 1990). This raises the question of what the precise distribution and prevalence of the liver flukes is in Vietnam, and the need to more research on these zoonoses.
Records of FZT metacercariae in fish in Vietnam are also very few. The status of fish infections, however, is of major concern, because of the increasing prominence of aquaculture in the country's economy. Freshwater fish production in Vietnam has increased from 41,750 tons in 1962 to 390,000 tons in 2005 (a 9.3-fold increase) (
Keiser and Utzinger, 2005). With this growth in the aquaculture industry, there may be substantial risk of enhancing FZT infections, because intensification of aquaculture will probably facilitate the proliferation and availability of the snail and fish intermediate hosts, resulting in increased transmission of the parasites, especially if current aquaculture practices, such as the use of manures are unchanged (
WHO, 2004). Fertilization of ponds with human and animal manure is attractive to fish farmers because of it low cost. The risk of infection in a country with well-established preferences for raw fish, such as Vietnam, therefore, may be increased significantly.
As part of a national program on assessing the occurrence and risk of FZTs in the Vietnamese aquaculture sector that is currently being conducted by various Vietnamese institutions under a partnership with the Ministry of Fisheries, and with Danish institutions (
www.fibozopa.ria1.org), a survey of especially high-value cultured fish, Tra catfish and snakehead fish was carried out in An Giang province, a major fish production area in the Mekong Delta. A limited survey of wild fish in the same area was also carried out. The results demonstrated that there is an appreciable risk of acquiring zoonotic flukes from the consumption of either farmed and wild fish in this important fish producing region.
MATERIALS AND METHODS
Survey area and sample collection
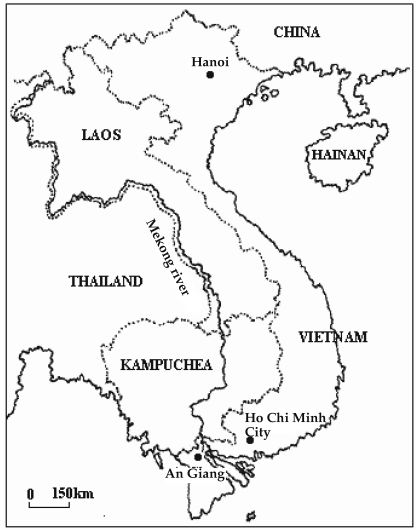
An Giang province is located in the upper reaches of the Mekong Delta of south-western Vietnam, and shares a border with Cambodia to the northwest (
Fig. 1). An Giang is a major production center for highvalue fish, such as Tra catfish and snakehead fish.
Fish collections were carried out from June 2005 to March 2006 in 4 districts of An Giang province: Chau Phu, Chau Thanh, Chau Doc and An Phu. The random selection of farms for fish collection was conducted according to standard methods by computerized selection of study households (farms) from a list of all households engaged in aquaculture. Fish were collected twice, first from June to August 2005, and later from October 2005 to March 2006. The second sampling was made to detect seasonal differences in trematode transmission. A total of 16 Tra catfish and 13 snakehead collection sites (farms) were selected at random from the total population of fish farms in An Giang province. The Tra catfish samples were obtained from 3 districts: Chau Phu (n = 371 fish), covering 10 communes; Chau Thanh (n = 55 fish) involving 4 communes; and Chau Doc (n = 33 fish) involving 2 communes (
Table 2). A total of 13 communes in 3 of these districts were sampled for snakeheads: Chau Phu (n = 15 fish), Chau Thanh (n = 265 fish) and Chau Doc (n = 5 fish) (
Table 2). Whenever possible, a minimum of 5 fish, were collected from each site.
In addition, 11 species of wild fish (n = 108) (
Table 2), caught in the Song Hau River and sold in 8 local markets distributed throughout the 4 districts (Chau Phu, Chau Thanh, Chau Doc and An Phu) were examined. The fish were purchased while still alive and processed for recovery of metacercariae as described below. Before purchasing a fish at the market, the trader confirmed the origin of the fish (i.e. that it was wild caught). These fish served as "indicator fish" to assist in determining the presence of FZTs in the study area.
