Abstract
Allergen extracts from dust mites and cockroaches commonly found in Korean homes were used to evaluate their enzymatic activity as they are believed to influence allergenicity. Allergen extracts were prepared from 3 dust mite species (Dermatophagoides farinae, D. pteronyssinus, and Tyrophagus putrescentiae) and 3 cockroach species (Blattella germanica, Periplaneta americana, and P. fuliginosa) maintained in the Korea National Arthropods of Medical Importance Resource Bank. Proteins were extracted in PBS after homogenization using liquid nitrogen. The activities of various enzymes were investigated using the API Zym system. No significant difference in phosphatase, lipase, or glycosidase activity was observed among the 6 allergen extracts, but much difference was observed in protease activity. Protease activity was assessed in more detail by gelatin zymography and the EnzChek assay. Extract from T. putrescentiae showed the highest protease activity, followed by those of the cockroach extracts. Extracts from D. farinae and D. pteronyssinus showed only weak protease activity. Gelatinolytic activity was detected mainly in a 30-kDa protein in D. farinae, a 28-kDa protein in D. pteronyssinus, a > 26-kDa protein in T. putrescentiae, a > 20-kDa protein in B. germanica, and a > 23-kDa protein in P. americana and P. fuliginosa. The information on various enzymatic activities obtained in this study may be useful for future studies. In particular, the strong protease activity found in cockroach extracts could contribute to sensitization to cockroach allergens, which is known to be associated with the development of asthma.
-
Key words: Dermatophagoides farinae, Dermatophagoides pteronyssinus, Tyrophagus putrescentiae, Blattella germanica, Periplaneta americana, Periplaneta fuliginosa, allergen, protease, cockroach, dust mite
INTRODUCTION
Allergen extracts from dust mites and cockroaches are commercially available for diagnostic and immunotherapeutic purposes. However, heterogenicity of allergen extracts has been reported in terms of protein concentration and major allergen content leading to the variable allergenicity among manufacturers [
1,
2]. The Korea National Arthropods of Medical Importance Resource Bank (AMIB), housed in the Institute of Tropical Medicine, Yonsei University College of Medicine, Seoul, Korea, was established to collect, rear, and provide investigators with access to arthropods that directly or indirectly affect human health. Allergic disorders are commonly elicited by arthropods [
3]. Allergen extracts prepared by the addition of protease inhibitors and 1-phenyl-3-(2-thiazolyl)-2-thiourea to prevent protein degradation and melanization were first described in 2003 [
4]. Since then, allergen extracts from mites and cockroaches prepared at our resource bank have been shown to have various effects giving rise to allergic responses. Cockroach extracts were shown to induce human eosinophils to release inflammatory mediators [
5] independent of serine protease activity. Furthermore, proteases from cockroach extracts were found to activate protease-activated receptor 2 (PAR-2) in human airway epithelial cells resulting in calcium oscillations [
6], induce IL-8 expression in human airway epithelial cells [
7], and delay barrier recovery and lamellar body secretions in murine skin [
8]. Extract from the house dust mite,
Dermatophagoides pteronyssinus, was shown to activate human monocytic THP-1 cells to express MCP-1, IL-6, and IL-8 independent of protease activity [
9]. Mite extract was also shown to influence epidermal permeability barrier homeostasis via activation of PAR-2 [
8].
Proteolytic activity is one of the major concerns when preparing allergen extracts. Proteases may degrade protein components, including major allergens, when preparing allergen extracts. However, a large body of evidence suggests that enzyme activity is directly associated with the allergenicity of allergen extracts [
10]. Recently, it was shown that addition of protease inhibitors to protease-rich cockroach extracts used for diagnosis and therapy increased the shelf life of the extracts [
11].
In this study, we evaluated the enzyme activities of allergen extracts prepared in the AMIB and have been provided to the Korean researchers from 3 dust mite species (Dermatophagoides farinae, D. pteronyssinus, and Tyrophagus putrescentiae) and 3 cockroach species (Blatella germanica, Periplaneta americana, and Periplaneta fuliginosa) that are commonly found in Korean homes.
