Abstract
The circumsporozoite protein (CSP) of Plasmodium spp. is a diagnostic antigen and useful biomarker for monitoring short-term/seasonal changes to malaria transmission. Using P. vivax CSP antibody ELISA, epidemiological characteristics were analyzed in the residents of Ganghwa, Cheorwon, Paju, and Goseong from 2017 to 2018. In Ganghwa and Cheorwon, 1.6% and 1.2% of residents, respectively, were PvCSP-antibody-positive in 2018, which indicates a decrease of 0.4% in the positive rate compared to 2017. The annual parasite incidence (API) in Ganghwa and Cheorwon was 24.9 and 10.5 in 2017 and 20.3 and 10.7 in 2018, respectively. Although the changes were not significant, the API in Ganghwa decreased slightly by 4.5 in 2018 compared to the previous year. In Paju and Goseong, 3.9% and 2.0% of residents were positive for the PvCSP antibody. The API in Paju was 13.1 in 2017 and 16.0 in 2018, although no malaria patients were reported for the 2 years. Therefore, the results suggest that PvCSP is a useful antigen for confirming initial malaria infection. Additionally, considering that the antibody is relatively transient, it can be employed for sero-epidemiological studies to determine the extent of malaria transmission in the current year.
-
Key words: Plasmodium vivax, vivax malaria, circumsporozoite protein, elimination study, Korea
Vivax malaria is endemic in the Korean Peninsula. The Malaria Eradication Service, jointly implemented by the Korean Government and the WHO since 1959, reported that malaria has continued to decline since the late 1970s and has almost disappeared from Korea. In particular, since 2 outbreaks occurred in 1984, indigenous malaria was reported to be completely wiped out and was considered to have been eradicated from Korea [
1]. However,
Plasmodium vivax re-emerged in 1993, with one case diagnosed in a soldier who served in the northern Gyeonggi-do Province of Korea [
2]. Korea was deemed to have achieved the pre-elimination of malaria in 2013, because the number of malaria cases in this year was 453, which satisfied the WHO standard (less than 1 outbreak per 1,000 people in endemic areas). However, to achieve complete eradication and WHO certification, it is necessary to (1) investigate parasitemia in the blood of patients from the past and present, (2) confirm the distribution of sporozoites in natural mosquitoes, (3) provide scientific evidence such as the circulation of antibodies to circumsporozoite protein (CSP) in high-risk malaria areas, and (4) provide management strategies and policies. In general, malaria eradication requires new policies to reach the pre-elimination stage and a shift in management policy after eradication. In addition, after eradication, continuous management is needed to prevent malaria from becoming reinstated.
The CSP is a surface membrane protein expressed in the sporozoite stage of the malaria parasite and is a candidate target of the RTS, S/AS01 pre-erythrocytic malaria vaccine [
3–
6], which was developed in 1987 as part of a collaboration between GlaxoSmithKline and the Walter Reed Institute of Research [
7]. Sporozoite-specific antigens provide potentially useful markers for monitoring the short-term/seasonal changes in malaria transmission [
8]. Antibodies to CSP are important in reducing the prevalence of malaria among persons of increasing age in endemic areas [
9]. It is necessary to investigate and analyze the CSP-antibody-positive rate of the blood of at-risk individuals to identify the malaria-mediated mosquito exposure intensity during the summer in the Ganghwa and Cheorwon regions, close to the border with North Korea. These findings would help to determine the extent of malaria transmission during the winter season (a malaria-free period) and to establish data for an early detection network in these regions. In addition, CSP analysis could be used to compare the prevalence of malaria among regions and to predict the outbreak of malaria epidemics in the following years. In this study, we analyzed the correlation between
PvCSP antibody titers and malaria prevalence by comparing the retention rate of
PvCSP antibodies by region among residents living in malaria epidemic areas.