The types of fish collected from different districts in the province are presented in
Table 1. Cultured fish were collected either from farm ponds or river cages. Three different sizes of wooden cages are used for the culture of Tra catfish; small, 3 × 2 × 2 m; medium, 6 × 3 × 3 m; and large, 8 × 15 × 4 m, and the fish are stocked at a density of about 200 fish/m
3. The 2 main species of Tra catfish cultured in cages were
Pangasius hypophthalmus and
Pangasius bocourti, while the species raised in ponds were
P. hypophthalmus and
P. larnaudii. Pond stocking density was 10-20 fish/m
2 for these fish species. Snakehead fish,
Channa striata and
Channa micropeltes, were also grown in pond systems with a density of 15-20 fish/m
3. The farmed fish were fed either a mix of trash fish and rice, or commercial pelleted feed.
Following collection of fish from farms or markets, they were placed on ice and transported to the Research Institute for Aquaculture No. 2 in Ho Chi Minh City, where they were placed in cold storage (4℃) until they could be examined for metacercariae, normally within 7 days of collection. In the laboratory, the length and weight of each fish was recorded. Metacercariae were isolated and recovered using the standard pepsin digestion procedure described in Annex 6 of WHO (
1995). For fish weighing less than 150 g, the entire body was ground and digested. Fish weighing more than 150 g were subdivided into 5 sub-samples, as described in Annex 6 of WHO (
1995). All samples were minced by grinding in a mortar with a pestle or a meat grinder until a paste-like consistency was obtained. This was then digested using a solution of 1% pepsin and 0.06 M HCl in distilled water at 37℃ for at least 2-3 hr. The digested material was then filtered through a 1 × 1 mm mesh brass sieve, allowed to settle, and the sediment was subsequently re-suspended in 0.86% saline. The sediment was again allowed to settle. This washing step was repeated as necessary until the supernatant became almost clear. The supernatant was then carefully poured off and discarded. The sediment was then transferred into a small Petri dish containing 6-7 ml 0.85% saline, and examined under a stereomicroscope. Metacercariae recovered were counted and identified by mounting on a glass slide under a coverslip and viewing with a stereo or a compound microscope. When encysted metacercariae were not readily identifiable, they were excysted by either physical pressure (pressing on the coverslip) or by placing them in trypsin digestion fluid (0.4% sodium hydrogen carbonate, 1.0% trypsin, 0.85% NaCl) until they emerged from the cyst and could be examined in an extended condition under a microscope.
Identification of the metacercariae was assisted by use of morphological criteria detailed in Kaewkes (
2003), Pearson and Ow-Yang (
1982), Schell (
1970), Scholtz et al. (
1991), Velasquez (
1973), and Yamaguti (
1971).
The collected data were analyzed statistically with SPSS software (Statistical Package for Social Sciences version 10; SPSS Inc., Chicago, Illinois, USA) for Windows 11.5 using, where appropriate, a variety of parametric and non-parametric tests (Chi-square test, Student t-test, Kruskai-Walls test).
RESULTS
Prevalence of metacercariae in Tra catfish
A total of 459 Tra catfish were collected and examined for the presence of metacercariae (
Table 1). The weights and lengths of the fish are given in
Table 2. The prevalence of all types of metacercariae in Tra catfish was 2.6% (12/459) (
Table 3); metacercariae were recovered only from the cage-reared catfish. The only species of zoonotic metacercariae recovered was
Haplorchis pumilio, whose prevalence was 0.7% (3/459). These encysted metacercariae were identified by their elliptical shape, size (0.16-0.19 × 0.14-0.16 mm), possession of 36-42 hooklets on the ventral sucker, and O-shaped excretory bladder. The putative non-zoonotic
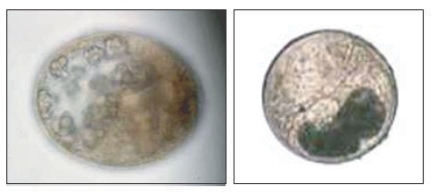
Exorchis oviformis was identified by its 0.18-0.20 × 0.14-0.15 mm size, thin transparent cyst wall, prominent eye spots lateral to the pharynx, and ventral sucker smaller than the oral sucker; it occurred in 0.9% of catfish (4/459). An unidentified metacercaria species (Us-1,
Fig. 2a) was present in 1.1% (5/459). Among the 3 districts, the prevalence of
H. pumilio was 0.5% in Chau Phu (involving 2 communes), and 1.8% in Chau Thanh district (one commune); no metacercariae were recovered from catfish obtained from Chau Doc district (
Table 3). The prevalence of all types metacercariae, including
H. pumilio, in Tra fish did not differ significantly (
P > 0.05) between the districts.
