Abstract
Liver function tests were performed in 61 vivax, 54 malariae and 15 ovale malaria patients who were admitted to Bangkok Hospital for Tropical Diseases between 2001 and 2004. The objective of the study was to evaluate changes in hepatic biochemical indices before and after treatment with artemisinin derivatives. On admission and prior to treatment, hepatic dysfunction was found among the 3 groups. Serum liver function tests and physical examinations were performed weekly during the 28-day follow-up period. Initially elevated serum bilirubin and diminished albumin returned to normal within 2 weeks of treatment. Serum alkaline phosphatase and aminotransferases returned to within normal limits within 3 weeks. We conclude that patients with Plasmodium vivax, P. malariae and P. ovale infections had slightly elevated serum bilirubin, aminotransferase and alkaline phosphatase levels, and hypoalbuminemia. These minor abnormalities returned to normal within a few weeks after treatment with therapies based on artemisinin derivatives.
-
Key words: Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, malaria, liver profile dysfunction, artemisinin derivatives
INTRODUCTION
Malaria is a major health problem in the tropics. Liver dysfunction has been a common finding in malaria patients since the disease was described clinically and histologically (
Marchiafava and Bignami, 1894;
World Health Organization, 2000). However, opinions differ as to the extent and clinical importance of liver damages in malaria (
Molyneux et al., 1989;
Davies et al., 1996). Although there have been some reports of the liver dysfunction in malaria observed using modern clinical laboratory instruments (
Wilairatana et al., 1994;
Ravichandiran et al., 1996;
Premaratna et al., 2001), the serial liver profile for 28 days follow-up of patients infected with
Plasmodium vivax,
P. malariae and
P. ovale have not been fully investigated. We performed a prospective laboratory evaluation of changes in liver biochemical indices among patients infected with
P. vivax,
P. malariae and
P. ovale and treated with artemisinin derivatives.
MATERIALS AND METHODS
Study site and recruitment
The study was conducted from March 2001 to December 2004 at the Bangkok Hospital for Tropical Diseases, Thailand. All patients included fulfilled inclusion criteria (acute uncomplicated vivax, malariae and ovale malaria infections, either male or female; if female, pregnancy test negative before enrolment into the study, positive only asexual forms of
P. vivax,
P. malariae, or
P. ovale in blood smears, weight > 35 kg and age ≥ 13 years). Informed consent for the study was obtained from the patients or their guardians before enrolment into the study. The patients were admitted to hospital for 28 days to exclude reinfection. We excluded severe malaria cases according to WHO criteria (
World Health Organization, 2000), i.e., mixed malaria infections and G6PD deficiency. Sideeffects were defined as signs and symptoms that occurred or worsened after treatment. Adverse events would also be treated by standard procedures at the Bangkok Hospital for Tropical Diseases. This study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Upon admission the patients received the following treatments with artemisinin combination therapy (ACT) as part of clinical trials using alternative regimens of antimalarial drugs for vivax, malariae or ovale malaria:
P. malariae infection: A single dose of oral artesunate (200 mg/day) was given once a day for 3 days together with mefloquine 8 mg/kg/day for 3 days (
Silachamroon et al., 2005).
P. vivax or
P. ovale infections: A single dose of oral artesunate, 200 mg, on the first day, followed by single daily doses of oral artesunate, 100 mg, for the next 4 days, after which primaquine (0.6 mg/kg) was given once a day for 14 days (
Silachamroon et al., 2003).
Laboratory measurements
The following parameters were measured using the Integra 400 and commercial reagents by Roche diagnostic : total bilirubin (normal values 0.1-1.2 mg/dL), direct bilirubin (normal values 0.03-0.50 mg/dL), indirect bilirubin (normal values 0.07-0.70 mg/dL), aspartate aminotransferase and alanine aminotransferase (AST and ALT, normal values 7-40 U/L), alkaline phosphatase (normal values 40-150 U/L), and albumin (normal value 3.5-5.0 g/dL). Serial liver profiles were taken on days 0 (before antimalarial treatment), 7, 14, 21, and 28 during the study period.
Statistical analysis
Statistical analysis was performed using the Analyze It Add-Ins (Version 1.65) for Excel for Windows. All
P-values reported were from 2-tailed testing, and the statistically significant level was set at a 0.05 probability. The distribution of data did not generally show a normal distribution by using the Schapiro-Wilks test. Therefore, data were expressed as the median and range. Two statistical tests were performed: chi-square test to test differences among qualitative variables and Kruskal-Wallis test to test the differences among quantitative variables (
Tabachnick, 2001).