Blood samples were collected from participants residing at 25 villages in 2 administrative areas in Ganghwa and at 15 villages in 4 administrative areas in Cheorwon. The sampling was conducted in November and December of 2017 and 2018. In addition, blood samples were collected in 10 villages of 3 administrative areas in Paju and 9 villages of 2 administrative areas in Goseong in December 2018. To evaluate the diagnostic performance of the PvCSP recombinant protein, 1,233 blood samples were collected from residents in Ganghwa and Cheorwon in 2017 and 1,845 blood samples were collected from all 4 study areas in 2018. The sera were separated by centrifugation at 13,000 rpm and 4°C for 5 min and stored at −20°C for the serological tests.
Informed consent was obtained from all individuals who participated. All samples were collected using protocols that were reviewed and approved by the Human Ethics Committee of Inha University Hospital (INHAUH 2018-12-019-001). After blood collection, all blood samples were deposited at the Global Resource Bank of Parasitic Protozoan Pathogens (GRBPPP; National Research Foundation Grant funded by the Korean Government, NRF-2017M3A9B8069530), and the experiment was performed after parceling-out.
An ELISA was performed to detect
PvCSP antibodies from the sera of residents. The methods for
PvCSP recombinant protein expression and ELISA are described in our previous study [
10]. The
PvCSP recombinant protein (0.5 μg/well) was coated onto a 96-well plate (Corning, New York, USA) using 0.05 M carbonate-bicarbonate buffer (pH 9.4) and incubated for 12 hr at 4°C. The plate was washed 3 times at 5 min intervals with phosphate-buffered saline (pH 7.4) containing 0.05% tween 20 (PBST), and then the plate was blocked with blocking buffer (PBST containing 3% bovine serum albumin) for 1 hr at room temperature. After the wells were washed with PBST 3 times, as described previously, human serum sample in blocking buffer at a dilution of 1:100 (vol/vol) was added to each well (100 μl/well). In addition, 4 positive and 4 negative control serum samples were added to each plate. After 2 hr of incubation at room temperature, the plates were washed 3 times, and peroxidase-conjugated anti-human IgG (Sigma, 1:2,000, vol/vol) diluted in blocking buffer was added to each well (100 μl/well). The plates were re-incubated for 1 hr at room temperature, after which the reaction was stopped by washing the plates as described previously. To develop the color, 100 μl of tetramethylbenzidine substrate buffer (Invitrogen, Carlsbad, USA) was added, and the plates were covered with foil and incubated for 5 min at room temperature. The reaction was stopped with stop solution (1 N HCl, 100 μl/well). Absorbance was measured at 450 nm, and the cut-off value for positivity was defined as the mean +3 standard deviations of the negative control samples.
We performed PCR to confirm whether the
PvCSP-ELISA-positive samples were infected with
P. vivax. The genomic DNA of the malaria parasite
P. vivax was extracted from 200 μl of whole-blood samples using the HiYield Genomic DNA Mini Kit (Real Biotech Corporation, Banqiao City, Taiwan) followed by the manufacturer’s instructions. Amplification of the 18S rRNA gene of the malaria parasite was performed with primers designed according to the methods of Snounou et al. [
11]. The rPLU5 (forward) and rPLU6 (reverse) primers were 5′-CCT GTT GTT GCC TTA AAC TTC-3′ and 5′-TTA AAA TTG TTG CAG TTA AAA CG-3′, respectively. The thermal cycling parameters for the PCR were as follows: 1 cycle of initial denaturation at 95°C for 5 min, 30 cycles of 95°C for 30 sec, annealing at 60°C for 1 min, and extension at 72°C for 1 min, followed by a final extension at 72°C for 5 min. Nested-PCR was then performed using species-specific forward and reverse primers for
P. vivax: 5′-CGC TTC TAG CTT AAT CCA CAT AAC TGA TAC-3′ and 5′-ACT TCC AAG CCG AAG CAA AGA AAG TCC TTA-3′, respectively. The thermal cycling parameters for the nested PCR were as follows: 1 cycle of initial denaturation at 95°C for 5 min, 30 cycles at 95°C for 30 sec, annealing at 58°C for 30 sec, and extension at 72°C for 30 sec, followed by a final extension at 72°C for 5 min. Each PCR product was analyzed by electrophoresis on 1% agarose gel.