The intensity of H. pumilio infection was 5.0 ± 5.0 metacercariae/100 g. Although the size of fish infected with H. pumilio appeared to influence the infection rate (small fish, ≤ 100 g; prevalence = 1.7%; large fish, > 100 g; prevalence = 0.9%) the difference between sizes was not significant (P > 0.05).
Prevalence of metacercariae in farmed snakehead fish
A total of 285 pond-reared snakeheads were sampled (225
C. striata and 60
C. micropeltes) (
Table 1). The average weights and lengths of these fish are detailed in
Table 2. No metacercariae were recovered from any of these snakehead fish.
Eleven species of wild fish (n = 108 fish) were collected from 8 different markets in 4 districts (
Table 1). The mean lengths and weights of each fish species examined are presented in
Table 2. The prevalence data on specific metacercaria infections for each species of wild fish are presented in
Table 4. The prevalence for all metacercarial types was 30.6%; for zoonotic metacercariae the prevalence was 10.3%, i.e.,
H. pumilio 2.8% and
Procerovum sp. 5.6%.
Procerovum metacercariae, although similar to
Haplorchis, were distinguished by the lack of spines on the ventral sucker, but possessing spines on the gonotyl.
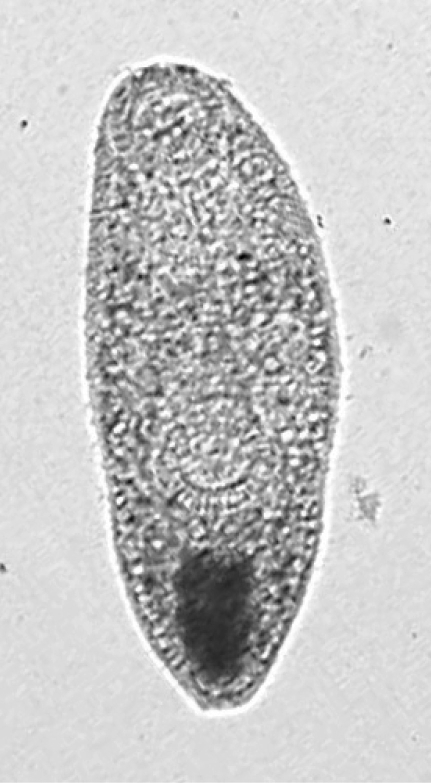
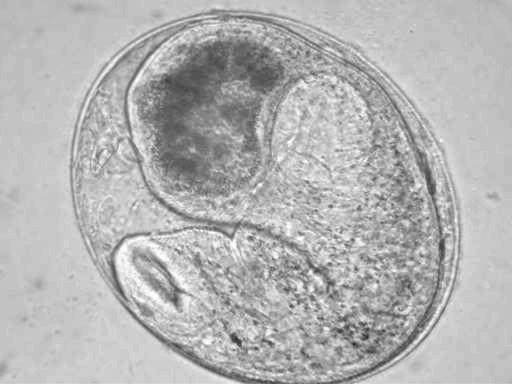
Opisthtorchis metacercariae were recovered from 1.9% of the fish (
Figs. 3 and
4), and based on gross morphology (elliptical cyst shape, 0.19-0.25 × 0.15-0.22 mm size, nearly equal size oral and ventral suckers, and O-shaped excretory bladder occupying large part of posterior body) were identified provisionally as
O. viverrini (see Discussion).
E. oviformis was recovered from 17.6% of the wild fish. Two unidentified species of metacercariae were found in 12.1% of the wild fish: Us-1, (10.2%) and Us-2, (1.9%) (
Fig. 2a, 2b).