MATERIALS AND METHODS
Extract preparation
Mites (
D. farinae,
D. pteronyssinus, and
T. putrescentiae) and cockroaches (
B. germanica,
P. americana, and
P. fuliginosa) were cultured in an insect rearing facility in the AMIB, Department of Environmental Medical Biology, Yonsei University College of Medicine, Seoul, Korea, as described previously [
12,
13]. Frozen mites and cockroaches (30 µg each) were pulverized in liquid nitrogen, and extracts were obtained by overnight incubation with PBS containing 6 mM 2-mercaptoethanol, protease inhibitor set III (1/1,000 volume; Calbiochem, San Diego, California, USA), and 1 mg/ml 1-phenyl-3-(2-thiazolyl)-2-thiourea (Sigma-Aldrich, St. Louis, Missouri, USA) at 4℃ after defatting with ethyl ether and ethyl acetate. Thereafter, the extracts were sieved through 2 pieces of cheesecloth and centrifuged, and the insoluble matter removed by using a syringe filter (0.22 µm, Millipore, Bedford, Massachusetts, USA). The extracts were aliquoted and stored at -70℃ until use.
The protein concentrations of the extracts were determined using the Bradford assay (Bio-rad, Hercules, California, USA). SDS-PAGE analysis was performed to investigate the protein profiles of the extracts. Samples were run on 10% gels under reducing conditions. Ten µg of each allergen extract was loaded on the gel and visualized by staining with Coomassie Brilliant Blue R250.
For the analysis of gelatinolytic protease activity, samples were run on 10% SDS-PAGE gels containing 0.1% gelatin. Five µg of mite extract or 1 µg of cockroach extract was loaded per well. Subsequently, the gels were incubated for 60 min in renaturing buffer (2.7% w/v Triton X-100) at room temperature and then in developing buffer (Invitrogen, Carlsbad, California, USA) overnight at 37℃. Gels were stained with Coomassie Brilliant Blue R250.
ApiZym assay
The activities of 19 different enzymes, including 5 proteases, 3 lipases, 3 phosphatases, and 8 glycosidae activities, were semiquantitatively estimated using the API Zym assay (bioMerieux, Marcy l'Etoile, France). Procedures and interpretations were followed to the manufacturer's instructions. Values ranged from - (no activity) to +++ (maximum enzymatic activity).
EnzChek assay
The protease activities of mite and cockroach extracts were compared using the EnzChek Protease Assay kit (Molecular Probes Inc., Eugene, Oregon, USA) according to the manufacturer's instructions. Tosyl-L-phenylalanine chloromethyl ketone-treated trypsin (TPCK-trypsin, Pierce, Rockford, Illinois, USA), a chymotrypsin activity-inhibited trypsin isolated from bovine pancreas, was used as a reference protease. Protease activity was measured using pH-insensitive green fluorescent BODIPY-FL-conjugated casein as a substrate in a total reaction volume of 200 µl. All enzymes and extracts were diluted in PBS (pH 7.4). After addition of 100 µl of substrate solution and 100 µl (up to 10 µg) of enzyme or extract into 96-well plates, the reaction mixtures were incubated for one hour at room temperature and protected from light. Fluorescence was read using a luminescence spectrometer (LS50B, PerkinElmer Inc., Waltham, Massachusetts, USA) at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
RESULTS
SDS-PAGE and gelatin zymogram analysis
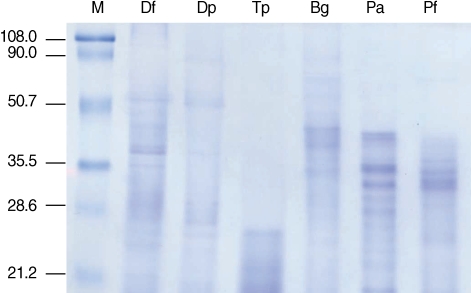
Several proteins in the molecular weight range of 20-100 kDa were detected from extracts of 2 (
D. farinae and
D. pteronyssinus) of the 3 mite species. In contrast, proteins smaller than 25 kDa were detected only in
T. putrescentiae extracts.
P. americana and
P. fuliginosa cockroach extracts had strongly stained protein bands in the 20-40 kDa range. A broader range of protein sizes and strongly stained protein bands in the 30-40 kDa range were visualized in
B. germanica extracts (
Fig. 1).
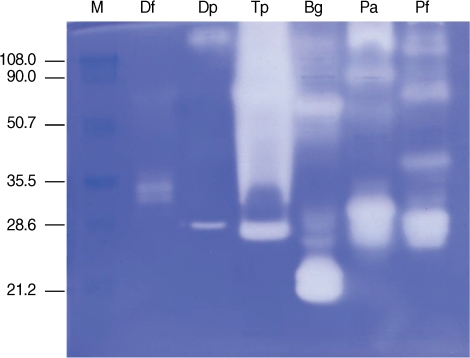
Gelatinolytic activity was detected primarily in proteins with molecular weights of 28-38 kDa in
D. farinae, 28 kDa in
D. pteronyssinus, 26 to > 100 kDa in
T. putrescentiae, 20-23 and 60 kDa in
B. germanica, and 26-33 kDa in
P. americana and
P. fuliginosa. Five µg of 2
Dermatophagoides spp. mite extracts produced a weak hollow in a gelatin zymogram. In contrast, 1 µg of cockroach extract produced multiple strong hollows, implying strong proteolytic activity.
