Abstract
This study describes the isolation of a Toxocara canis species-specific excretory-secretory (ES) antigen and the development of an enzyme-linked immunosorbent assay (ELISA) based on this antigen. Analysis of the ES antigens of T. canis, Toxocara vitulorum, Ascaris lumbricoides and Necator americanus larval antigen was performed by SDS-PAGE followed by western blotting. A 57 kDa T. canis-specific antibody fraction (TcES-57) was identified by western blotting and labelling with anti-Toxocara antibodies (from experimental rabbits and human patients) and tracing with anti-human or anti-rabbit peroxidase conjugate. No protein fraction of 57 kDa was detected in ES or larval antigens collected from T. canis, T. vitulorum, A. lumbricoides and N. americanus. Using TcES-57, a specific anti-serum was produced in rabbits and a double sandwich ELISA was developed. This test was validated using known seropositive sera from toxocariasis patients, sera from A. lumbricoides or N. americanus patients, and 50 serum samples from cats. These tests revealed that TcES-57 antigen is specific to T. canis infection and does not cross react with sera of other related infections. Thus, ELISA based on TcES-57 antigen was proven to be an effective tool in the diagnosis of toxocariasis and studies on the role of T. canis in the epidemiology of human toxocariasis.
-
Key words: Toxocara canis, visceral larva migrans, ELISA, excretory-secretory protein, diagnosis, 57 kDa, Sri Lanka
INTRODUCTION
Toxocara canis, a common ascaridoid parasite of dogs, was considered by Beaver et al. (
1952) to cause visceral larva migrans syndrome (VLM) in humans. Because of the occult nature of VLM, serology is the only realistic means of diagnosis. The most widely used assays detect antibodies to parasite antigens released in vitro, i.e., the so-called excretory-secretory (ES) antigens obtained in
T. canis larval cultures (TcES). This is an excellent source of antigen as the ES production by each larva in culture (based on protein) has been estimated at 200 pg per day (
Meghji and Maizels, 1986;
Rajapakse et al., 1992). An ELISA based on this antigen (TcES-ELISA) has proved to be the most sensitive and specific immunodiagnostic tool for toxocariasis and is the assay used extensively to date (
de Savigny, 1975;
de Savigny et al, 1979;
van Knapen et al., 1983;
Radman et al., 2000). Glickman et al. (
1978) reported 78% sensitivity and 92% specificity for this test, while Speiser and Gottstein (
1984) showed a higher sensitivity and specificity of 80% and 93%, respectively. Jacquier et al. (
1991) reported a high level of sensitivity (91%), but the specificity recorded in their study was less, being 86%. Assays using TcES, however, cannot distinguish between infections due to different species of
Toxocara, i.e.,
T. canis,
T. cati, and
T. vitulorum (
Page et al., 1991). Although extensive cross reactions have been demonstrated between
T. canis and
T. cati using SDS-PAGE with a radioimmunoprecipitation assay using TcES antigen, Kennedy et al. (
1987) produced a monoclonal antibody (Ten 2), which demonstrated specificity for
Toxocara. Although Ten 2 was shown not to bind to ES antigens of
T. cati or other closely related parasites, cross reactions have been reported using Ten 2 in capture ELISA (
Gillespie et al., 1993). Furthermore, extensive cross reactions of antibodies to ES antigens of
T. canis and
T. vitulorum were demonstrated in gel diffusion (
Rajapakse et al., 1994) and in western blot following SDS-PAGE (
Starke-Buzetti and Ferreira, 2004). Cross reactions have also been reported with other helminth infections (
Jacquier et al., 1991;
Gillespie et al., 1993;
Lynch et al., 1988). In a recent study using a purified recombinant antigen based on
T. canis 38 kDa ES protein, cross reactions with anisakiasis (a fish nematode infection) sera were excluded only with pre-absorption of sera with the
Anisakis antigen (
Yamasaki et al., 1998).
The existence of non-specific reactions justifies continued investigation to identify antigens lacking crossreacting epitopes. This is especially applicable to tropical countries, such as Sri Lanka, where intestinal parasitoses are common and more than one species of nematode infection in each person. Although
T. canis is the most important of the
Toxocara species causing visceral larva migrans in humans (
Glickman and Schantz, 1981), the role of different species of
Toxocara in the etiology of toxocariasis needs definition. Such information is vital for the proper understanding of the epidemiology of toxocariasis and the implementation of correct preventive measures. Therefore, this study was carried out to isolate and identify a
T. canis ES species-specific antigen of diagnostic value and to develop an ELISA based on this antigen for the diagnosis of human toxocariasis.