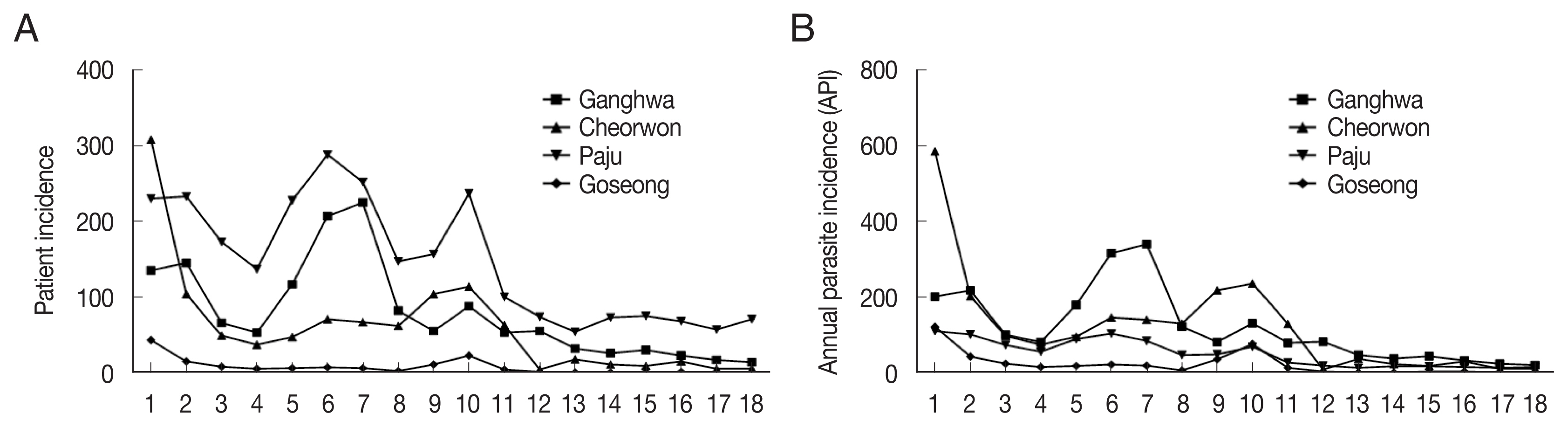
During 2001–2018, the patient incidence by year fluctuated between 135 and 14 and the annual parasite incidence (API; number of cases per 100,000 people) ranged between 201.6 and 20.3 in Ganghwa; the patient incidence was between 308 and 5 and API was between 202.7 and 10.7 in Cheorwon; patient incidence was between 230 and 71 and API was between 110.5 and 16.0 in Paju; and patient incidence was between 43 and 0 and API was between 122.8 and 0 in Goseong (
Fig. 1A, B). In the Goseong, malaria case did not occur in 2017 and 2018. Overall, it was confirmed that the patient incidence and API of malaria had continuously decreased. Therefore, to understand the correlation between the incidence of malaria and the prevalence of the
PvCSP antigen, serological studies were conducted on the inhabitants of these 4 areas.
A total of 1,476 blood samples were collected from Ganghwa residents in 2017 (922 samples) and 2018 (554 samples). The ELISA results for the Ganghwa resident’s sera showed 1.20% positivity in 2017 and 0.72% positivity in 2018 (
Table 1). The API in 2017 (24.86) was higher than that in 2018 (20.34) in Ganghwa (
Table 1). In Cheorwon, ELISA revealed 2.20% of residents carried the
PvCSP antibody in 2017, and 1.62% were seropositive in 2018. Furthermore, the API values for Cheorwon were 10.50 and 10.68 in 2017 and 2018, respectively. Overall, 1.62% of people in 2017 and 1.22% of people in 2018 were
PvCSP-positive (
Table 1). Moreover, the API in Ganghwa and Cheorwon was 24.86 and 10.50 in 2017, and 20.34 and 10.68 in 2018, respectively. Ganghwa showed a slight decrease in API (4.52) in 2018 (
Table 1), although the change was not significant. However, the results of the nested PCR used to detect the
P. vivax were all negative in 2017 and 2018.
A total of 612 blood samples were collected from the residents of Paju (308 samples) and Goseong (304 samples) in 2018, but no samples were collected from either area in 2017. The antibody-positive rate was 4.00% in Paju and 1.97% in Goseong in 2018. Overall, 2.94% of samples were positive for the
PvCSP antibody (
Supplementary Table S1). However, the results of the nested PCR were negative in both Paju and Goseong. Moreover, the API in Paju was 13.12 and 15.96 in 2017 and 2018, respectively, but it was 0 in Goseong for the 2 years.
