INTRODUCTION
Culicoides biting midges (Diptera: Ceratopogonidae) are the smallest blood-sucking arthropods and their length rarely exceeds 3 mm [1]. The wings of adult Culicoides display various patterns by species, which can be useful for classifying the species [2]. Culicoides are vectors that transmit epizootic arthropod-borne viruses (arboviruses) such as the Akabane virus, the bovine ephemeral fever, the bluetongue virus, and the Schmallenberg virus to livestock [1,3,4]. Arboviruses are major pathogens in the veterinary field and ruminants infected with arboviruses have a high fever, reduced appetite, respiratory abnormalities, and salivation. Also, the infections cause abortion, stillbirth, and congenital malformation in the fetus, resulting in a significant economic loss in the industrial animal field [5]. Culicoides has a wide range of activities across the globe, and sporadic outbreaks of the disease are serious international problems. Therefore, constant monitoring and control of Culicoides should be required.
Many previous studies have been conducted to control mosquitoes of various species that cause direct damage to humans. Aedes aegypti, as a representative example, is known as a vector of not only Zika virus but also dengue fever and yellow fever viruses [6–8]. Aedes aegypti is widely distributed around the world because they are easily adaptable to a variety of environments, and many studies have been conducted on test substances that could be used to develop control methods. One bioactivity study using plant essential oils suggested that the leaf and bark essential oils of Camellia japonica were an effective larvicide against Aedes aegypti [9]. A previous study by Gillij et al. [10] proposed conditions that showed the most effective repellent activity against Aedes aegypti using aromatic plant essential oils of different species and concentrations. Other studies have also demonstrated that ammonia and carbon dioxide (CO2) were attractants of Aedes aegypti [11,12]. These studies were conducted on mosquitoes such as Culex pipiens molestus and Anopheles stephensi, not just on Aedes aegypti, and many mosquito repellents have been developed based on the results [13,14].
Few attractions or repellent tests have been conducted on Culicoides, which causes significant damage to industrial animals currently. Therefore, we developed a useful device that can be used to investigate the attraction and repellent tendencies of Culicoides and performed attraction and repellent tests using essential oils, cow dung, and CO2.
MATERIALS AND METHODS
Collection of Culicoides biting midges
Culicoides biting midges were collected using a CDC black light trap (SNC, Hanam, Korea) from June to August in cattle farms located in the Jinan (35°51′17.6353″ N; 127°20′19.2646″ E) area, Jeonbuk Province, where the most Culicoides were collected in the previous study [15]. The light trap consisted of an 8 W ultraviolet fluorescent light and had a downdraft suction fan at the bottom. The trap chamber had 4 attachment holes for connecting to the branching chambers. The diameter of branching chamber was 9 cm and height was 7 cm except test material space. Additionally, the branching chamber was divided into an upper space and a lower space by a mesh membrane that prevents Culicoides from touching with the test substances. The diameter of bridge passage was 2 cm and length was 5 cm. The lid holes were closed during collection. The trap was set up in the afternoon before dark and removed the next morning after sunrise.
Preparation of test substances
Cow dung and CO2 were used as potential attractant substances for Culicoides. The fresh cow dung (50 g) was obtained on the day of Culicoides collection, and a dry ice block (about 2 cm3) was used as the CO2 source [16]. To effectively spread CO2, 50 ml of distilled water (DW) was added to a dry ice block shortly before the test.
Three pure (100%) essential oils (lavender, lemongrass, and eucalyptus) were purchased from NOW Food (Bloomingdale, Illinois, USA). Each was formulated as a 0.2% solution (v/v, 100 μl of essential oil in 50 ml of DW).
Movement test of Culicoides biting midges
We made in-house a specially designed mobile chamber to investigate the movement of Culicoides (Korean Patent No. 10-2141006, 2018) [17]. It consisted of a trap chamber where Culicoides were collected and 4 branching chambers in which test material could be placed (Fig. 1A). When collected Culicoides, the trap chamber was brought to the laboratory, test material was put into 2 branching chambers, and DW into the other 2 branching chambers as controls (Fig. 1B). The whole chamber was placed in a dark room for 30 min until the Culicoides moved sufficiently.
After 30 min, each branching chamber was detached from the trap chamber, and frozen for at least 5 hr (Fig. 1C). The Culicoides species among the insects in each chamber were identified under a dissecting microscope and counted [15,18].
The test was repeated 3 times per test substance. The results of each test were calculated to average and presented as the means with standard deviation. The attraction and repellent percentage were calculated in 2 ways using the following formulas. A was the total number of collected Culicoides and B was the total number of Culicoides moved to the test substance. C was the total number of Culicoides moved to DW.
In compared to total collected number,
In compared to only control group except for no moved,
Statistical analysis
The differences between each group were compared with a 2-tailed t-test using Prism version 7.04 (GraphPad Software, San Diego, California, USA). A P value <0.05 was considered to be statistically significant.
