Abstract
Trypanosomiasis is caused by a pathogenic protozoan of the genus Trypanosoma, being Trypanosoma vivax the most important agent for cattle. The aim of the present study was to demonstrate the expansion of T. vivax infection in different mesoregions of Minas Gerais, Brazil, and describe the clinicopathological findings of trypanosomiasis in cattle. The diagnosis was based on visualization of the parasite in blood smears and DNA detection of T. vivax in the blood of live cows and tissues of necropsied animals by the polymerase chain reaction (PCR). Thirty suspected herds were tested, of which 11 were positive for T. vivax. The most frequent clinical signs were anemia, apathy, drop in milk production, weight loss, reproductive disorders, and nervous signs. Concomitant diseases, such as malignant edema, pneumonia and increased cases of mastitis were associated with T. vivax infection. Three cows were necropsied and the most significant findings were low body condition score, pale mucous and spleen with white pulp hyperplasia. The results demonstrated the expansion of T. vivax infection in Minas Gerais, that PCR-associated blood smears are promising for diagnosis, and that other diseases often occur concomitantly to T. vivax infection in regions with trypanosomiasis in cattle.
-
Key words: anemia, parasitic diseases, ruminants, trypanosomiasis
Trypanosomiasis is a disease caused by pathogenic protozoa of the genus
Trypanosoma, being
Trypanosoma vivax the most important etiological agent for cattle [
2]. This parasite is morphologically characterized by presenting a sickle shape, obtuse posterior extremity, undulating membrane, central large nucleus, terminal kinetoplast and free flagellum [
17]. It is widely distributed and economically important in African countries, especially in regions where its biological vector occurs, the tsetse fly [
6]. The adaptation to mechanical transmission by Tabanidae and
Stomoxys sp. allowed expanding
T. vivax to Central and South America [
2].
