Abstract
Excretory-secretory products (ESP) of T. vaginalis have been shown to inhibit sperm motility, viability, and functional integrity, leading to a decreased fertilization rate in vitro. This study investigated whether T. vaginalis induce apoptosis and ultrastructural changes of sperm using flow cytometry and electron microscopy. Incubation of sperm with T. vaginalis ESP increased phosphatidylserine externalization and DNA fragmentation, and decreased mitochondrial membrane potential. Transmission electron microscopy of sperm incubated with ESP revealed abnormal features such as distorted heads, broken necks, and acrosomes exocytosis. This is the first report that demonstrates a direct impact of T. vaginalis ESP on sperm apoptosis and architecture in vitro.
-
Key words: Trichomonas vaginalis, excretory-secretory products, apoptosis, sperm
Trichomonas vaginalis infection is recognized as an important cause of infertility in men as well as women [
1,
2]. Many clinical studies have demonstrated decreased sperm motility and viability in men infected with
T. vaginalis [
3–
7]. We previously demonstrated that
T. vaginalis excretory-secretory products (ESP), which are composed of extracellular polymeric substances, reduced sperm motility, viability, and functional integrity, thus leading to a decreased fertilization rate in vitro [
8]. The present study examined the impact of the ESP on the apoptosis of mouse spermatozoa.
Mouse sperm were obtained from 8- to 10-week-old 12 male ICR mice (Samtako Biokorea, Osan, Korea) and prepared as previously described [
8]. Animal care followed institutional guidelines, and the Hanyang University IACUC approved all procedures, including animal experiments (HY-IACUC-090043). ESP were prepared as previously described [
8]. Briefly, ESP were collected after incubating
T. vaginalis (T016 isolate), which was kindly provided by Prof. John F. Alderete (Department of Microbiology, University of Texas Health Science Center at San Antonio, Texas, USA) for 1 h in 1 ml of Hank’s balanced salt solution (HBSS, pH 7.2) (Gibco, New York, New York, USA).
To investigate the effect of ESP on apoptosis of spermatozoa in vitro, sperm was prepared as follows. After exposure of the peritoneal cavity, the cauda epididymis was removed and washed immediately in prewarmed 1ml of collection medium (Whitten’s HEPES-buffered medium), and transferred to a 200 μl drop of human tubal fluid (HTF) medium (Quinn’s Advantage Fertilization, In-vitro Fertilization Inc, Trumbull, Connecticut, USA) containing 10% fetal bovine serum in a 35 mm culture dish equilibrated overnight under embryo-tested mineral oil in 5% CO2 humidified atmosphere at 37°C. Sperm were gently squeezed out of the epididymis using a 26-gauge needle, and the residual caudal tissue was discarded. The sperms were allowed to disperse for 15 min and large aggregations of immotile sperm in the culture drops were removed under a dissecting microscope. Aliquots of the sperm suspension (1.5×108 per ml) were incubated in the presence or absence of ESP for 3 h in a 37°C CO2 incubator and labelled with a fluorescence probe to detect apoptotic changes. The fluorescence signals of labelled sperm were evaluated by flow cytometry (FACSCalibur; BD Bioscience, San Jose, California, USA). A minimum of 20,000 events were measured for each sample at a flow rate of 200 to 300 events sec-1 using CellQuest pro software (BD Bioscience).