RESULTS
Three groups of malaria patients were enrolled as follows: 61 vivax, 54 malariae and 15 ovale patients. All patients completed the follow-up schedule. No treatment failures or fatalities occurred. There was no major adverse event during the follow-up period.
Table 1 listed demographic and clinical data on admission. Hepato-splenomegaly was frequently observed among all 3 groups. Before treatment, mild anemia was frequently found in
P. vivax and
P. malariae groups. The results also showed thrombocytopenia among the 3 groups (normal values of platelet count 150,000-350,000 per µL). An increase of serum bilirubin was observed among 3 study groups, however, abnormal clinical significance was not observed. The other laboratory results of total patients were within normal limit.
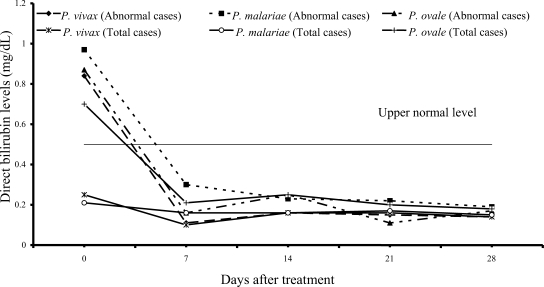
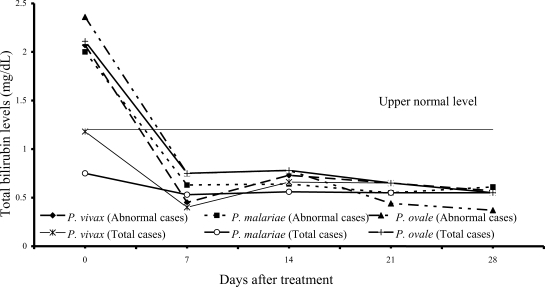
On admission, 14 of 61 vivax patients (23%), 7 of 54 malariae patients (13%), and 9 of 15 ovale patients (60%) had increased values of both direct and total bilirubins (
Figs. 1,
2); median (range) values were 0.84 mg/dL (0.55-6.00), 0.97 mg/dL (0.67-1.50), 0.87 mg/dL (0.70-1.76) for direct, and 2.07 mg/dL (1.23-10.60), 2.00 mg/dL (1.21-2.68) and 2.36 mg/dL (1.72-4.32) for total bilirubin, respectively. However, there were no complications in those patients. During the follow-up, the total and direct birilubin levels fell within the range of normal values by day 7 after treatment.
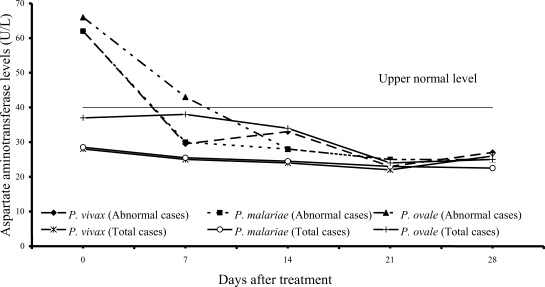
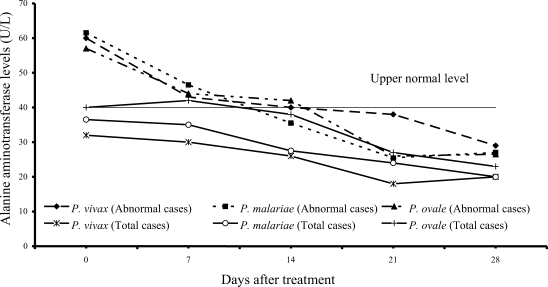
Transient hypertransaminasemia (AST and ALT) was observed in most patients (
Figs. 3,
4). Increased AST levels on admission were observed in 16 of 61
P. vivax patients (26%), 17 of 54
P. malariae patients (31%), and 6 of 15
P. ovale patients (40%); median (range) values were 62 U/L (43-324), 62 U/L (41-216) and 66 U/L (45-299), respectively. Increased ALT levels were found in 13 of 61 vivax (21%), 16 of 54 malariae (30%), and 6 of 15 ovale patients (40%); median (range) values were 60 U/L (43-476), 61.5 U/L (42-385) and 57 U/L (50-522), respectively. However, serum AST and ALT fell within the range of normal levels within 3 weeks of treatment in all groups.