Trypanosomiasis has played an important role as a cause of acute anemia, weight loss, decreased milk production, and other clinical signs that may lead to the death of cattle in some Brazilian regions. Some risk factors contribute to the infection and transmission of the agent to cattle by vectors, such as the grouping of animals from different properties in resting places during long trips [
16] and increase in the population of horsefly (
Tabanus sp.) and stable flies (
Stomoxys calcitrans), predisposing to the occurrence of outbreaks [
2]. The aim of the present study was to demonstrate the expansion of
T. vivax infection in Minas Gerais, Brazil and describe the clinicopathological findings of trypanosomiasis in cattle.
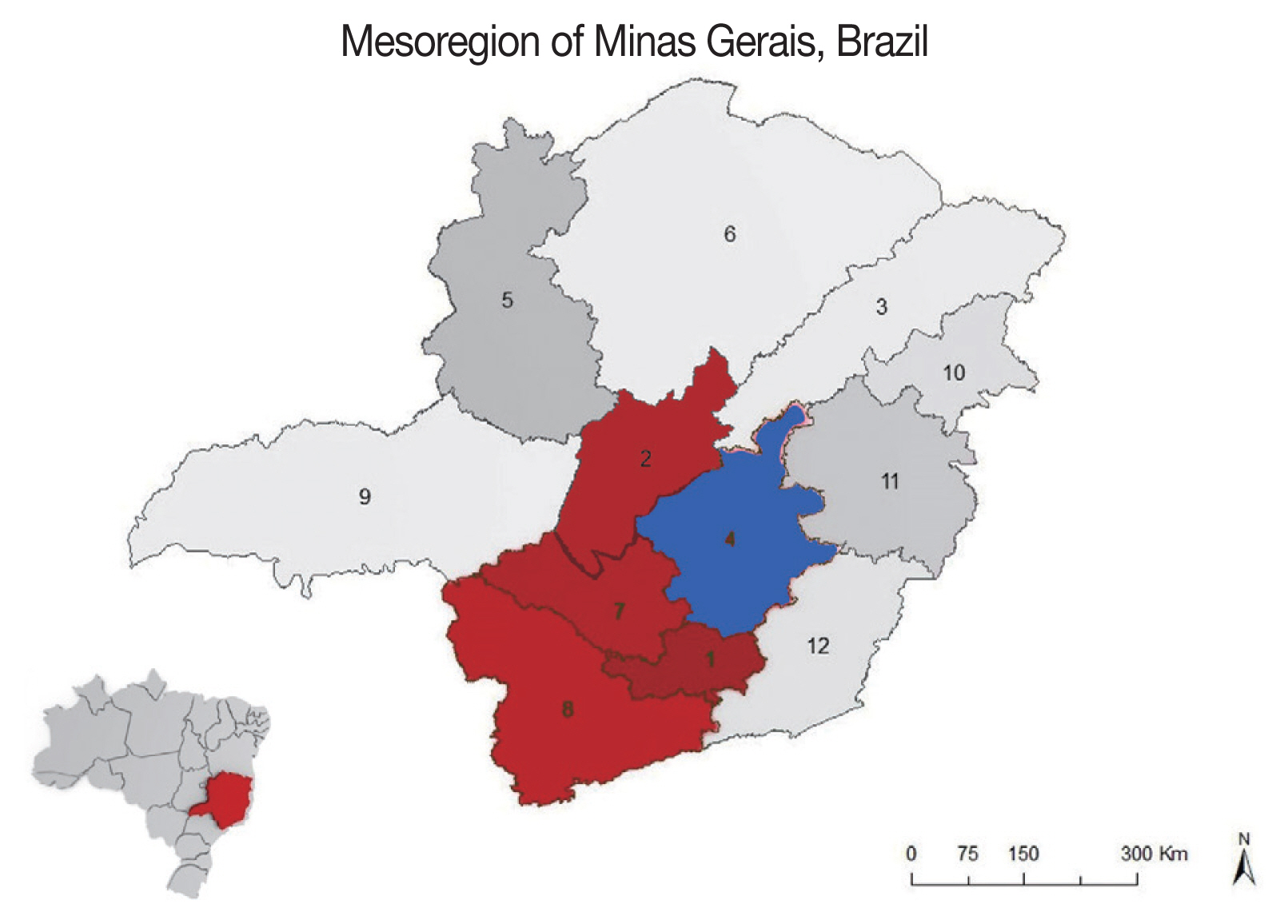
The epizootic of trypanosomiasis in cattle occurred from 2016 to 2017 in Minas Gerais in mesoregions located at latitude (17°45′36″ S to 22°46′10″ S) and longitude (47°16′59″ W 43°31′13″ W) (
Table 1;
Fig. 1). Thirty dairy farms with suspected trypanosomiasis were visited, and epidemiological and clinical data obtained from veterinarians and owners (
Tables 1,
2).
Blood samples from sick and healthy cattle were collected using a vacutainer tube with EDTA as anticoagulant, from the median caudal, jugular or superficial epigastric vein of 25 to 100% of the animals per dairy farm. Blood samples were checked for the presence of trypanosomes by blood smears and were stored in 1.5 ml microtubes at −20°C for molecular analysis. Three cows were necropsied (dairy farms A, H, and I) and fragments of central nervous system (CNS), heart, lung, lymph nodes, liver, spleen, kidney, intestines, uterus, forestomachs were fixed in 10% formaldehyde, paraffin embedded, cut to 3 μm and stained with hematoxylin and eosin (HE). Spleen, liver, kidney, CNS, heart, lung and blood were collected and stored at −20°C for molecular tests. Trypanocidal treatment (1 ml for 20 kg body weight intramuscularly, repeated 4 months after the first application), was recommended in all farms.