The infection intensities for each fish species are shown in
Table 5. Although the sample sizes for each fish species was small, statistical analyses suggest that wild fish were generally more heavily infected than cultured fish; the heaviest infections were observed in climbing perch (
Anabas testudineus) (mean = 1.66 metacercariae/g of tissue). A comparison of infection intensities between Tra catfish (
P. hypophthalmus) and wild snakehead fish (
C. striata) and climbing perch (
A. testudineus) indicated significant differences (
P < 0.05) among these species (
Table 5).
DISCUSSION
The results from this study extend our understanding on the geographic distribution of zoonotic liver and intestinal FZTs in Vietnam, and in Asia. The examination of cultured and wild fish collected in An Giang province revealed the presence of
O. viverrini and, for the first time in Vietnam, 2 species of intestinal flukes, i.e.,
H. pumilio and
Procerovum sp. The identification of the recovered
Opisthtorchis metacercariae as
O. viverrini is provisional, because it was not possible to infect laboratory animals for adult worm recovery, a stage which can provide conclusive morphological features for identification. Also, other non-zoonotic species of the genus are reported in Vietnam (
Yamaguti 1971), and could be present in fish. However, we feel this identification is likely, because of the lack of evidence for other species in the Mekong Delta region. Yamaguti (
1971) accepted 18 species of
Opisthtorchis from mammals, birds and fishes, but only 2 of these,
O. viverrini and
O. felineus, were recorded from Southeast Asia; the latter, however, is not currently considered endemic to Southeast Asia (
Kwaekes, 2003). Two species have been reported from China, one from a bird and one from fish, but little is known or reported on these since the 1940s. The identification, of metacercariae recovered in this study as
O. viverrini, is supported by the morphological features of the metacercariae (
Figs. 3 and
4), and the numerous reports on the presence of this species in humans and fish in South East Asia (
De et al., 2003;
Chai et al., 2005a,
b;
Scholtz et al., 1991). It is proposed that this identification be regarded as provisional, however, until the metacercariae are again found and studies such as adult worm examinations (by animal infection studies) can be carried out to confirm this identification.
The study results unequivocally document for the first time the occurrence of
H. pumilio and
E. oviformis in the economically important Tra catfish (
P. hypophthalmus). However, it should be noted that the occurrence of
H. pumilio was low (0.6%). Infections with
E. oviformis have been reported from different species of snakeheads (
Ophicephalus argus) in Japan (
Komiya, 1965). To date there are no reports of
E. oviformis infections in humans.
The recovery of the 2 species of metacercariae (Us-1 and Us-2,
Figs. 2a, 2b), which could not be identified, makes it difficult to provide a more complete biodiversity profile of zoonotic metacercariae in Vietnamese fish. Difficulties in trematode identifications based solely on metacercarial stages, especially for the heterophyids, are well known. The identity of Us-2 (
Fig. 2b) is of particular interest, because of its close morphological similarity to
Metagonimus, which is widely reported from freshwater fish in Asia (
WHO, 1995;
Chai et al., 2005a;
Cho et al., 2006). Because it was not feasible in this investigation to perform timely animal infection experiments to obtain adult stages of these trematodes for specific identification, further surveys in this region are needed to provide the parasite material necessary to carry out these needed animal infection experiments. The development of new molecular diagnostic techniques is also needed to facilitate the accuracy of species identifications of zoonotic metacercariae (
Maleewong et al., 2003, cited in
Chai et al., 2005a;
Hoa et al., 2006).