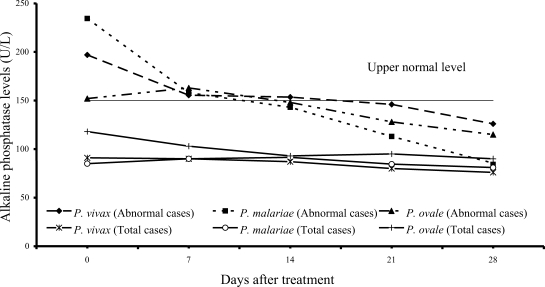
Increased serum alkaline phosphatase levels were observed in 12 of 61 vivax (20%) and 12 of 54 malariae patients (22%), and 3 of 15 ovale patients (20%); median (range) values were 197 U/L (166-318), 234.5 U/L (153-322), 152 U/L (152-318), respectively. After treatment, serum alkaline phosphatase fell within the range of normal values within 3 weeks after treatment (
Fig. 5).
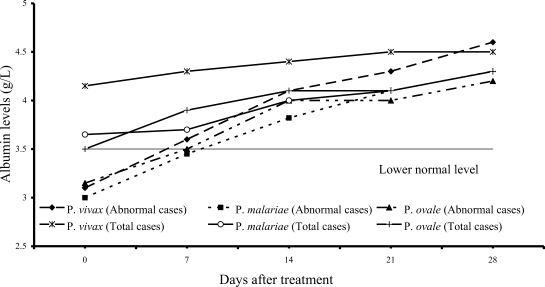
Fig. 6 shows 7 of 61
P. vivax patients (11%), 22 of 54
P. malariae patients (41%), and 7 of 15
P. ovale patients (47%) had hypoalbuminemia; median (range) values were 3.10 g/dL (2.80-3.40), 3.00 g/dL (2.10-3.40) and 3.15 g/dL (2.70-3.20), respectively. The albumin levels in 3 study groups fell within the range of normal values within 2 weeks after treatment.
Among the 83 of 130 patients having normal liver profiles on admission (64 %) did not show any liver profile dysfunction on follow-ups. All patients were cured after the treatment. In addition, there was no serious adverse event reported during this study.
DISCUSSION
We have shown that liver function profiles among patients with vivax, malariae and ovale malaria are often abnormal. Previous studies have documented liver dysfunctions in
P. falciparum malaria (
Mishra et al., 1992;
Davies et al., 1996;
Premaratna et al., 2001), and it has been documented that abnormal liver function profiles return to normal a few weeks after treatment (
Wilairatana et al., 1994). Our findings with the other human malarias accorded with falciparum malaria. Liver function profile abnormalities in vivax, malariae and ovale malaria patients were observed at admission and returned to normal within 3 weeks after treatment.
Liver abnormalities are a relatively common finding in severe falciparum malaria (
Harinasuta and Bunnag, 1988;
Miller et al., 1994;
Enwere et al., 1999). Our work showed that liver dysfunctions also occurred in the other 3 malaria species (
P. vivax,
P. malariae and
P. ovale). However, in this study, most patients had abnormal liver profiles without clinically significant complications.
In severe
P. faciparum malaria, jaundice (total bilirubin > 3 mg/dL) is a common feature attributed in part to liver damage and hemolysis of both parasitized and non-parasitized erythrocytes (
Wilairatana et al., 1994). In this study, there were only 8 mild jaundiced patients (4 vivax and 4 ovale malaria patients). Although the direct parameters for hemolysis were not measured in this study, a decrease of hematocrit (without bleeding) concomitant with an increase of predominantly unconjugated bilirubinemia for a few days after admission was suggestive of hemolytic jaundice.
The abnormal serum AST and ALT levels in the patients at admission were usually observed in all 3 study groups (
Figs. 3,
4). The elevation of transaminase levels returned to normal within 3 weeks. The increased transaminase levels, especially AST level were also compatible with increased hemolysis and hepatic injury or hepatitis.