The mean immune response intensity among 11 positive samples obtained from 922 residents of Ganghwa in 2017 was 0.7177±0.0272 (
P<0.0001), which decreased to 0.5240± 0.0089 (
P<0.0001) among 5 positive samples obtained from 554 residents in 2018 (
Fig. 2A). The mean immune response intensity in Cheorwon also decreased from 0.6566±0.0610 (
P<0.0001) in 2017 to 0.5314±0.0086 (
P<0.0001) in 2018 (
Fig. 2B). Furthermore, when we compared the
PvCSP reactivity of these 2 study areas, the immune intensity in Ganghwa was 0.6208±0.0180, which was slightly higher than that in Cheorwon (0.5240±0.0777). There was no
PvCSP immune response intensity data for Paju or Goseong in 2017, which made it impossible to compare them with Ganghwa and Cheorwon. However, Paju showed a similar immune response intensity compared with Ganghwa and Cheorwon (0.5583± 0.1113 [
P<0.0001]) among 12 positive samples obtained from 308 residents in 2018 (
Supplementary Fig. S1), while that in Goseong was 0.9810±0.0215 (
P=0.0007) among 6 positive samples from 304 residents in 2018, which was the highest among the study areas (
Supplementary Fig. S1).
Nested PCR targeting the 18S rRNA was performed to detect the malaria parasite in the samples that reacted to the PvCSP antigen. Genomic DNA was extracted from a total of 33 positive samples (Ganghwa: 4, Cheorwon: 11, Paju: 12, and Goseong: 6), but the malaria parasite was not detected in any of these samples. Only the positive control showed a band at around 120 bp on agarose gel electrophoresis.
Serological tests to detect antibodies reactive to malaria-specific antigens can be useful tools for epidemiological studies, screening for donors in areas without malaria, and/or diagnosing infected individuals. In addition, most malaria patients have antibodies against malaria parasites, even those with low parasitemia levels, and these serological tests can be applied to serodynamic investigations [
12,
13]. Moreover, the mean incubation period of
P. vivax has been reported to be 279±41 days (153 to 452 days) [
14]. These findings suggest that antibody levels for the CSP antigen could become deficient or reduced over the long-term incubation period: the proportions of antibodies lost over short- and long-term incubation periods were 25% and 75%, respectively, in Korea [
15]. However, after a short incubation period (within 1 month of exposure to the CSP antigen delivered through an infectious anopheline mosquito) patients can have elevated CSP antibody titers, which suggests that the CSP-antibody-positive rate for a particular year could be related to the malaria-positive results of the following year. In this study, a recombinant CSP protein of
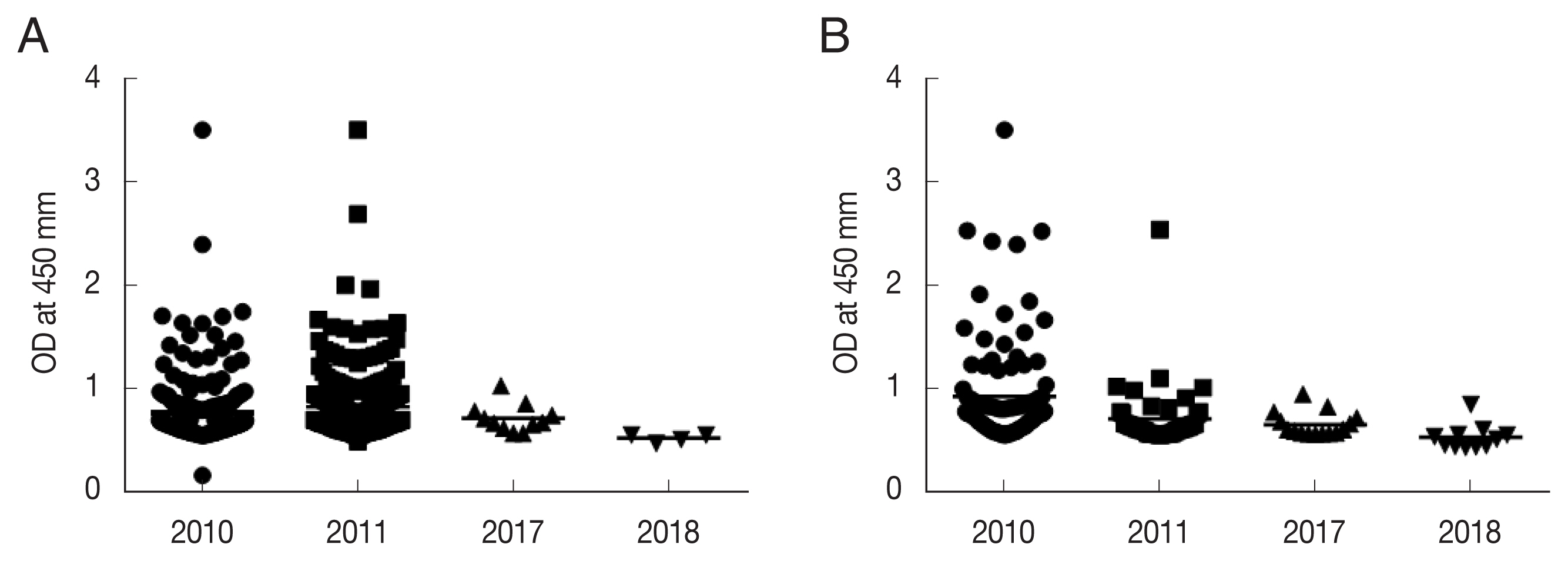