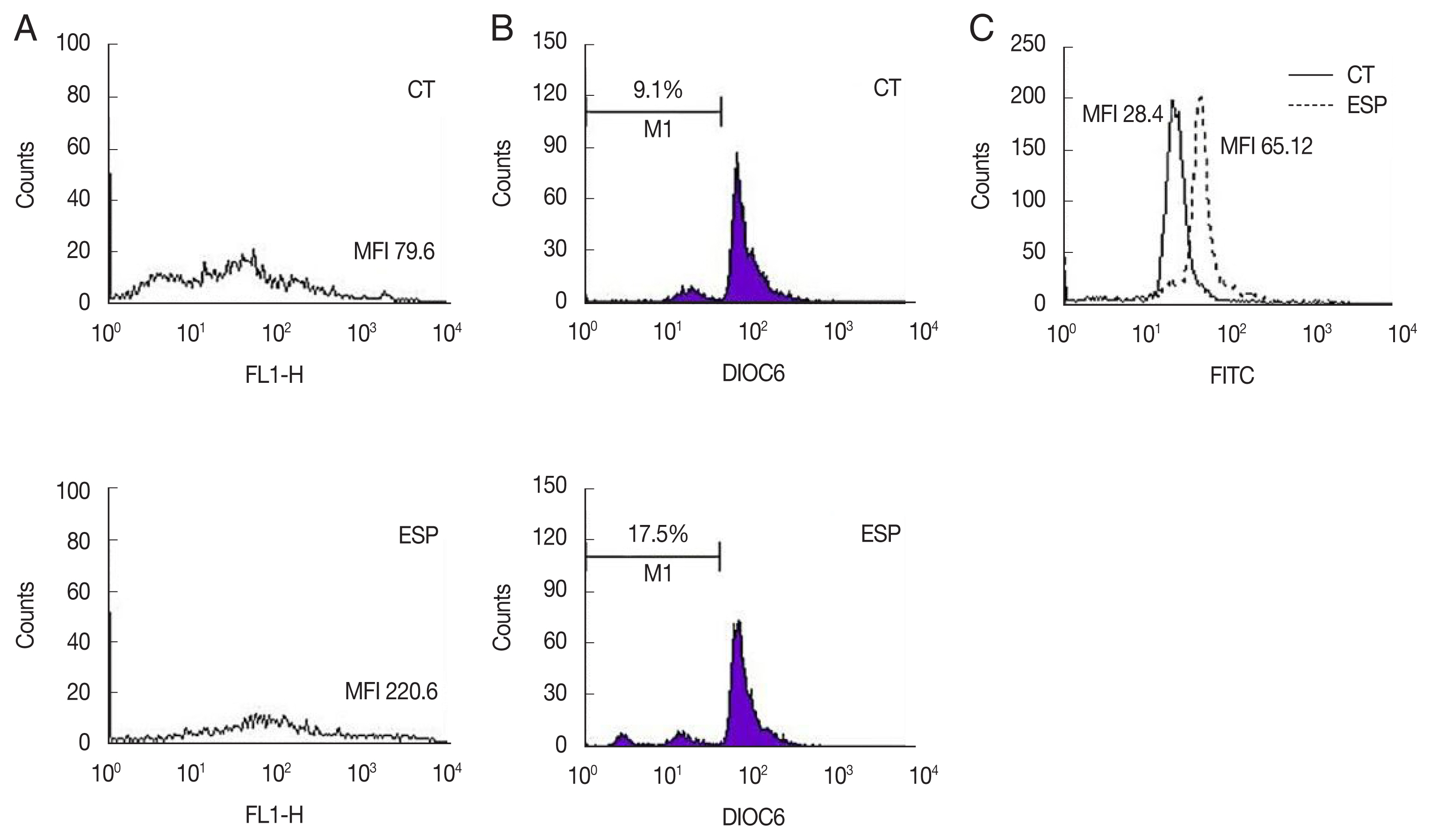
Since the release of phosphatidylserine (PS) from the plasma membrane is a hallmark of apoptosis [
9], structural changes of the plasma membrane of spermatozoa were analyzed with an Annexin V-FITC apoptosis detection kit (BD Bioscience). The proportion of spermatozoa with PS externalization was markedly increased in the ESP-exposed group (ESP) compared to the control (CT) (MFI; CT, 79.6 vs. ESP, 220.6) (
Fig. 1A). It has been reported that many factors including infection and toxins can alter the intermembrane permeability of mitochondria, triggering the intrinsic mitochondrial pathway of apoptosis [
10]. To assess the integrity of the mitochondrial membrane potential (MMP), the green fluorescence (FL-1 channel) of sperm stained with fluorescein isothiocyanate (FITC)-conjugated 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes, Eugene, Oregon, USA) was evaluated. ESS increased the proportion of spermatozoa with low MMP compared to the control (CT, 9.1% vs. ESP, 17.5%) (
Fig. 1B). As loss of sperm motility is closely correlated with decreased MMP integrity [
11], low MMP induced by ESP can explain how ESP decreases sperm motility. On the other hand, integrity of sperm DNA has been associated with male fertility in vivo and in vitro [
12]. Damage to DNA, such as by fragmentation and denaturation, can cause infertility [
13]. We therefore evaluated sperm DNA integrity using Terminal deoxynucleotidyl transferase-mediated dUTP Nick End Labelling (TUNEL) assays (Roche Diagnostics, Mannheim, Germany).
Fig. 1C showed that ESP increased DNA fragmentation (MFI; CT, 28.4 vs. ESP, 65.12).