Important for the growing Vietnamese aquaculture industry is a better understanding of the magnitude of problems with FZTs. Although this survey was limited in size and geographical scope, the results clearly show that these parasites are present in cultured catfish, a potentially important food safety problem. However, the prevalence of infection among different fish species differed markedly between farmed fish and between wild fish (30.6% for the group), although the sample size of the latter was too small for statistical comparisons. Infection was low (2.6%) in cultured Tra fish (P. hypophthalmus) and absent in cultured snakeheads (C. striata and C. micropeltes).
It would have been interesting to compare statistically the prevalence of metacercariae in Tra fish reared in ponds to those reared in river cages to gain more insight on the ecological conditions in different production systems that favor transmission, but the number of Tra fish from cages was too low (n = 13) to allow this. Subsequent to the initiation of this study, the formerly wide-spread practice of cage-rearing Tra fish was markedly reduced, because it became economically less attractive in comparison to pond culture.
The high prevalence of FZTs in wild fish, compared to farmed fish, especially in snakeheads (C. striata), does indicate, however, that factors favoring transmission of these FZTs to susceptible fish hosts are influenced by certain aquatic ecological and farming factors which are not as yet well characterized. It is possible that fish raised in rivers, canals, streams and lakes may be more exposed to infected snails/cercariae, and reservoir hosts, compared to fish raised in farms, where the pond environment may not favor the presence of snails (e.g., husbandry practices, such as hand picking to remove snails or exposure to snail-eating ducks). The authors hope that the results of this study will help to stimulate the undertaking of further comparative studies, employing larger sampling schemes and involving more comprehensive evaluations of various aquatic/ecological factors (such as the interplay of snail populations and water types and sources, in determining transmission rates).
Another potentially important influence on transmission of FZTs in aquaculture is the presence of reservoir hosts, such as cats, dogs and pigs, which may contaminate the environment with FZT eggs (
Chai et al., 2005a). In Vietnam, such livestock-fish-vegetable systems utilizing manures as fertilizers (VAC system) are encouraged. The role of these hosts needs much closer examination, especially since recent strategies for control of FZTs place strong emphasis primarily on mass drug treatment of the human population (
WHO, 2004). However, domestic animal excreta containing FZTs eggs may also contaminate water bodies, leading to snail and fish infections, and a sustainable transmission of FZTs to fish. Unfortunately, for risk assessment analyses, the actual importance of these reservoir hosts has not been thoroughly evaluated.
Complicating the epidemiological picture of sources of eggs for fish culture systems is contamination of water sources by infected snails located outside of the ponds themselves, perhaps though exchange of flood-water or irrigation water. The mobility of the cercariae of FZTs in these types of water systems is not well known. The impact of the common practice in the Mekong Delta of discharging human excreta directly into canals and rivers (sewage) on FZT transmission also is unknown. A comprehensive understanding of more aspects of the ecology and epidemiology of FZT transmission in aquaculture will require evaluation of these potential sources.
In conclusion, the prevalence of zoonotic liver and intestinal metacercariae in cultured high-value fish in An Giang province, although low in Tra catfish (and absent in snakeheads), presents a risk to humans consuming inadequately prepared fish. Even low rates of FZT infection in popular cultured fish can pose a public health problem because over a long time span repeated consumption of raw fish could result in a substantial accumulation of parasites, especially liver flukes (
WHO, 1995;
Chai et al. 2005a). It is recommended that fish farms operating in high risk liver and intestinal fluke areas adopt as a minimum husbandry practices designed to reduce contamination of ponds with trematode eggs. These should include the cessation of the practice of using untreated or non composted fecal material as pond fertilizer, the use of a safe water supply, the removal or control of snail populations, and prevention of exposure of domestic animals to raw fish unless a fool-proof method of preventing their feces from contaminating the ponds is instituted.
Notes
-
This study was financially supported by the Fishborne Zoonotic Parasites in Vietnam (FIBOZOPA) project no. 91140/file no. 104.Dan.L.8.f and the Danish International Development Assistance (DANIDA).