The results from study have shown that serum alkaline phosphatase rose during the first 2 weeks (
Fig. 5) and returned to normal within 3 weeks. Alkaline phosphatase elevation during the first 2 weeks of admission might be due to intrahepatic cholestasis during acute malaria infection as a consequence of mononuclear infiltration in the liver. The slight decrease in serum albumin observed in all 3 groups of the patients might be due to either malnutrition before treatment or the malaria infection itself. At present, there is no study showing increased vascular permeability in
P. vivax,
P. malariae and
P. ovale infection and causing hypoalbuminemia. Since the patients in this study were not suffering severe malaria, further investigation to exclude other possible causes of abnormal liver profiles, such as liver ultrasonography were not done. Further investigation may clarify other contributing factors to abnormal liver profiles.
The patients in this study received different treatment regimens. Some patients treated with primaquine showed a decrease in hematocrit (without bleeding), however, hematocrit returned to normal a few days after the treatment. Serum transaminases did not significantly increase in patients given mefloquine. Therefore, mefloquine did not show hepatotoxicity in the patients as in previous reports (
Looareesuwan et al., 1996;
Tangpukdee et al., 2005).
In conclusion, patients with P. vivax, P. malariae and P. ovale infections had minimal abnormalities in liver profiles, increased levels of bilirubin, aminotransferases, and alkaline phosphatases, and hypoalbuminemia. These minor abnormalities returned to normal within a few weeks after antimalarial treatment.
Notes
-
This study was supported in part by the grant of the Ministry Health Labour and Welfare of Japan and a Mahidol University Research Grant.
ACKNOWLEDGMENTS
We thank the staff of the Bangkok Hospital for Tropical Diseases for their help and support of the study. We thank Prof. Kevin Baird for reviewing this manuscript.
References
Fig. 1Median of serum direct bilirubin levels in vivax, malariae and ovale malaria patients.
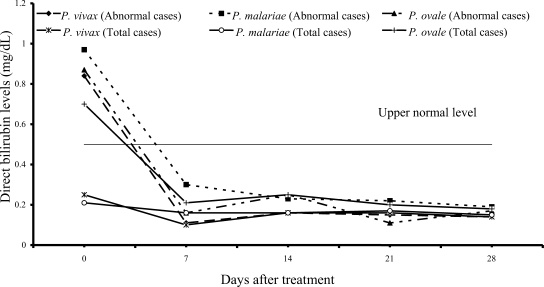
Fig. 2Median of serum total bilirubin levels in vivax, malariae and ovale malaria patients.
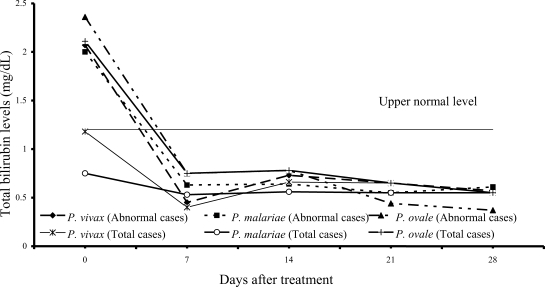
Fig. 3Median of serum aspartate aminotransferase levels in vivax, malariae and ovale malaria patients.
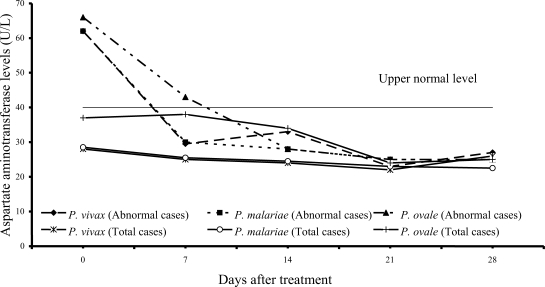
Fig. 4Median of serum alanine aminotrasferase levels in vivax, malariae and ovale malaria patients.
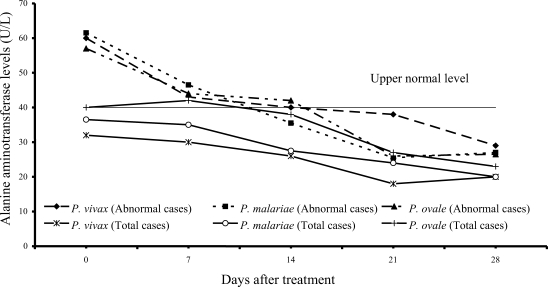
Fig. 5Median of serum alkaline phosphatase levels in vivax, malariae, ovale malaria patients.