RESULTS
Attraction of Culicoides to cow dung or CO2
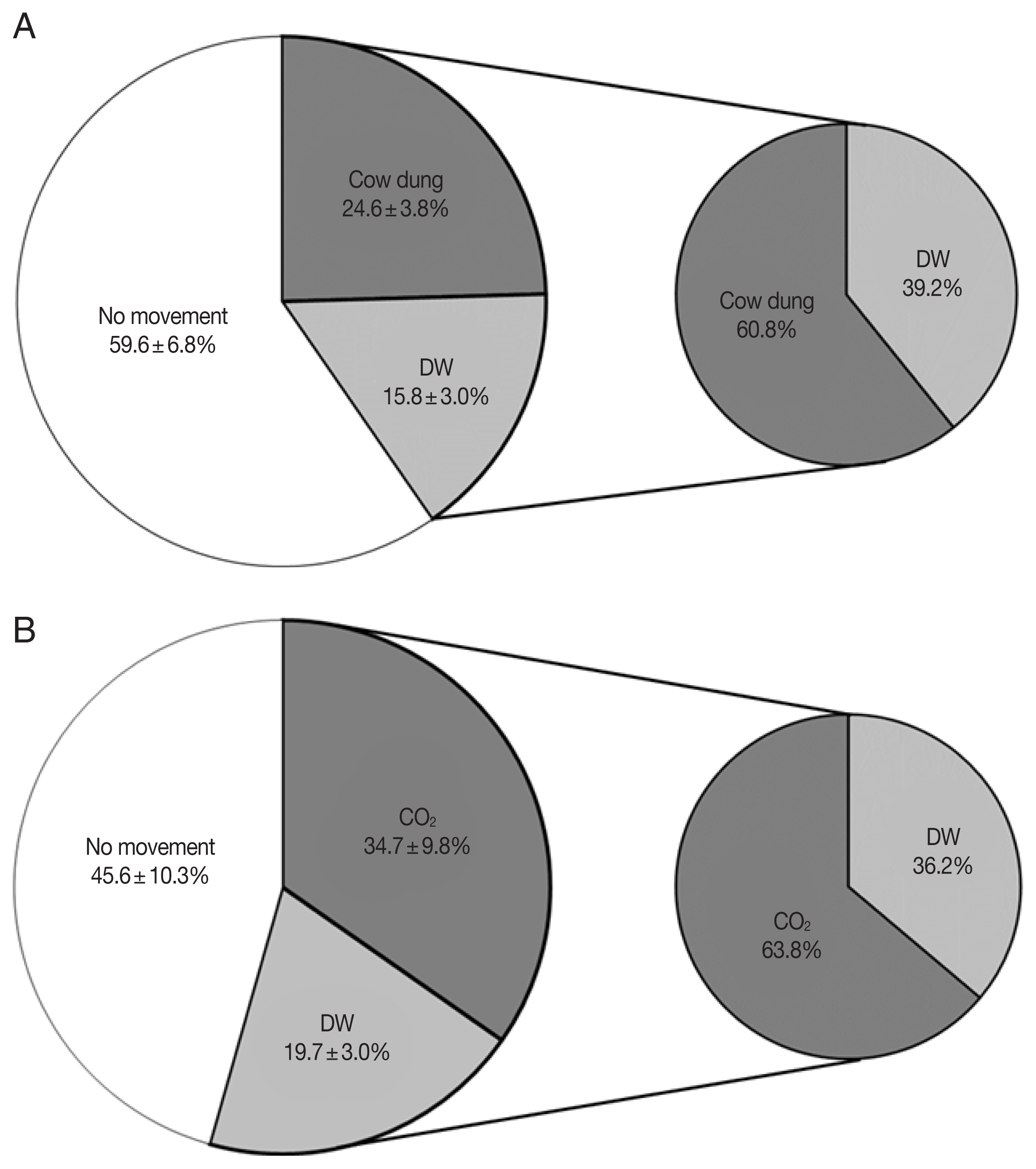
In the cow dung group, 59.6±6.8% (n=164, 98, and 153) of the total collected Culicoides did not move, 24.6±3.8% (n=49, 48, and 72) moved to the cow dung chamber, and 15.8±3.0% (n=30, 31, and 48) moved to the DW chamber (Fig. 2A). As a result of comparing the movement rate between DW and cow dung, a mean of 60.8% (P<0.0001) of the Culicoides moved to the cow dung chamber and a mean of 39.2% moved to the DW chamber.