P. vivax sporozoites was used to compare the positive rate of anti-CSP antibodies in high-risk malaria areas in Korea: Ganghwa, Cheorwon, Paju, and Goseong. Our previous study indicated that the anti-CSP-antibody-positive rates in 2010 and 2011 were related to the API for the subsequent year in Ganghwa and Cheorwon. The anti-CSP-antibody-positive rates were 18.6% (2010) and 18.9% (2011) in Ganghwa and 17.1% and 7.3% in Cheorwon, respectively [
10]. However, in the 2017 and 2018 surveys, the rates of anti-CSP-antibody-positive sera were significantly reduced to 1.20% and 0.72% in Ganghwa and 2.20% and 1.62% in Cheorwon, respectively. Interestingly, the API values for 2017 and 2018 were significantly decreased compared to those in 2010 and 2011. In Ganghwa, the API values of 131.2 (2010) and 79.2 (2011) decreased to 24.86 (2017) and 20.34 (2018), respectively. Moreover, this was also the case for Cheorwon, with a decrease in API from 236.0 and 129.9 (2010 and 2011) to 10.50 and 10.68 (2017 and 2018).
The CSP-ELISA positivity rate for 2010 to 2018 is expected to be significantly related to the malaria incidence rates in Ganghwa and Cheorwon during this period. These results suggest that malaria development in the following year can be predicted by the current rate of sera positive for anti-CSP, and that some of the cases could affect the incidence of cases with long-term incubation periods because the APIs of the present year include short-term-incubation cases with as well as patients with long-term incubation periods who were infected in the previous year. In other words, the anti-CSP-positive response can be estimated using the infection rate of
Plasmodium spp. sporozoites from mosquitoes. However, even if the CSP-ELISA assay returns positive results, it is difficult to calculate how many people will develop malaria after having been bitten by malaria-infected mosquitoes. Cho et al. [
10] reported that this number was highly dependent on the individual’s immune capacity, and infection did not necessarily end in parasite-positive results in a qPCR analysis of 632 anti-CSP antibody-positive samples. Our results are similar to these findings, as the 33 individuals identified as CSP-antibody-positive in 2018 did not show evidence of
Plasmodium parasite infection in the rapid diagnostic test and nested-PCR analyses. In addition, Wijesundera et al. [
12] reported that only 63% of patients with
P. falciparum infection had antibodies for the CSP antigen, which is consistent with our results. These findings suggest that a current high CSP-antibody-positive rate indicates a malaria outbreak occurred in the previous year and is more closely related to community response than individual response.