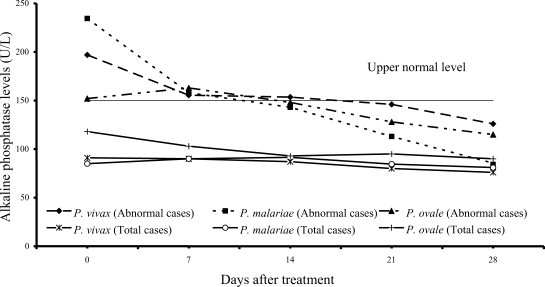
Fig. 6Median of serum albumin levels in vivax, malariae, ovale malaria patients.

Table 1.Clinical and laboratory characteristics of study groups before treatments
Table 1.
|
P. vivax patients (N = 61) |
P. malariae patients (N = 54) |
P. ovale patients (N = 15) |
P-values |
|
Gender (Male/Female) |
40/21 |
39/15 |
6/9 |
0.068 |
|
Age (yr) |
|
|
|
|
|
Median |
24.0 |
23.0 |
34.0 |
0.149 |
|
(Range) |
(19.0-56.0) |
(13.0-50.0) |
(20.0-64.0) |
|
|
Height (cm.) |
|
|
|
|
|
Median |
162.0 |
160.0 |
160.0 |
0.334 |
|
(Range) |
(123.5-170.0) |
(141.0-179.0) |
(154.5-170.0) |
|
|
Body weight (kg.) |
|
|
|
|
|
Median |
50.0 |
50.7 |
53.5 |
0.089 |
|
(Range) |
(37.5-70.0) |
(38.0-75.0) |
(40.0-69.0) |
|
|
Highest fever before treatment (°C) |
|
|
|
|
|
Median |
37.7 |
37.6 |
38.0 |
0.628 |
|
(Range) |
(36.2-40.7) |
(36.0-40.2) |
(37.2-40.0) |
|
|
Number (%) of patients with: |
|
|
|
|
|
Splenomegaly |
4 (6.5) |
8 (14.8) |
2 (13.3) |
0.342 |
|
Hepatomegaly |
5 (8.2) |
6 (11.0) |
6 (20.0) |
0.143 |
|
First malaria attack |
27 (44.2) |
31 (57.4) |
6 (40.0) |
0.278 |
|
Parasites on admission (per μL) |
|
|
|
|
|
Median |
8,960 |
4,000 |
588 |
0.156 |
|
(Range) |
(88-95,920) |
(55-23,160) |
(221-26,100) |
|
|
Laboratory data {Median (Range)} |
|
|
|
|
|
Packed cell volume (%) |
36.9 |
33.5 |
44.0 |
< 0.001 |
|
(28.0-45.6) |
(21.0-51.0) |
(30.0-46.0) |
|
|
Leukocyte count (x103 per μL) |
5.8 |
5.0 |
4.2 |
< 0.001 |
|
(2.7-11.6) |
(2.7-11.0) |
(2.7-5.6) |
|
|
Platelet count (x103 per μL) |
89.0 |
137.0 |
59.0 |
< 0.001 |
|
(40-420) |
(43-320) |
(45-160) |
|
|
Total bilirubin (mg/dL) |
1.18 |
0.76 |
2.11 |
< 0.001 |
|
(0.30-10.60) |
(0.25-2.68) |
(0.90-4.32) |
|
|
Indirect bilirubin (mg/dL) |
0.96 |
0.55 |
1.48 |
< 0.001 |
|
(0.20-4.60) |
(0.19-2.29) |
(0.52-3.60) |
|
|
Direct bilirubin (mg/dL) |
0.25 |
0.22 |
0.70 |
< 0.001 |
|
(0.09-6.00) |
(0.06-1.50) |
(0.38-1.76) |
|
|
Aspartate aminotransferase (U/L) |
28.0 |
28.5 |
37.0 |
0.329 |
|
(12.0-324.0) |
(8.0-216.0) |
(23.0-299.0) |
|
|
Alanine aminotransferase (U/L) |
32.0 |
36.5 |
40.0 |
0.015 |
|
(15.0-476.0) |
(7.0-385.0) |
(33.0-522.0) |
|
|
Alkaline phosphatase (U/L) |
91.0 |
85.0 |
118.0 |
0.346 |
|
(50.0-318.0) |
(43.0-322.0) |
(82.0-318.0) |
|
|
Albumin (g/dL) |
4.15 |
3.65 |
3.50 |
< 0.001 |
|
(2.80-5.00) |
(2.10-5.10) |
(2.70-4.30) |
|