T. putrescentiae extract showed strong gelatinolytic activity compared to extract of
Dermatophagoides spp. mites (
Fig. 2).
Enzymatic activities as measured by the ApiZym assay are summarized in
Table 1. The results are graded as negative (-), weakly positive (+), moderately positive (++), or strongly positive (+++). All 6 extracts showed similar degrees of phosphatase and glycosidase activities but differed from protease and lipase activities. For lipases, extracts of all 6 species showed weak or moderated degree of both esterase (C4) and esterase lipase activity. However,
T. putrescentiae and
B. germanica exhibited weak lipase (C14) activities. In case of proteases, overall,
T. putrescentiae extract showed the strongest protease activity, followed by the cockroach extracts. In particular, strong trypsin and α-chymotrypsin activities were detected in
T. putrescentiae extract, but extracts from
Dermatophagoides spp. mites showed no α-chymotrypsin activity and weak trypsin activity.
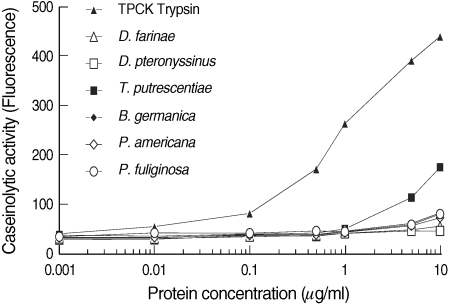
The caseinolytic activities of 10 µg of
D. farinae,
D. pteronyssinus,
T. putrescentiae,
B. germanica,
P. americana, and
P. fuliginosa extracts were equivalent to 0.048, 0.005, 0.574, 0.166, 0.124, and 0.157 µg of TPCK-trypsin, respectively, as measured by the EnzChek assay (
Fig. 3). Overall, caseinolytic activities assessed by the EnzChek assay were similar with the protease activities estimated by the ApiZym assay.
DISCUSSION
Proteolytic enzymes can contribute to the development of allergic disorders [
10], and elimination or reduction of protease activity preserving antigenicity of allergen extracts might be helpful when developing allergy vaccines [
14]. However, enzyme activity should be preserved when investigating allergic responses and may even provide new therapeutic targets. Furthermore, allergen extraction procedure can affect the quality of the extracts. The present study report the enzyme activity of the allergen extracts prepared from AMIB, which provide allergen extracts to Korean researchers.
Previous studies demonstrated that protease allergens from fungi, house dust mites, and cockroaches play an important role in driving allergic responses by providing an adjuvant effect to the protease allergen itself as well as to bystander allergens [
15-
18]. Mite and cockroach proteases in particular are known to activate p44/p42 MAP kinases in human airway epithelial cells, resulting in elevated production of IL-8 [
19].
Mite group 1, 3, 6, and 9 allergens and cockroach group 10 allergens are known to have protease activity, probably associated with a digestive function [
20,
21]. Allergen extracts with strong gelatinolytic activities have been reported to have strong allergenicities from dust mite and cockroach extracts [
22]. Furthermore, various protease activities were detected in
T. putreascentiae extracts, and serine protease inhibitors were shown to be able to retard mite development [
23].
Recently, mechanisms by which some non-proteolytic allergens, such as Der p 2 [
24,
25], Der p 5 [
26], and Per a 7 [
27], elicit allergic responses have been investigated. However, much more effort should be devoted to studies that identify the mechanisms by which protein allergens trigger allergic responses. Insight into the interactions between allergens and immune cells might provide novel targets for the treatment of allergic disorders.
In this study, we demonstrated various enzyme activities in cockroach and mite extracts using gelatin zymography (
Fig. 2), the ApiZym assay (
Table 1), and the EnzChek assay (
Fig. 3). There were no significant differences in phosphatase, lipase, or glycosidase activity among the 6 allergen extracts.