MATERIALS AND METHODS
Preparation of antigens
Excretory-secretory antigens of
T. canis (TcES),
T. vitulorum (TvES),
Ascaris lumbricoides (AlES) were prepared by the method described in Rajapakse et al. (
1992).
Necator americanus larval antigen (NaL) was prepared using the Harada-Mori culture technique (
Ash and Orihel, 1987). The protein content of all antigen preparations was estimated by the Bicinchoninic acid protein assay kit (Sigma Chemicals, UK).
One dimensional polyacrylamide gel electrophoresis (PAGE) was carried out using Mini-PROTEAN II Electrophoresis Cells (Bio-Rad, USA). Briefly, 10% polyacrylamide gel with an acrylamide/bis ratio of 36.5 : 1 in the presence of 10% sodium dodecyl sulphate (SDS) supplemented with Temed (Sigma, T-9281) and ammonium persulfate in Tris-HCl buffer pH 8.8, according to Laemmli (
1970). Molecular weight standard mixtures (MW 15,000-150,000, Sigma M-0671) were used for calibrating the gel. The antigen was diluted in a Tris (pH 6.8) sample buffer (0.1M Tris-HCl, 2% SDS, 10% glycerol, 0.2 M 2-mercaptoethanol and 0.1% bromophenol blue) and then loaded in the gel with 20 µg protein/lane. The electrophoresis was monitored using 0.1% bromophenol blue and the current was set at 30 mA. The protein fractions were visualized by staining with 0.10% Coomassie Brilliant Blue R 250 (Sigma, B-0149).
The relative molecular weights were calculated using prestained protein of standard molecular weight according to the relative electrophoretical mobility (RM), using the following equation:
The RM values (ordinate) were related to known molecular weights of the standard proteins (abscissa) in a semi-logarithmic graph to provide a basis for interpolation of the data from proteins of the Pe-antigen.
Western blot
Gels with parasite antigen were electrophoretically transferred onto nitrocellulose sheets (0.22 µm) for immunoblotting according to the procedure described by Towbin et al. (
1979). The transfer was performed in a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad apparatus) for 12 h in a constant current of 35 V in a transfer buffer (Tris-glycine-methanol). The nitrocellulose papers were blocked by a blocking solution with 5% non-fat dried milk in TBS-Tween (0.01 M Tris, 0.15 M NaCl, 0.05% Tween-20) and then incubated for 90 min with primary antibodies (pool of 10 serum samples from buffalo cows and calves) diluted to 1/50 in the blocking solution and 5% normal rabbit serum in a rotating homogenizer. Subsequently, the nitrocellulose was washed 3 times (15 min each) in TBS-Tween and milk solution. Specifically bound antibodies in all filters were detected with anti-bovine alkaline phosphatase conjugate (Sigma, A-7914) diluted 1 : 30,000 in the blocking solution for 90 min. After rinsing 3 times in TBS-Tween and milk solution, the blots were incubated at room temperature for about 10 min in enzymatic substrate (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium B).
Anti-TcES rabbit serum was prepared against TcES. Two New Zealand white rabbits (3-4 months old) were used. Antigen was adjusted to a protein concentration of 2 mg/ml in PBS and was emulsified with an equal volume of Freund's incomplete adjuvant (Sigma Chemicals, USA). Each rabbit was inoculated on the axillary region of all 4 limbs intramuscularly (0.25 ml at each site) at weekly intervals for 4 weeks. Before immunization 3 ml of blood was collected from each rabbit from the ear-marginal vein. Rabbits were bled weekly before every subsequent injection for serum collection. All serum samples were stored at -20℃ until use.
Human serum samples
Test sera came from 20 patients with a history suggestive of visceral toxocariasis and positive on the T. canis L2 larval ES antigen ELISA (TES-ELISA). Sera from 20 healthy subjects that were negative on TES-ELISA were used as negative controls.
Cat serum samples
Sera were collected from 50 cats at animal clinics, where minimum attention had been given to anthelmintic medication by their owners. Samples were collected from the cephalic vein and, after separation, stored at -20℃ until used.
Isolation of T. canis species-specific protein band (TcES-57) on SDS-PAGE
All antigens, namely TcES, TvES, AlES and NaL, were subjected to SDS-PAGE in a gel containing a molecular weight marker, followed by western blot against anti-TcES rabbit serum, as well as TES-ELISA positive human serum with appropriate conjugate in order to identify the T. canis species-specific antigen fraction we named TcES-57. All SDS-PAGE and western blots were repeated at least 3 times.