Notes
-
The authors declare no conflict of interest related to this study.
Supplementary Information
Supplementary Table S1.
Positive rate of circumsporozoite protein and annual parasite incidence in Goseong-gun and Paju-si
ACKNOWLEDGMENTS
We would like to thank the staffs at the Public Health Centers of Ganghwa, Cheorwon, and Goseong for their contributions and technical supports in blood collection. This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean Government (NRF 2019 K1A3A9A01000005 and NRF 2017M3A9B8096530) and the Inha University Research Fund (65321-1).
Fig. 1(A) Patient incidence and (B) annual parasite incidence from 2001 to 2018 in Ganghwa, Cheorwon, Paju, and Goseong.
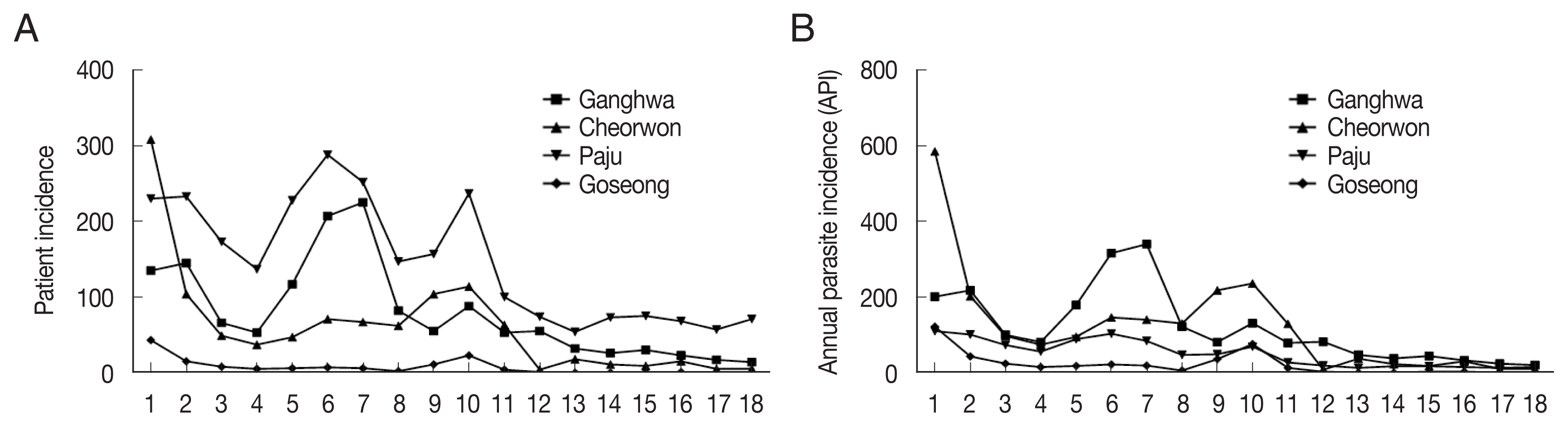
Fig. 2Anti-CSP antibody titers in 2017 and 2018: (A) Ganghwa and (B) Cheorwon.