ACKNOWLEDGMENTS
Andrew Shinn at Stirling University, Scotland is thanked for excellent supervision of the senior author during her MSc studies. In Vietnam, Phan Thi Hong Hai, and Le Minh Thuc provided assistance in the collection and metacercarial diagnosis of fish samples. We also would like to thank the staff in the FIBOZOPA project secretariat, especially Jesper Clausen, Jacob Fjalland and Bui Thanh, and those at the Research Institute for Aquaculture No.2 (RIA2), together with colleagues at the Fishery Department in An Giang province, for their assistance and cooperation throughout this study. Appreciation is expressed to Professor Jong-Yil Chai, Department of Parasitology, Seoul National University College of Medicine, and Professor Woon-Mok Sohn, Department of Parasitology, College of Medicine, Gyeongsang National University, Korea for their expert advice and counsel on the recovery and identification of metacercariae.
References
Fig. 1Map of Vietnam indicating the location of An Giang Province.

Fig. 2Photos of an unidentified metacercaria type 1 (Us-1) (left) and an unidentified metacercaria type 2 (Us-2) (right). × 40.

Fig. 3An excysted Opisthtorchis metacercaria. × 100.

Fig. 4An encysted Opisthtorchis metacercaria. × 100.

Table 1.Details on the fish sampled throughout An Giang province, Vietnam
Table 1.
|
Common name |
Fish species examined |
Type of sample site (No. of fish) |
No. of fish sampled (% of fish sample per district)
|
|
Chau Phu |
Chau Thanh |
Chau Doc |
An Phu |
|
Tra fish |
Pangasius hypophthalmus
|
Cage (13) |
371 (80.8) |
55 (12.0) |
33 (7.2) |
0 |
|
|
Pond (446) |
|
|
|
|
|
Snakehead |
Channa striata
|
Pond (225) |
15 (6.7) |
205 (91.1) |
5 (2.2) |
0 |
|
Snakehead |
Channa micropeltes
|
Pond (60) |
0 |
60 (100) |
0 |
0 |
|
Climbing perch |
Anabas testudineus
|
Market (35) |
20 (57.1) |
0 |
15 (42.9) |
0 |
|
Tiger botia |
Botia helodes
|
Market (5) |
5 (100) |
0 |
0 |
0 |
|
Snakehead |
Channa striata
|
Market (39) |
10 (25.6) |
14 (35.9) |
10 (25.6) |
5 (12.8) |
|
Mud carp |
Cirrhinus jullieni
|
Market (3) |
3 (100) |
0 |
0 |
0 |
|
Peacock eel |
Macrognathus siamensis
|
Market (3) |
3 (100) |
0 |
0 |
0 |
|
Zig-zag eel |
Mastacembelus armatus
|
Market (1) |
1 (100) |
0 |
0 |
0 |
|
Mytus |
Mystus albolineatus
|
Market (5) |
5 (100) |
0 |
0 |
0 |
|
Tilapia |
Oreochromis mossambicus
|
Market (3) |
0 |
3 (100) |
0 |
0 |
|
Silver sharkminnow |
Osteochilus hasselti
|
Market (5) |
5 (100) |
0 |
0 |
0 |
|
Catopra |
Pristolepis fasciatus
|
Market (2) |
2 (100) |
0 |
0 |
0 |
|
Three-lined rasbora |
Rasbora trilineata
|
Market (7) |
7 (100) |
0 |
0 |
0 |
|
Total (852 fish) |
|
Cage (13) |
447 (52.5) |
337 (39.5) |
63 (7.4) |
5 (0.6) |
|
|
Pond (731) |
|
|
|
|
|
|
Market (108) |
|
|
|
|
Table 2.Weight and length of Tra catfish, snakehead and wild caught fish examined in An Giang province, Vietnam
Table 2.