In the CO2 group, 45.6±10.3% (n=62, 50, and 65) of the Culicoides stayed in the trap chamber without movement, 34.7±9.8% (n=78, 37, and 30) moved to the CO2 chamber, and 19.7±3.0% (n=32, 26, and 20) moved to the DW chamber (Fig. 2B). Excluding the Culicoides that did not move, a mean of 63.8% (P<0.01) of the Culicoides moved to the CO2 chamber and 36.2% moved to the DW chamber.
Culicoides tended to move to cow dung (1.6 times) and CO2 (1.8 times) more than DW and showed more active movement to CO2 than to cow dung.
Repellent behavior of Culicoides to essential oils
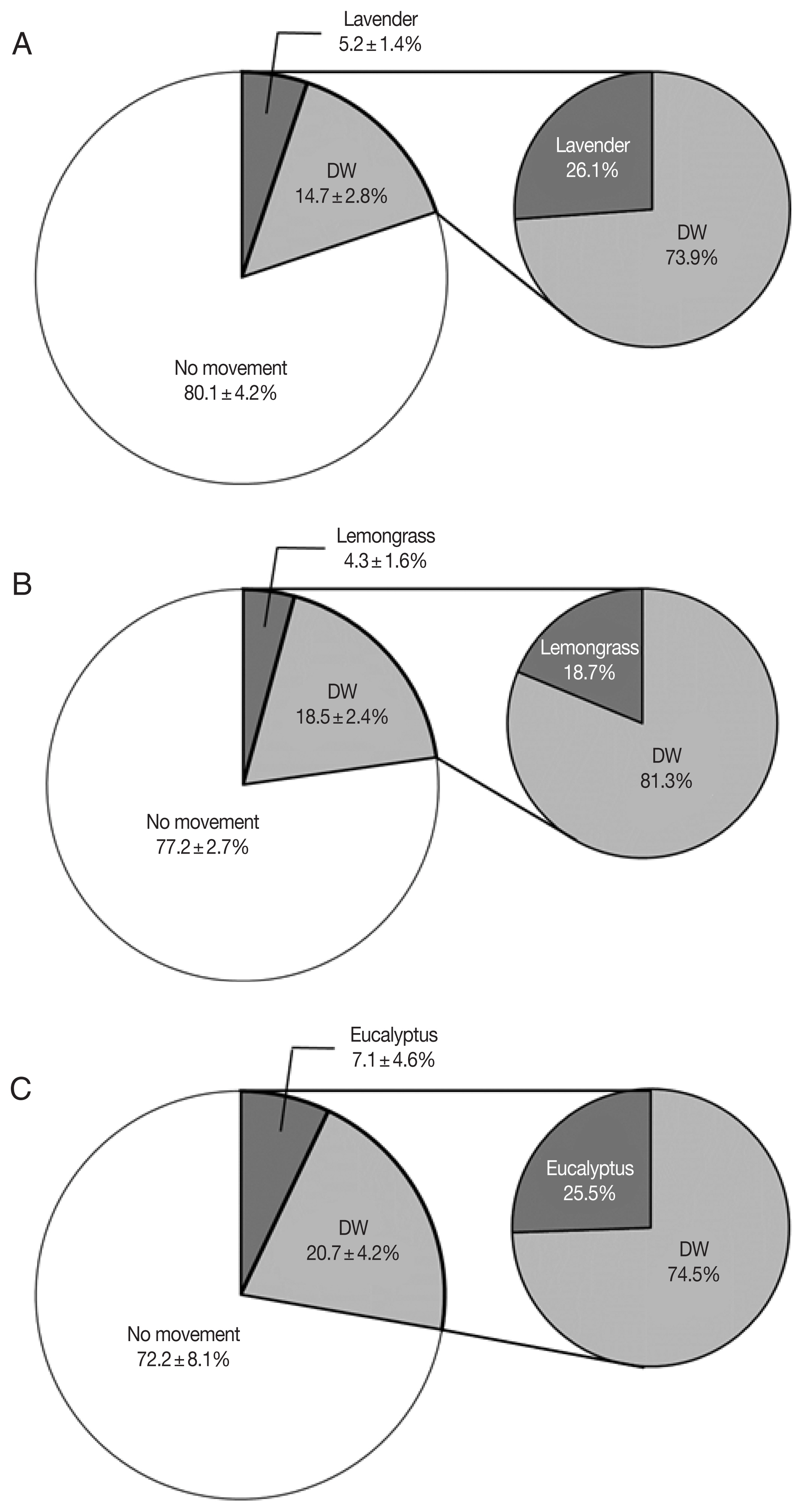
In the lavender oil group, 80.1±4.2% (n=475, 444, and 440) of the collected Culicoides did not move but 14.7±2.8% (n=96, 60, and 95) moved to the DW chamber and 5.2± 1.4% (n=34, 19, and 36) moved to the lavender oil chamber (Fig. 3A). A mean of 73.9% among the moved Culicoides moved to the DW chamber and a mean of 26.1% (P<0.0001) moved to the lavender oil chamber.