Preparation of anti-TcES-57 rabbit serum
Two New Zealand white rabbits (3-4 months old) were used. The gel strip containing the 57 kDa band was crushed in PBS and mixed with an equal volume of Freund's incomplete adjuvant. Each rabbit was inoculated on the axillary region of all 4 limbs intramuscularly with 0.25 ml of the mixture at each site, at weekly intervals for 4 weeks. Pre-immunization blood was collected from the marginal ear vain, sera separated and stored at -20℃. Four 4 weeks after rabbits were bled (4 ml), serum was separated and stored at -20℃. Thereafter, the rabbits were given booster immunizations weekly for one month and were bled weekly, serum collected and stored at -20℃.
Double sandwich ELISA using TcES-57
The following human serum samples were used in this assay: (a) 50 TES-ELISA positives, (b) 20 TES-ELISA negatives, (c) sera from 6 parasitologically proven (eggs in feces)
A. lumbricoides infected patients, (d) sera from 3 parasitologically proven (eggs in feces) hookworm infected patients. In addition, sera from 50 cats were used. The assay was optimized by checkerboard titration. The microtitration plate wells were coated with anti-TcES-57 rabbit serum diluted 1/800 in carbonate buffer, pH 9.6. A hundred µl of antigen was added to each well and plates were incubated at 4℃ overnight. Following this plates were washed 3 times in phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBS-Tween) pH 7.4. After the last wash, plates were inverted and blotted onto absorbent paper to empty the wells completely. The wells were post coated with 120 µl of PBS containing 1% bovine serum albumin and 2.5% sucrose and incubated at room temperature for 1 hr. A hundred µl of TcES antigen (diluted to 10 µg/ml of protein in PBS) was placed in each well and plates incubated for 1 hr at room temperature (RT). Plates were then washed 3 times in washing buffer and emptied as before. A hundred µl of diluted serum samples were added to the test wells in duplicate and incubated for 1 hr at RT. Following this the washing step was repeated to remove unbound serum. To assay specifically bound antibodies, wells were incubated for 1 hr at RT with 100 µl of peroxidase-conjugated either anti-human or anti-cat IgG as appropriate, diluted 1 : 250, and washed as before. Then, 100 µl of enzyme substrate o-phenylenediamine dihydrochloride and 3% hydrogen peroxide solution (Sigma Aldrich Corporation, India) was added. The enzyme hydrolysis of substrate was monitored. After an appropriate interval, as determined by the rate of reaction of a reference standard serum, hydrolysis was stopped by addition of 100 µl of 3 M H
2SO
4. Optical density (OD; ELISA values) was determined photometrically at 490 nm in an ELISA reader (Minireader 11, Dynatech Laboratories Inc.). Interpretation of ELISA results was based on OD values determined for the local population as described by Iddawela et al. (
2003) as follows: < 0.2, no serological evidence of toxocariasis; 0.2-0.7, compatible with past infection or current light infection; > 0.7, compatible with recent infection.
RESULTS
The banding pattern obtained with all 4 antigens on SDS-PAGE and stained with Coomassie blue is shown in
Fig. 1. A minimum of 11 bands, ranging between 28 to 260 kDa, was revealed in TcES. The 57 kDa band was one of the most prominent (
Fig. 1).
The western blot using anti-TcES rabbit serum is shown in
Fig. 2. When the nitrocellulose paper bound proteins were probed with anti-TcES rabbit sera, bands were seen in TcES, TvES, AlES and NaL lanes. Eleven bands were detected in the TcES lane. Of these, 5 bands were darkly stained and the others were lightly stained. The band at 57 kDa was prominent. In the TvES lane, 5 bands were detected with a prominent band at 28 kDa. In the AlES lane, one clear band at 21 kDa was detected, and in the NaL lane, one lightly stained band was detected at 66 kDa. Western blot of TcES, TvES, AlES and NaL antigens using serum of patients with clinical toxocariasis, who were seropositive on TES-ELISA, are shown in
Fig. 2. In the TcES antigen lane, the bands were very similar to that of anti-TcES rabbit serum except that a band at 28 kDa was not seen and a band at 80 kDa was darkly stained. In the AlES lane, 2 bands were seen, one at 65 kDa that was darkly stained and the other at 21 kDa. In TvES lane, 5 bands were detected with human serum and 4 of the bands were very similar to that detected with anti-TcES rabbit serum, i.e., 48 kDa, 43 kDa, 32 kDa and 28 kDa. With human serum, a band at 80 kDa was detected, while with the rabbit serum, a 73 kDa band was seen. In the NaL antigen lane, 2 bands were seen, one prominent at 54 kDa and the other band at 66 kDa. The banding pattern was similar in high and low ELISA positive serum samples except that, in high positive serum samples, the bands were more darkly stained. No bands were seen on nitrocellulose paper with the control sera (pre-immunized rabbit serum and TcES-ELISA negative serum samples, conjugate and substrate control).