DNA from blood and tissues were extracted with a commercial kit (Blood & Tissue DNA Mini Kit, Meep Bioscience, Shenzhen, China) according to the manufacturer’s instructions. Detection of
T. vivax was performed using specific primers for the parasite, Tvi2 (forward: 5′ GCC ATC GCC AAG TAC CTC GCC GA 3′) and DTO156 (reverse: 5′ TTA GAA TTC CCA GGA GTT CTT GAT GAT CCA GTA 3′), which amplifies 177 base pairs (bp), as previously described [
5]. Blood samples of
T. vivax free-cattle were used as negative control and ultrapure Milli-Q water as blank control. The positive control was obtained from cows with a high parasitic load in blood smears and confirmed by DNA sequencing. Reactions were performed on a thermal cycler (Applied BiosystemsVeriti 96-Well Thermal Cycler, Applied Biosystems, Foster City, California, USA) according to the following protocol: denaturation at 94°C for 5 min; 35 cycles at 94°C for 1 min, annealing at 56°C for 1 min, extension at 72°C for 1 min and final extension at 72°C for 10 min. The PCR product was separated and the band size was identified with a standard molecular weight marker (Ladder, Sigma® 50 pb 500 μl). A new PCR was performed on 5 properties after trypanocidal treatment to test its effectiveness. Sequencing was performed by the enzymatic method [
11], using ABI 3730 equipment, with 50 cm capillary.
A total of 971 bovids were evaluated through blood smears in the 30 dairy farms of the studied area (
Fig. 1), from which 11 ones had cattle infected by the trypomastigote form compatible with
Trypanosoma vivax, totaling 178 infected animals. Epidemiological and clinical data are presented in
Tables 1,
2. Concomitant diseases, such as malignant edema (Farm H), pneumonia (Farm I) and increased cases of mastitis (Farms A, D, and I) were observed in affected animals.
The necropsied animals (bovids 1, 2, and 3) showed poor body condition score, pale mucosa, spleen with increased volume and white pulp evidenced. All 3 were previously positive for Trypanosoma sp. at blood smears and PCR. Bovid 1 (Farm A) was euthanized due to poor prognosis and had also brown kidneys with whitish spots disseminated in the cortex; heart with discrete dilation of the right ventricle; and liver with evidenced lobular pattern. Microscopic examination showed moderate hyperplasia of lymphoid follicles in the spleen and lymph nodes, moderate multifocal lymphoplasmacytic interstitial nephritis, membranous glomerulonephritis, and intratubular proteinaceous material; moderate congestive hepatopathy, predominantly centrilobular, and lymphoplasmacytic periportal infiltrate. T. vivax DNA was identified in the liver, kidney, spleen, heart, lung, and CNS. Bovid 2 (Farm H) was euthanized due to gas gangrene/malignant edema secondary to the use of contaminated needles. At necropsy, the muscles of the sternal and abdominal regions were grayish, interspersed with yellowish areas, swollen with gas bubbles and fetid odor. The microscopic findings included diffuse hepatocellular vacuolation; edema and histiocytic infiltrate in lymph nodes; moderate lymphoplasmacytic interstitial nephritis, besides lesions of gas gangrene/malignant edema. PCR positive results were found in the liver, kidney and lung. The necropsy of bovid 3 (Farm I) revealed dilation of heart ventricles and cranioventral consolidation of the lungs reaching approximately 50% of the right lung and 80% of the left, in addition to bronchiectasis with abundant mucopurulent exudate, which extended to the trachea. At microscopy, were observed splenic congestion, discrete lymphocytic interstitial nephritis, and mild multifocal lymphoplasmacytic myocarditis. The rostral colliculi showed discrete multifocal gliosis; obex, cerebellum and telencephalon discrete multifocal lymphoplasmacytic perivascular cuffs, especially in meningeal vessels; and there were atrophy of centrilobular hepatocytes, moderate periportal fibrosis with lymphoplasmacytic infiltrate; and chronic suppurative bronchopneumonia with bronchiectasis. The CNS was subjected to the IHC anti-rabies virus technique, since rabies is frequent in the region, however, resulted negative. The PCR was negative to T. vivax in tissues samples probably due to DNA degradation. No Trypanosoma sp. was seen in histopathologic exam of the 3 cows.