|
Fish species |
Location (District or Market) |
No. of fish examined |
Weight (g)
|
Length (cm)
|
|
Range |
Mean ± SD |
Range |
Mean ± SD |
|
Tra catfish |
|
|
|
|
|
|
|
Pangasius hypophthalmus
|
Chau Phu |
371 |
0.5-1,080.0 |
126.6 ± 200.8 |
5.0-53.5 |
19.3 ± 9.6 |
|
Pangasius hypophthalmus
|
Chau Thanh |
55 |
7.0-895.0 |
110.4 ± 151.4 |
6.0-39.0 |
16.6 ± 7.3 |
|
Pangasius hypophthalmus
|
Chau Doc |
33 |
6.2-365.0 |
110.1 ± 119.2 |
8.7-31.0 |
17.9 ± 6.7 |
|
Total |
|
459 |
0.5-1,080.0 |
123.4 ± 190.6 |
5.0-53.5 |
18.9 ± 9.2 |
|
|
Snakehead |
|
|
|
|
|
|
|
Channa striata
|
Chau Phu |
15 |
3.5-138.7 |
42.0 ± 50.5 |
7.6-25.4 |
14.7 ± 6.1 |
|
Chau Thanh |
205 |
0.6-352.8 |
39.4 ± 58.5 |
4.4-34.4 |
13.1 ± 7.4 |
|
Chau Doc |
5 |
17.0-30.0 |
22.4 ± 5.1 |
10.0-13.0 |
11.6 ± 1.2 |
|
Channa micropeltes
|
Chau Thanh |
60 |
0.3-171.8 |
22.0 ± 32.2 |
3.0-26.3 |
11.1 ± 5.4 |
|
Total |
|
285 |
0.3-352.8 |
35.6 ± 57.6 |
3.0-34.4 |
12.7 ± 6.9 |
|
|
Wild caught fish |
|
|
|
|
|
|
|
Anabas testudineus
|
Market |
35 |
15.0-80.0 |
50.4 ± 20.2 |
7.5-14.2 |
10.9 ± 1.8 |
|
Botia helodes
|
Market |
5 |
2.5-3.9 |
3.2 ± 0.6 |
6.6-8.2 |
7.3 ± 0.6 |
|
Channa striata
|
Market |
39 |
25.0-430.0 |
147.6 ± 97.4 |
11.5-31.5 |
20.6 ± 5.3 |
|
Cirrhinus jullieni
|
Market |
3 |
1.1-3.6 |
2.1 ± 1.3 |
5.6-7.5 |
6.5 ± 1.0 |
|
Macrognathus siamensis
|
Market |
3 |
9.6-13.2 |
10.9 ± 2.0 |
14.2-16.4 |
15.0 ± 1.2 |
|
Mastacembelus armatus
|
Market |
1 |
5.9 |
5.9 ± 0.0 |
12.0 |
12.0 ± 0.0 |
|
Mystus albolineatus
|
Market |
5 |
3.2-5.4 |
4.1 ± 0.9 |
8.0-9.2 |
8.3 ± 0.5 |
|
Oreochromis mossambicus
|
Market |
3 |
235.0-250.0 |
241.7 ± 7.6 |
18.0-19.5 |
18.8 ± 0.8 |
|
Osteochilus hasselti
|
Market |
5 |
2.6-4.7 |
3.6 ± 1.0 |
5.7-6.7 |
6.2 ± 0.4 |
|
Pristolepis fasciatus
|
Market |
2 |
1.7-2.8 |
2.2 ± 0.8 |
5.3-5.6 |
5.5 ± 0.2 |
|
Rasbora trilineata
|
Market |
7 |
2.3-6.0 |
4.2 ± 1.5 |
6.8-8.7 |
7.7 ± 0.7 |
|
Total |
|
108 |
1.1-430.0 |
77.6 ± 88.6 |
5.3-31.5 |
13.8 ± 6.5 |
Table 3.Prevalence of different species of metacercariae in pond-reared Tra catfish from different districts in An Giang province, Vietnam
Table 3.