A single band at 57 kDa was seen in the TcES lane while no bands were detectable in the other lanes (
Fig. 3).
Of the 50 TES-ELISA positive sera tested using the antigen TcES-57 on the double sandwich ELISA, 45 (90%) were positive. The 20 TES-ELISA negative sera remained negative on the TcES-57 double sandwich ELISA as well. Sera from patients infected with A. lumbricoides and N. americanus were negative with the TcES-57 double sandwich ELISA. None of serum samples from cats were positive in sandwich ELISA with TcES-57 captive primary serum. However, when TcES captive primary serum was used, 11 serum samples were positive in ELISA.
DISCUSSION
Of the
Toxocara spp. infecting humans,
T. canis and
T. cati, parasites of canines and felines, respectively, are the most important in the domestic environment. A further species,
T. vitulorum, is commonly found in the small intestine of buffaloes.
T. vitulorum is a highly fecund worm producing a large number of eggs, which frequently contaminate the environment, but its effect on VLM is not investigated yet. However, VLM is worldwide and high prevalence is reported in children (
Gillespie et al., 1993).
As humans are not natural hosts, the parasite fails to develop to maturity and is arrested in the larval stage. Diagnosis of toxocariasis is currently based on serology to detect antibodies. A widely used assay is ELISA test based on ES antigens of 2nd stage larvae derived from culture. However, cross reaction with ES antigens of other related species within the genus has been reported. SDS-PAGE of
T. canis ES has produced variable results between laboratories. Sugane and Oshima (
1983) described a single band at 35 kDa, while Maizels et al. (
1984) demonstrated 5 major components (ES labelled with radio-iodination) at 32, 55, 70, 120 and 400 kDa. Meghji and Maizels (
1986), carrying out extensive molecular and biochemical characterization of ES from long-term cultures, using labelled ES, concluded that there were a number of macromolecules secreted, of which the major components were glycoproteins that differed in essential characteristics, i.e., 32 kDa, 120 kDa and 400 kDa. Starke-Buzetti and Ferreira (
2004) reported 4 bands ranging between 29 kDa and 125 kDa, while in this study, the TcES separation on SDS-PAGE identified 11 bands ranging from 28 kDa to 280 kDa. The differences in the gel used for separation, namely gradient or monomer, could partly account for this, but the more important and critical factor is the age of the in vitro larval culture used for collection of ES antigen. Larvae are viable in these cultures for long periods, over 18 mo (
Rajapakse et al., 1992), and would continue to secrete antigenic material. With long-term culture, turnover of ES antigen is also likely (
Smith et al., 1981). SDS-PAGE of TvES antigen revealed a minimum of 7 bands detected at 80 kDa, 73 kDa, 61 kDa, 48 kDa, 43 kDa, 38 kDa, and 28 kDa. For each species of
Toxocara tested, the banding pattern obtained was very similar with shared bands at 28, 43, 48 and 80 kDa. However, the banding pattern was different with
Ascaris ES and larval antigen of
N. americanus. This confirms that sharing of protein in ES antigen is limited to the genus level.
With western blot, using rabbit antisera collected following oral infection of
T. canis eggs, Starke-Buzetti and Ferreira (
2004) showed extensive cross reactions between
T. canis and
T. vitulorum ES. In the western blot of the present study using both more specifically prepared anti-TcES rabbit sera and human toxocariasis sera, 5 common bands were identified. However, the 57 kDa band in the
T. canis ES antigen lane was unique to that species.
It could be suggested that cats giving a positive result using TcES had been exposed to
T. cati infection and antibodies of
T. cati may have shared cross reactivity with TcES (but not with TcES-57). If so, the specificity of the TcES-57 protein fraction has been demonstrated. Although in-depth investigations of ES antigens has demonstrated shared antigens with related ascaroids, the use of suitably diluted ES antigen of a particular species in ELISA would not yield cross reactions between related species. Furthermore, when carrying out ELISA, use of suitable cut-off titres is essential to limit non-specific reactions (
Maizels and Robertson, 1991).