The results of the blood samples analyzed by PCR and smears are demonstrated in
Table 1. The sequenced blood and liver samples showed 100 and 99% identity with
T. vivax. Treatment with isometamidium chloride was done in all properties with
T. vivax positive bovids. The PCR resulted negative for
T. vivax in the blood of cows tested 6 months after the first treatment and 2 months after the second treatment in 5 of the farms.
The diagnosis of trypanosomiasis was based on epidemiological, clinical and pathological findings associated with protozoa compatible with
T. vivax in blood smears, as well as PCR positive for
T. vivax. The first report of the infection in Brazil occurred in buffaloes in the state of Pará in 1972 [
12]. Afterwards, trypanosomiasis cases by
T. vivax were diagnosed in cattle in the states of Amapá [
13], Mato Grosso [
14], Mato Grosso do Sul [
10], Tocantins [
7], Paraíba [
2], Rio Grande do Sul [
15], São Paulo [
3], and Maranhão [
8]. In Minas Gerais, the first diagnosis occurred in the region of Igarapé, in the metropolitan mesoregion of Belo Horizonte (mesoregion 4;
Fig. 1) [
4]. Generally, the reports describe few affected properties, being the disease considered rare in Brazil [
1]. However, in this study the disease was diagnosed in several mesoregions of the State, considered as free of infection up to the present, demonstrating its expansion in the state of Minas Gerais.
No evidence that indicates the possible origin of this epizootic was found in the studied mesoregions, but some owners report purchasing of animals, being probable that animals with subclinical infection have been introduced in some properties, spreading to other areas. In contrast, the disease expanded within the properties by the use of shared needles, especially by the application of oxytocin to aid in the ejection of milk from zebu cows. In the property that did not use oxytocin, the suspicion was that flies were transported alongside trucks that make the property-property transportation of milk (
Table 1).
In all positive properties of this study, clinical signs associated with cattle death were observed. The most frequent clinical signs were apathy, anorexia, anemia, weight loss, abrupt drop in milk production, reproductive disorders and nervous signs (
Table 2). These signs were also described by other authors [
1,
9]. Neurological changes, such as blindness and ataxia, were also reported, as well as muscle tremors, fasciculations, opisthotonus and strabismus [
1,
16]. Reproductive disorders are associated to transplacental passage of the agent [
9,
16], fetuses and placental histological lesions [
16].
In this study, it was not possible to assess fetuses or placenta, but it should be considered that abortions are associated with poor body condition of T. vivax infected cows, which were suffering from severe weight loss and anemia.
In the necropsied animals, either alone or in combination, spleen enlargement due to lymphoid hyperplasia, lymphoplasmacytic interstitial nephritis, meningitis or meningoencephalitis were observed, with lymphocytes, plasma cells and macrophages infiltrates, as previously described in cases of trypanosomiasis [
1]. However, these lesions are non-specific ones, becoming necessary to use parasitological or molecular diagnostic techniques.
Concomitant diseases were also observed, as increasing cases of mastitis in the period, one case of gas gangrene/malignant edema, and one case of pneumonia. Animals with severe anemia, as occur in trypanosomiasis, are more susceptible to secondary infection by bacteria and viruses as there is competition among precursor cells for erythroid and granulocyte differentiation [
18].
The results demonstrated an expansion of T. vivax infection in the State of Minas Gerais, with significant economic losses. The blood smear associated with the PCR technique was promising for diagnostic confirmation. The occurrence of concomitant diseases should not be excluding the investigation of T. vivax infection in regions with trypanosomiasis.
Notes
-
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest with respect to the article, authors, and publication.
ACKNOWLEDGMENTS
The authors thank all veterinarians and dairy farmers who contributed to this study, the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for its financial support (Process n˚ CVZ PPM 00763/16). To Myleus Biotechnology Company that sequenced samples, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the MSc grant and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Iniciação Científica grant.