T. putrescentiae extract showed the highest protease activity, followed by those of the cockroach extracts, while
D. farinae and
D. pteronyssinus extracts only had weak protease activity. The strong protease activity of cockroach extracts may play an important role in allergies to cockroach antigens, which are associated with development of asthma. Different enzymatic activities can be detected based on the use of different extraction buffers and preparation procedures. Microbes in the guts of mites and cockroaches can also influence the enzymatic activities of extracts [
28,
29]). Protease-activated receptor 2 is of special interest in allergic inflammation induced by protease allergens [
30]. We anticipate that the enzymatic activities of mite and cockroach extracts reported in this study could be helpful for future studies, e.g., investigation of the effects of selected extracts on various immune cells.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090028).
References
- 1. Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy 2010;65:184-190.
- 2. Patterson ML, Slater JE. Characterization and comparison of commercially available German and American cockroach allergen extracts. Clin Exp Allergy 2002;32:721-727.
- 3. Jeong KY, Hong CS, Yong TS. Domestic arthropods and their allergens. Protein Pept Lett 2007;14:934-942.
- 4. Jeong KY, Lee J, Lee IY, Ree HI, Hong CS, Yong TS. Allergenicity of recombinant Bla g 7, German cockroach tropomyosin. Allergy 2003;58:1059-1063.
- 5. Sohn MH, Lee YA, Jeong KY, Sim S, Kim KE, Yong TS, Shin MH. German cockroach extract induces activation of human eosinophils to release cytotoxic inflammatory mediators. Int Arch Allergy Immunol 2004;134:141-149.
- 6. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, Shin DM. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol 2004;113:315-319.
- 7. Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH. Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp Allergy 2007;37:1364-1373.
- 8. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol 2008;128:1930-1939.
- 9. Lee JS, Kim IS, Ryu JS, Yun CY. House dust mite, Dermatophagoides pteronyssinus increases expression of MCP-1, IL-6, and IL-8 in human monocytic THP-1 cells. Cytokine 2008;42:365-371.
- 10. Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep 2007;7:363-367.
- 11. Sudha VT, Srivastava D, Arora N, Gaur SN, Singh BP. Stability of protease-rich Periplaneta americana allergen extract during storage: formulating preservatives to enhance shelf life. J Clin Immunol 2007;27:294-301.
- 12. Ree HI, Lee IY, Kim TE, Jeon SH, Hong CS. Mass culture of house dust mites, Dermatophagoides farinae and D. pteronyssinus (Acari: Pyroglyphidae). Med Entomol Zool 1997;48:109-116.
- 13. Ree HI, Lee IY. Development of mass rearing technique of Tyrophagus putrescentiae (Acari: Acaridae) found in house dust. Korean J Parasitol 1997;35:149-154.
- 14. Fernandez-Caldas E, Gallego M, Carnes J, Iraola V. Enzymatic activity of Dermatophagoides pteronyssinus extracts after acidic treatment. Int Arch Allergy Immunol 2008;145:298-304.
- 15. Clarke AH, Thomas WR, Rolland JM, Dow C, O'Brien RM. Murine allergic respiratory responses to the major house dust mite allergen Der p 1. Int Arch Allergy Immunol 1999;120:126-134.
- 16. Kurup VP, Xia JQ, Crameri R, Richaby DA, Choi HY, Fluckiger S, Blaser K, Dawson CA, Kelly KJ. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin Immunol 2001;98:327-336.
- 17. Schwab CJ, Cooley JD, Brasel T, Jumper CA, Graham SC, Straus DC. Characterization of exposure to low levels of viable Penicillium chrysogenum conidia and allergic sensitization induced by a protease allergen extract from viable P. chrysogenum conidia in mice. Int Arch Allergy Immunol 2003;130:200-208.
- 18. Sudha VT, Arora N, Singh BP. Serine protease activity of Per a 10 augments allergen-induced airway inflammation in mouse model. Eur J Clin Invest 2009;39:507-516.
- 19. Kuderer NM, San-Juan-Vergara HG, Kong X, Esch R, Lockey RF, Mohapatra SS. Mite and cockroach proteases activate p44/p42 MAP kinases in human lung epithelial cells. Clin Mol Allergy 2003;1:1.
- 20. Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett 2007;14:943-953.
- 21. Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Peripleneta americana. Allergy 2008;63:768-776.
- 22. Iraneta SG, Duschak VG, Rodriguez SM, Seoane MA, Albonico JF, Alonso A. Proteinase and gelatinolytic activieis of house dust mite and cockroach extracts. J Investig Allergol Clin Immunol 1999;9:235-240.