In the lemongrass oil group, 77.2±2.7% (n=403, 209, and 198) of the collected Culicoides did not move but 18.5±2.4% (n=81, 58, and 48) moved to the DW chamber and 4.3± 1.6% (n=26, 15, and 6) moved to the lemongrass oil chamber (Fig. 3B). Comparing the movement rate between DW and lemongrass oil, a mean of 81.3% moved to the DW chamber but a mean of 18.7% (P<0.001) moved to the lemongrass oil chamber.
Lastly, in the eucalyptus oil group, 72.2±8.1% (n=141, 130, and 255) of the collected Culicoides stayed in the trap chamber without movement, but 20.7±4.2% (n=45, 47, and 50) moved to the DW chamber and 7.1±4.6% (n=10, 25, and 12) moved to the eucalyptus oil chamber (Fig. 3C). Excluding the Culicoides that did not move, a mean of 74.5% moved to the DW chamber, and a mean of 25.5% (P<0.01) moved to the eucalyptus oil chamber.
The movement of Culicoides based on 3 essential oils showed that about 76.6±4.0% of the total collected Culicoides did not move at all, and a few Culicoides moved to lavender, lemongrass, and eucalyptus oil at 5.2±1.4%, 4.3±1.6%, and 7.1±4.6%, respectively. In addition, the Culicoides that moved to the 3 essential oil chambers showed markedly low activity.
DISCUSSION
Previous studies have conducted attraction or repellent tests using various essential oils and components in mosquitoes that directly attack humans such as the Culex and Aedes genera. We focused on the significance of Culicoides in the industrial animal field and conducted tests specific to Culicoides. In this study, Culicoides tended to prefer CO2 and cow dung more than DW, the control group. Dense breeding of a large number of ruminants in a small space reduces the activity radius of the animal and increases the temperature inside the farms, which can generate a large amount of CO2 by increasing the respiration rate. To prevent intensive access to Culicoides, it is necessary to maintain a pleasant environment by appropriately controlling the number of livestock in a particular breeding ground. In addition, regular cleaning of cow dung may reduce the spread of ammonia, which mosquitoes prefer. This study was evaluated by excluding the characteristics of Culicoides biting midge that exist in various habitats depending on species [1]. Therefore, it seems that more efficient prevention is possible if the attraction behavior test according to species is investigated.
Investigations of the attraction or repellent tendencies of mosquitoes using test substances have been conducted in a variety of ways. Newhouse et al. [19] compared the number of mosquitoes collected by installing light traps with or without dry ice at the same time. Several studies have evaluated the efficacy of test substances by counting the number of bites after applying a test substance or control substance directly to the human forearm or mouse skin [20–22]. However, it may be difficult to obtain a constant result in an outdoor environment using these methods and it is hard to rear mosquitoes in the laboratory. In our study, we developed a convenient device for conducting Culicoides collection and attraction or repellent tests at the same time. It is possible to directly investigate attraction or repellent tendencies by connecting the branching chambers containing the test substance on the collection day, without transferring the Culicoides that are collected. The concentration of the test substance should be high enough to allow Culicoides to notice it and move. In this study, we initially had used essential oils at concentration of 1%. That concentration was too high to test because the scent spread even to the control chamber and Culicoides did not move to any chamber. Therefore, the experiment was carried out by modifying the final concentration to 0.2% through a concentration-determining experiment. As indicated above, proper concentration settings are important and the conditions suitable for the experiment should be established. Additionally, many Culicoides did not move even though the appropriate concentration was used. It is thought that they did not move simply because they stayed in the unfamiliar environment. Comparing the Culicoides immobility rate between the attraction and the repellent behavior test, the percentage was larger in the repellent test than in the attraction test. It is thought that the immobility rate in the repellent test may be higher than that in the attraction behavior test because Culicoides did not prefer the odor of essential oils. In the attraction behavior test using a substance preferred by Culicoides, it is supposed that the movement rate was higher than in the repellent test since they were more likely to recognize the odor and moved to test substance actively.
Due to the toxicity and environmental risk of N, N-diethyl-m-toluamide (DEET), the most effective and widely used insect repellent [23,24], the development of harmless natural repellents for humans and animals is increasing. This study confirmed that lavender, lemongrass, and eucalyptus essential oils have repellent effects on Culicoides. Plant-derived natural essential oils are harmless when applied to human skin with proper condition (concentration, ratio, or pH) [25–27]. Even for natural materials, it is important to use with a dose that does not cause irritation or side effects when applied to humans or animals. Since these are only a few of the essential oils known to have mosquito repellent effects, we expect that further research can be conducted on the repellent effects of various essential plant oils. The development of non-toxic natural repellents using essential oils that have been found to have repellent effects against Culicoides will effectively prevent Culicoides access and the spread of epizootic diseases.