Fig. 1Mesoregions of the State of Minas Gerais, Brazil. Map showing the first diagnosis of T. vivax occurrence in cattle (mesoregion 4-in blue) and the disease expansion for the other mesoregions (1, 2, 7 and 8 - in red; Latitude: 17°45′36″ S to 22° 46′10″ S and longitude: 47°16′59″ W to 43°31′13″ W). Brazilian map at the bottom left highlighting the state of Minas Gerais.
Table 1Trypanosomiasis in cattle from the State of Minas Gerais, Brazil: Findings regarding epidemiological data, diagnosis and treatment
Table 1
|
Dairy farm |
State Mesoregion |
Breed |
Total herd |
Smears (P) |
PCR (blood) |
Deaths |
Predisposing factors |
Treatment |
|
A |
8 |
Holstein |
350 |
139 (5) |
P |
1 |
Infestation of flies |
Yes |
|
B |
8 |
Girolando |
100 |
30 (10) |
P |
30 |
Shared needles |
NI |
|
C |
8 |
Holstein |
200 |
50 (20) |
P |
70 |
Shared needles |
Yes |
|
D |
8 |
Girolando |
120 |
34 (17) |
P |
50 |
Shared needles |
Yes |
|
E |
8 |
Girolando |
900 |
230 (43) |
P |
5 |
Shared needles |
Yes |
|
F |
8 |
Girolando |
300 |
75 (24) |
P |
15 |
Shared needles |
Yes |
|
G |
8 |
Girolando |
160 |
42 (14) |
P |
2 |
Shared needles |
Yes |
|
H |
1 |
Holstein |
124 |
102 (28) |
P |
1 |
Shared needles |
Yes |
|
I |
7 |
Holstein |
30 |
21 (9) |
P |
5 |
Shared needles |
Yes |
|
J |
1 |
Holstein |
NI |
4 (3) |
P |
26 |
Shared needles |
Yes |
|
K |
2 |
Girolando |
30 |
9 (5) |
NR |
4 |
Shared needles |
NI |
Table 2Trypanosomiasis in cattle from the State of Minas Gerais, Brazil: Presented clinical signs
Table 2
|
Clinical signs/Dairy farms |
A |
B |
C |
D |
E |
F |
G |
M |
I |
J |
K |
|
Acute anemia |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
|
Apathy/Anorexia/Weight loss |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
|
Severe drop in milk production |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
|
Submandibular edema |
|
|
|
|
|
|
|
x |
x |
|
x |
|
Positive jugular pulse |
|
x |
|
|
|
|
|
|
|
|
|
|
Compulsive soil ingestion |
|
|
x |
x |
|
|
x |
|
|
|
|
|
Preterm calving |
x |
|
|
|
|
|
|
|
|
|
|
|
Abortions (any gestational phase) |
|
x |
|
|
x |
|
x |
|
x |
|
|
|
Fertility drop |
|
|
|
x |
|
|
x |
x |
|
|
|
|
Difficulty of locomotion/weakness |
|
|
x |
|
|
|
|
|
|
|
|
|
Blindness |
|
|
x |
|
|
|
x |
|
x |
|
|
|
Agitated/attacking people |
|
|
|
|
|
x |
|
|
|
|
|
|
Hypermetric |
|
|
|
|
|
|
|
|
x |
|
|
|
Go around in circles |
|
|
|
x |
|
|
|
|
|
|
|
References
- 1. Batista JS, Riet-Correa F, Teixeira MM, Madruga CR, Simões SD, Maia TF. Trypanosomiasis by Trypanosoma vivax in cattle in the Brazilian semiarid: description of an outbreak and lesions in the nervous system. Vet Parasitol 2007;143:174-181.
- 2. Batista JS, Bezerra FSB, Lira RA, Carvalho JRG, Rosado Neto AM, Petri AA, Teixeira MMG. Aspectos clínicos, epidemiológicos e patológicos da infecção natural em bovinos por Trypanosoma vivax na Paraíba. Pesq Vet Bras 2008;28:63-69. (in Portuguese).