|
District
|
Total |
|
Chau Phu
|
Chau Thanh
|
Chau Doc
|
|
No. |
(% infected) |
No. |
(% infected) |
No. |
(% infected) |
No. |
(% infected) |
|
No. of fish examined |
371 |
|
55 |
|
33 |
|
459 |
|
|
No. fish infected with metacercariae |
11 |
(2.4) |
1 |
(0.2) |
0 |
|
12 |
(2.6) |
|
Haplorchis pumilo
|
2 |
(0.4) |
1 |
(0.2) |
0 |
|
3 |
(0.6) |
|
Exorchis oviformis (Non-zoonotic) |
4 |
(0.9) |
0 |
|
0 |
|
4 |
(0.9) |
|
Unidentified species (Us-1) |
5 |
(1.1) |
0 |
|
0 |
|
5 |
(1.1) |
Table 4.Prevalence of zoonotic, non-zoonotic, and unidentified metacercariae recovered from wild fish in An Giang province, Vietnam
Table 4.
|
Fish species examined |
District |
No. positive/No. examined |
% infected |
Parasite species |
|
Anabas testudineus
|
Chau Phu, Chau Doc |
9/35 |
25.7 |
Hp, Ps, Us-1b, Us-2c
|
|
Botia helodes
|
Chau Phu |
5/5 |
100 |
Eod
|
|
Channa striata
|
Chau Phu, Chau Thanh, Chau Doc, An Phu |
5/39 |
12.8 |
Ov, Hp, Ps, Us-1, Us-2 |
|
Cirrhinus jullieni
|
Chau Phu |
0/3 |
0 |
- |
|
Macrognathus siamensis
|
Chau Phu |
3/3 |
100 |
Eo |
|
Mastacembelus armatus
|
Chau Phu |
1/1 |
100 |
Eo |
|
Mystus albolineatus
|
Chau Phu |
5/5 |
100 |
Eo |
|
Oreochromis mossambicus
|
Chau Phu, Chau Thanh |
0/3 |
0 |
- |
|
Osteochilus hasselti
|
Chau Phu |
5/5 |
100 |
Eo |
|
Pristolepis fasciatus
|
Chau Phu |
0/2 |
0 |
- |
|
Rasbora trilineata
|
Chau Phu |
0/7 |
0 |
- |
|
Total |
|
33/108 |
30.6 |
Ov, Hp, Ps, Eo, Us-1, Us-2 |
Table 5.Metacercarial intensities in infected wild fish in An Giang province, Vietnam
Table 5.
|
Fish species |
Parasite species |
Total no. of metacercariae recovered |
No. of metacercariae/g fish infected tissue
|
|
Range |
Mean ± SD |
|
Anabas testudineus
|
Haplorchis pumilio
|
5 |
0.02-0.06 |
0.04 ± 0.03 |
|
Procerovum sp. |
22 |
0.15-0.29 |
0.23 ± 0.05 |
|
Unidentified species Us-1 |
538 |
0.06-5.33 |
1.70 ± 2.14 |
|
Unidentified species Us-2 |
3 |
0.09 |
0.09 ± 0.0 |
|
Botia helodes
|
Exorchis oviformis
|
80 |
4.12-6.50 |
5.07 ± 0.96 |
|
Channa striata
|
Opisthorchis viverrini
|
19 |
0.18-0.27 |
0.22 ± 0.06 |
|
Haplorchis pumilio
|
1 |
0.01 |
0.01 ± 0.0 |
|
Procerovum sp. |
1 |
0.01 |
0.01 ± 0.0 |
|
Unidentified species Us-1 |
14 |
0.03-0.11 |
0.07 ± 0.04 |
|
Unidentified species Us-2 |
2 |
0.04 |
0.04 ± 0.0 |
|
Macrognathus siamensis
|
Exorchis oviformis
|
70 |
1.82-2.39 |
2.19 ± 0.32 |
|
Mastacembelus armatus
|
Exorchis oviformis
|
35 |
5.96 |
5.96 ± 0.0 |
|
Mystus albolineatus
|
Exorchis oviformis
|
6 |
0.22-0.37 |
0.29 ± 0.06 |
|
Osteochilus hasselti
|
Exorchis oviformis
|
71 |
3.00-5.41 |
4.13 ± 1.10 |
|
Total |
|
867 |
0.03-6.50 |
2.29 ± 2.24 |