- 23. Ortego F, Sanchez-Ramos I, Ruiz M, Castanera P. Characterization of proteases from a stored product mite, Tyrophagus putrescentiae. Arch Insect Biochem Physiol 2000;43:116-124.
- 24. Osterlund C, Gronlund H, Polovic N, Sundstrom S, Gafvelin G, Bucht A. The non-proteolytic house dust mite allergen Der p 2 induces NF-κB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy 2009;39:1199-1208.
- 25. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009;457:585-588.
- 26. Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy 2006;4:5.
- 27. Zhang Z, Zhang H, Yang H, Zhang L, Chen X, Zheng X, He S. Induction of T-helper type 2 cytokine release and up-regulated expression of protease-activated receptors on mast cells by recombinant American cockroach allergen Per a 7. Clin Exp Allergy 2008;38:1160-1167.
- 28. Peregrine PC. Host dietary changes and the hindgut fauna of cockroaches. Int J Parasitol 1974;4:645-656.
- 29. Martinez-Giron R, Ribas A, Astudillo-Gonzalez A. Flagellated protozoa in cockroaches and sputum: the unhygienic connection? Allergy Asthma Proc 2007;28:608-609.
- 30. Cocks TM, Moffatt JD. Protease-activated receptor-2 (PAR2) in the airway. Pulm Pharmacol Ther 2001;14:183-191.
Fig. 1Protein analysis of mite and cockroach extracts. Each (10 µg) allergen extract was separated on 10% SDS-PAGE. M, molecular mass marker (kDa); Df, D. farinae; Dp, D. pteronyssinus; Tp, T. putrescentiae, Bg, B. germanica; Pa, P. americana; Pf, P. fuliginosa.

Fig. 2
Gelatin zymography of dust mite and cockroach extracts. Five µg for mite extracts and 1 µg of cockroach extracts were analyzed on a 10% SDS-PAGE gel containing 0.1% gelatin.
M, molecular mass marker (kDa); Df, D. farinae; Dp, D. pteronyssinus; Tp, T. putrescentiae, Bg, B. germanica; Pa, P. americana; Pf, P. fuliginosa.

Fig. 3Caseinolytic activities of dust mite and cockroach extracts as measured by the EnzChek assay. Caseinolytic activity was measured by green fluorescent dye conjugated substrate and allergen extracts (1 ng-10 µg). TPCK-trypsin, Tosyl-L-phenylalanine chloromethyl ketone-treated (chymotrypsin-activity inhibited) trypsin.

Table 1.Enzymatic activity of mite and cockroach extracts determined by the Api Zym assay
Table 1.
Enzyme
|
Allergen extract
|
|
Classes |
Specific activity |
Df |
Dp |
Tp |
Bg |
Pa |
Pf |
|
Proteases |
Leucine arylamidase |
+ |
+ |
+++ |
++ |
++ |
+++ |
|
Valine arylamidase |
- |
- |
++ |
++ |
- |
+ |
|
Cystine arylamidase |
- |
- |
+ |
+ |
- |
+ |
|
Trypsin |
+ |
+ |
+++ |
+ |
++ |
++ |
|
α-Chymotrypsin |
- |
- |
+++ |
- |
- |
- |
|
Lipases |
Esterase (C4) |
++ |
++ |
+ |
+ |
+ |
+ |
|
Esterase lipase |
+ |
++ |
++ |
+ |
++ |
++ |
|
Lipase (C14) |
- |
- |
+ |
+ |
- |
- |
|
Phosphatases |
Alkaline phosphatase |
++ |
+ |
++ |
++ |
+ |
+ |
|
Acid phosphatase |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
|
Naphtol-AS-BI-phosphohydrlase |
++ |
++ |
++ |
++ |
++ |
++ |
|
Glycosidases |
α-Galactosidase |
+ |
++ |
+ |
+ |
+ |
+ |
|
β-Galactosidase |
+++ |
+++ |
++ |
+++ |
+++ |
+++ |
|
β-Glucuronidase |
++ |
++ |
++ |
+++ |
++ |
+++ |
|
α-Glucosidase |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
|
β-Glucosidase |
++ |
++ |
+++ |
+ |
+ |
+ |
|
N-acetyl-β-glucosaminudase |
++ |
++ |
++ |
++ |
++ |
++ |
|
α-Mannosidase |
++ |
++ |
+ |
+ |
+ |
+ |
|
α-Fucosidase |
+++ |
+++ |
+++ |
++ |
+++ |
++ |