- 3. Cadioli FA, Barnabé PA, Machado RZ, Teixeira MC, André MR, Sampaio PH, Fidélis OL Junior, Teixeira MM, Marques LC. First report of Trypanosoma vivax outbreak in dairy cattle in São Paulo State, Brazil. Rev Bras Parasitol Vet 2012;21:118-124.
- 4. Carvalho AU, Abrão DC, Facury Filho EJ, Paes PRO, Ribeiro MFB. Ocorrência de Trypanosoma vivax no estado de Minas Gerais. Arq Bras Med Vet Zootec 2008;60:769-771. (in Portuguese).
- 5. Cortez AP, Rodrigues AC, Garcia HA, Neves L, Batista JS, Bengaly Z, Paiva F, Teixeira MM. Cathepsin L-like genes of Trypanosoma vivax from Africa and South America--characterization, relationships and diagnostic implications. Mol Cell Probes 2009;23:44-51.
- 6. Gardiner PR, Assoku RK, Whitelaw DD, Murray M. Haemorrhagic lesions resulting from Trypanosoma vivax infection in Ayshire cattle. Vet Parasitol 1989;31:187-197.
- 7. Linhares GFC, Dias Filho FC, Fernandes PR, Duarte SC. Tripanossomíase em bovinos no município de Formoso do Araguaia, Tocantins: relato de caso. Ci Anim Bras 2006;7:455-460. (in Portuguese).
- 8. Melo SA, Barros AC, Costa FB, de Carvalho Neta AV, de Candanedo Guerra Rde M, Abreu-Silva AL. Bovine trypanosomiasis an emerging disease in Maranhão State-Brazil. Vector Borne Zoonotic Dis 2011;11:853-856.
- 9. Meléndez RD, Forlano M, Figueira W. Perinatal infection with Trypanosoma vivax in calf in Venezuela. J Parasitol 1993;79:293-294.
- 10. Paiva F, Lemos RD, Oshiro ET, Salvador SC, Nakazato L. Ocorrência de Trypanosoma vivax em bovinos no estado do Mato Grosso do Sul. Rev Bras Parasitol Vet 1997;6:349. (in Portuguese).
- 11. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 1977;74:5463-5467.
- 12. Shaw JJ, Lainson R.
Trypanosoma vivax in Brazil. Ann Trop Med Parasitol 1972;66:25-32.
- 13. Serra-Freire NM. Oiapoque-outro foco de Trypanosoma vivax no Brasil. Rev Bras Med Vet 1981;4:30-31. (in Portuguese).
- 14. Silva RA, Silva JA, Schneider RC, Freitas J, Mesquita D, Mesquita T, Ramirez L, Rivera Dávila AM, Pereira ME. Outbreak of trypanosomiasis due to Trypanosoma vivax (Ziemann, 1905) in bovine of Pantanal, Brazil. Mem Inst Oswaldo Cruz 1996;91:561-562.
- 15. Silva ASD, Costa MM, Polenz MF, Polenz CH, Teixeira MMG, Lopes STA, Monteiro SG. Primeiro registro de Trypanosoma vivax em bovinos no Estado do Rio Grande do Sul, Brasil. Cienc Rural 2009;39:2550-2554. (in Portuguese).
- 16. Silva TM, Olinda RG, Rodrigues CM, Câmara AC, Lopes FC, Coelho WA, Ribeiro MF, Freitas CI, Teixeira MM, Batista JS. Pathogenesis of reproductive failure induced by Trypanosoma vivax in experimentally infected pregnant ewes. Vet Res 2013;44:1.
- 17. Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. 7th ed. London, UK. Bailliére Tindall; 1982, p 809.
- 18. Valli VEO, Kiupel M, Bienzle D. Hematopoietic system. In Maxie MG ed, Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. St. Louis, USA. Saunders Elsevier; 2016, pp 103-268.