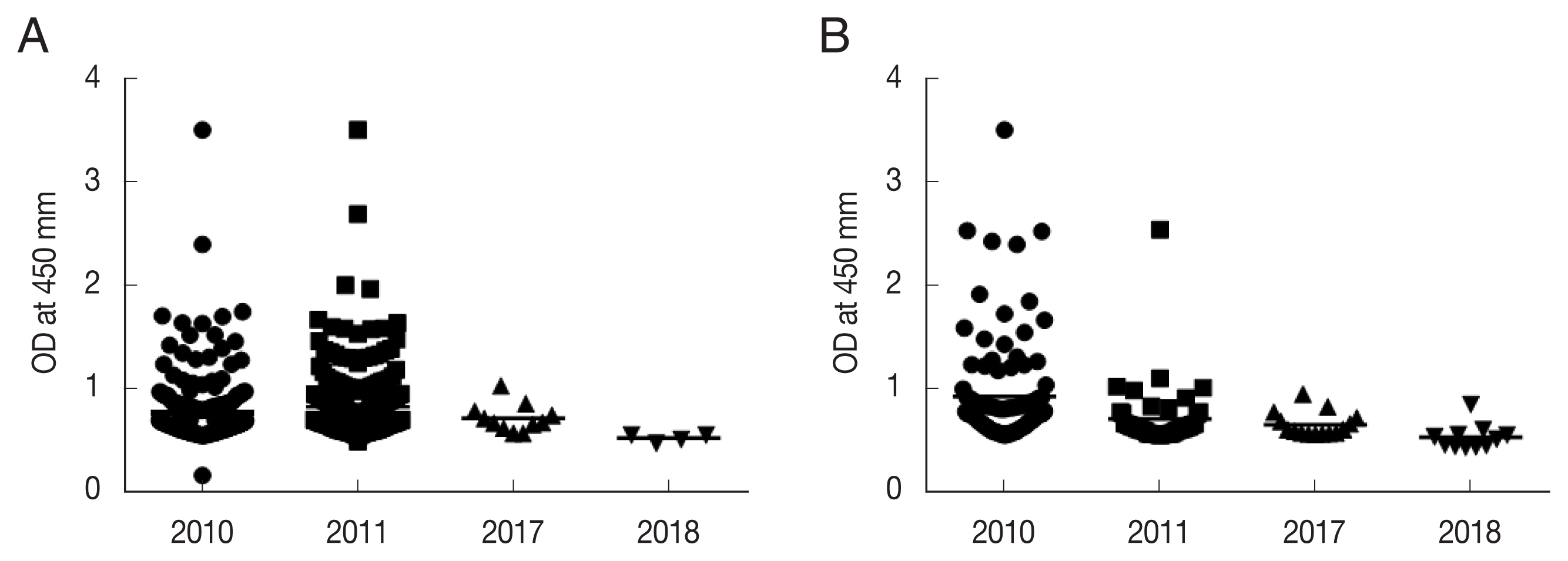
Table 1Positive rate of circumsporozoite protein and annual parasite incidence in Cheorwon-gun and Ganghwa-gun
Table 1
|
Area |
No. of sera tested |
No. of positive sera |
Positive rate (%) |
API |
|
|
|
|
|
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
2017 |
2018 |
|
Ganghwa |
922 |
554 |
11 |
4 |
1.20 |
0.72 |
24.86 |
20.34 |
|
|
Cheorwon |
680 |
679 |
15 |
11 |
2.20 |
1.62 |
10.50 |
10.68 |
|
|
Total |
1,602 |
1,233 |
26 |
15 |
1.62 |
1.22 |
17.68 |
15.51 |
References
- 1. Soh CT, Lee KT, Im KI, Min DY, Ahn MH, Kim JJ, Yong TS. Current status of malaria in Korea. Yonsei Rep Trop Med 1985;16:11-18.