Since the introduction of ELISA and use of ES antigens of
T. canis infective larvae (
de Savigny, 1975;
Maizels and Robertson, 1991), an acceptable immunodiagnostic test has become widely available. Estimation of sensitivity and specificity of ELISA for human toxocariasis is hampered by our inability to confirm a clinical diagnosis using alternative methods, such as tissue biopsy. In the evaluation of ELISA, cases of VLM as well as controls are selected based on the presence or absence of generally accepted clinical symptoms, signs and laboratory findings. However, using these criteria, it is difficult to ensure exclusion from the control group of persons who are infected, but are asymptomatic or have symptoms hitherto unrecognized as due to toxocariasis.
A recent clinical study in Sri Lanka has focused on ecchymoses as a clinical presentation in toxocariasis (
Wickremasinghe and Wijesundera, 2001). The significant association of several symptoms with seropositivity indicates the need to recognize covert toxocariasis as a disease entity in children. In our previous study on the seropositives for
T. canis species-specificity, a 91% were positive for
T. canis on the species-specific antigen test using TcES-57 double sandwich ELISA (
Iddawela et al., 2003). Thus, confirming
T. canis to be the most important etiological agent of toxocariasis in the area. However, 9% of the seropositives were not picked up by the test. Thus, the widely used TES-ELISA is not species-specific and gives positive results with other
Toxocara species, such as
T. canis of cats and
T. vitulorum of buffaloes or cattle. Therefore, in a country like Sri Lanka, where geohelminthic infections are common, the TcES-57 antigen would be more appropriate to avoid false positive reactions.
In conclusion, the work has clearly shown that isolation of species-specific antigens and development of ELISA is possible with the available technology. This could be extended to other species of Toxocara of local relevance, namely T. cati and T. vitulorum. TcES-57, a T. canis species-specific ES antigen of diagnostic value has been identified and an ELISA-based diagnostic test was developed. It is suggested that diagnostic tests based on this antigen will have a higher specificity in developing tropical countries like Sri Lanka and would be of value in differentiating and determining the role of T. canis in the epidemiology of human toxocariasis. TcES-57 is clearly a molecule of promise for use with other technologies, such as recombinant protein based diagnostic assays.
Notes
-
A financial support has been provided by the University of Peradeniya (Grant No: RG/98/39/PG/M).
ACKNOWLEDGMENTS
We wish to thank Prof. Dannis Jacobs, Royal Veterinary College, London, U.K. and Prof. S.N. Arseculeratne, Faculty of Medicine, University of Peradeniya, Sri Lanka, for their helpful comments on the manuscript.
References
Fig. 1Pattern of bands produced by antigens and molecular marker, separated on SDS-PAGE and stained with Coomassie blue. TcES, Toxocara canis ES antigen; AlES, Ascaris lumbricoides ES antigen; TvES, Toxocara vitulorum ES antigen; NaL, Necator americanus larval antigen. MW: molecular weight marker (high molecular weight range).
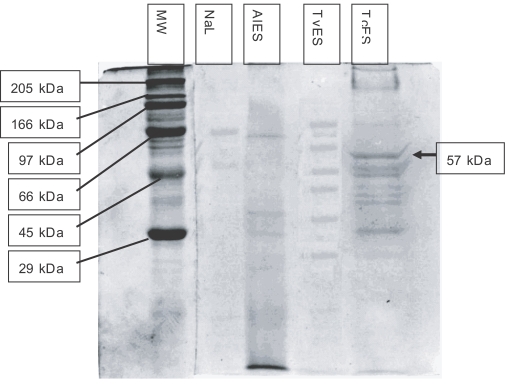
Fig. 2Western blot of antigens against anti TcES rabbit sera. TcES, T. canis ES antigen; AlES, A. lumbricoides ES antigen; TvES, T. vitulorum ES antigen; NaL, N. americanus larval antigen.
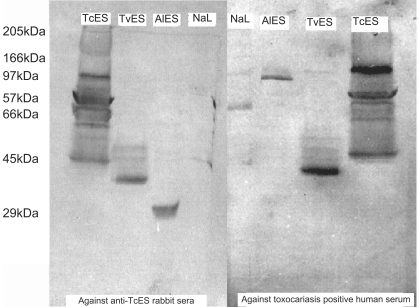
Fig. 3Western blot of antigens against toxocariasis positive human serum and anti TcES-57 rabbit serum. TcES, T. canis ES antigen; AlES, A. lumbricoides ES antigen; TvES, T. vitulorum ES antigen; NaL, N. americanus larval antigen.