- 2. Cho SY, Kong Y, Park SM, Lee JS, Lim YA, Chae SL, Kho WG, Lee JS, Shim JC, Shin HK. Two vivax malaria cases detected in Korea. Korean J Parasitol 1994;32:281-284. https://doi.org/10.3347/kjp.1994.32.4.281
- 3. Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. Circumsporozoite protein of Plasmodium vivax gene cloning and characterization of the immunodominant epitope. Science 1985;230:815-818. https://doi.org/10.1126/science.2414847
- 4. Porter MD, Nicki J, Pool CD, DeBot M, Illam RM, Brando C, Bozick B, De La Vega P, Angra D, Spaccapelo R, Crisanti A, Murphy JR, Bennett JW, Schwenk RJ, Ockenhouse CF, Dutta S. Transgenic parasites stably expressing full-length Plasmodium falciparum circumsporozoite protein as a model for vaccine down-selection in mice using sterile protection as an endpoint. Clin Vaccine Immunol 2013;20:803-810. https://doi.org/10.1128/CVI.00066-13
- 5. Brown AE, Webster HK, Krinchal K, Gordon DM, Wirtz RA, Permpanich B. Characteristics of natural antibody responses to the circumsporozoite protein of Plasmodium vivax. Am J Trop Med Hyg 1991;44:21-27. https://doi.org/10.4269/ajtmh.1991.44.21
- 6. Gordon DM, Cosgriff TM, Schneider I, Wasserman GF, Majarian WR, Hollingdale MR, Chulay JD. Safety and immunogenicity of a Plasmodium vivax sporozoite vaccine. Am J Trop Med Hyg 1990;42:527-531. https://doi.org/10.4269/ajtmh.1990.42.527
- 7. Laurens MB. RTS, S/AS01 Vaccine (Mosquirix™): an overview. Hum Vaccin Immunother 2020;16:480-489. https://doi.org/10.1080/21645515.2019.1669415
- 8. Kusi KA, Bosomprah S, Kyei-Baafour E, Dickson EK, Tornyigah B, Angov E, Dutta S, Dodoo D, Sedegah M, Koram KA. Seropervalence of Antibidoes against Plasmodium falciparum Sporozoite Antigen as Predictive Disease Transmission Markers in an Area Ghana with Seasonal Malaria Transmission. PLoS One 2016;11:e0167175. https://doi.org/10.1371/journal.pone.0167175
- 9. Hoffman SL, Wistar R Jr, Ballou WR, Hollingdale MR, Wirtz RA, Schneider I, Marwoto HA, Hockmeyer WT. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med 1986;315:601-606. https://doi.org/10.1056/NEJM198609043151001
- 10. Cho PY, Lee SW, Ahn SK, Kim JS, Cha SH, Na BK, Park YK, Lee SK, Lee WJ, Nam HW, Hong SJ, Pak JH, Kang YJ, Sohn Y, Bahk YY, Cho HI, Kim TS, Lee HW. Evaluation of circumsporozoite protein of Plasmodium vivax to estimate its prevalence in the Republic of Korea: an observational study of incidence. Malar J 2013;12:448. https://doi.org/10.1186/1475-2875-12-448
- 11. Snounou G, Pinheiro L, Gonçalves A, Fonseca L, Dias F, Brown KN, do Rosario VE. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg 1993;87:649-653. https://doi.org/10.1016/00359203(93)90274-t
- 12. Wijesundera MD, Peiris JSM, Ariyaratne YG, Verdini AS, Pessi A, Giudice GD. Antibodies to Plasmodium falciparum sporozoites following a malarial outbreak in a non-endemic area of Sri Lanka. Trans R Soc Trop Med Hyg 1990;84:35-39. https://doi.org/10.1016/0035-9203(90)90372-l
- 13. Druilhe P, Pradier O, Marc JP, Miltgen F, Mazier D, Parent G. Level of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect Immun 1986;53:393-397. https://doi.org/10.1128/IAI.53.2.393-397
- 14. Kho WG, Jang JY, Hong ST, Lee HW, Lee WJ, Lee JS. Border malaria characters of reemerging vivax malaria in the Republic of Korea. Korean J Parasitol 1999;37:71-76. https://doi.org/10.3347/kjp.1999.37.2.71
- 15. Lee JS, Kho WG, Lee HW, Seo M, Lee WJ. Current status of vivax malaria among civilians in Korea. Korean J Parasitol 1998;36:241-248. https://doi.org/10.3347/kjp.1998.36.4.241
 , Kyoung Jin2,†, Seong Kyu Ahn3,†
, Kyoung Jin2,†, Seong Kyu Ahn3,† , Sung-Keun Lee4, Hyung Wook Kwon5,6, Byoung-Kuk Na7,*
, Sung-Keun Lee4, Hyung Wook Kwon5,6, Byoung-Kuk Na7,* , Tong-Soo Kim1,6,*
, Tong-Soo Kim1,6,*