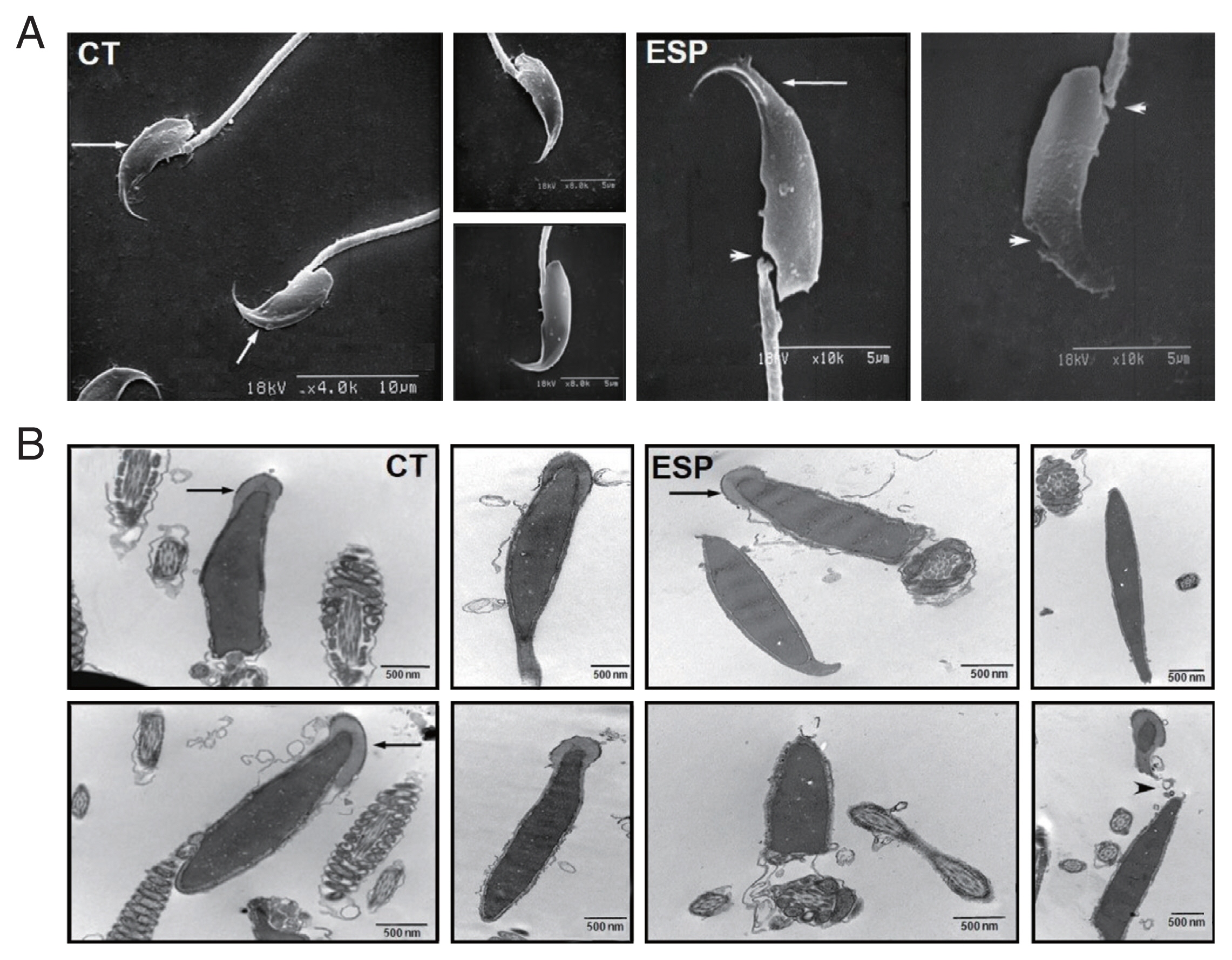
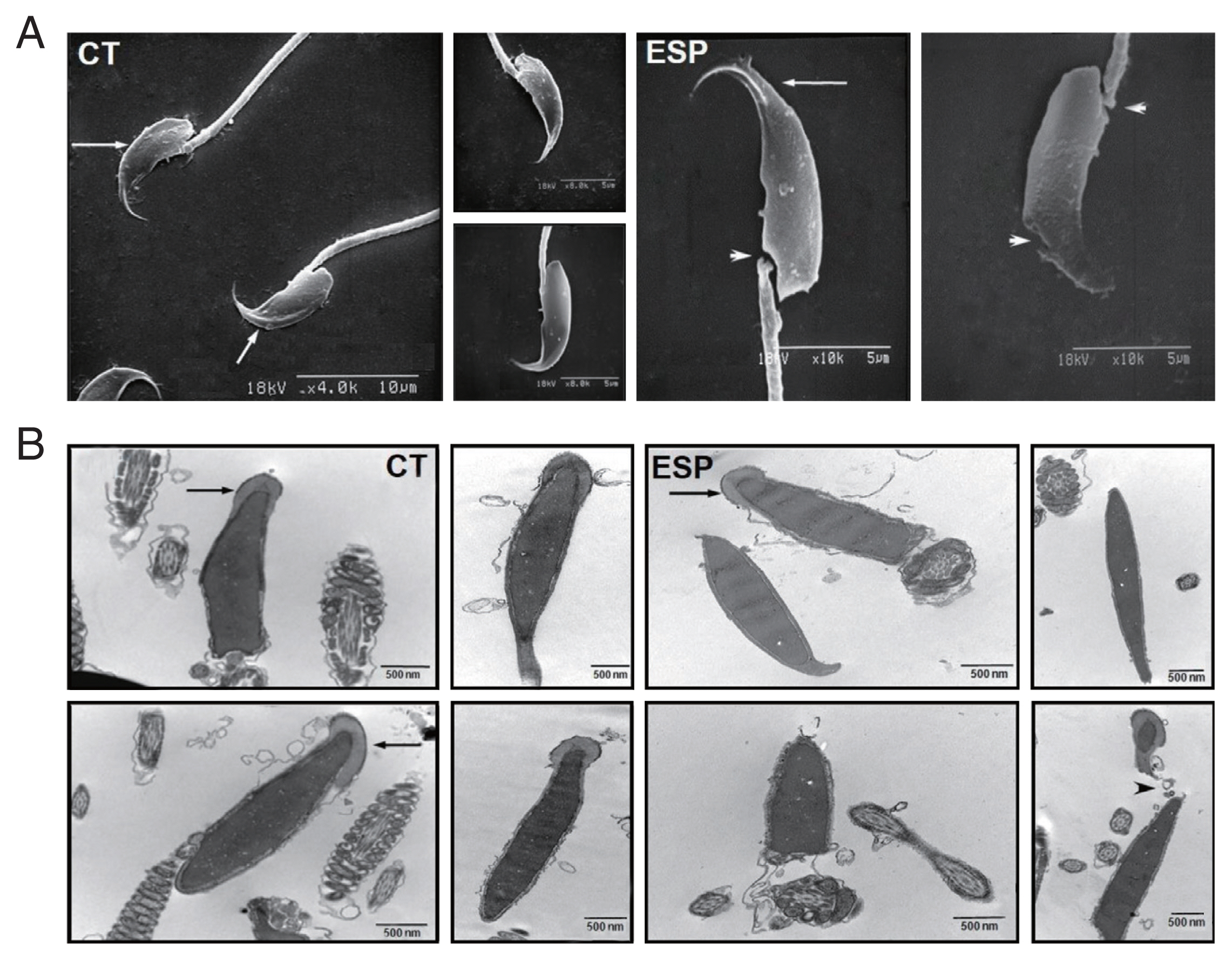
To further evaluate the effects of ESP on sperm, we examined their external and internal architecture after 3 h incubation with ESP using a scanning electron microscope (SEM; S-2380N, Hitachi, Tokyo, Japan) and a transmission electron microscope (TEM; Hitachi H-7600S, Hitachi). Ultrathin sections of the sperm were prepared as previously described [
14]. As shown in
Fig. 2, spermatozoa incubated with ESP had an abnormal appearance, with damaged or distorted heads and necks. The acrosome exocytosis was also evident. Considering that sperm membrane integrity and intact acrosomes are strongly associated with fertilization rate [
15], our results show how
T. vaginalis infection induces infertility and subfertility in women and men.
Many previous studies have concentrated on direct contact-dependent effects of
T. vaginalis on sperm [
16–
18], while little information has been available on the effects of ESP on sperm apoptosis. Apoptosis is an important and well-regulated form of cell death that occurs under a variety of physiological and pathological conditions [
14].
T. vaginalis ESP has also been shown to induce apoptosis in human vaginal epithelial cells [
19]. This study provides evidence that
T. vaginalis ESP can induce sperm apoptosis in vitro. If this phenomenon also occurs in vivo, it should lead to a decrease in the number of spermatozoa and a decline in fertility. Indeed, testicular trichomoniasis has been known to lead to azoospermia [
20]. A series of our studies employing
T. vaginalis ESP broadens insight at the subcellular levels into how
T. vaginalis infection causes infertility and subfertility in humans.
Notes
-
The authors declare no conflict of interest related to this study.
ACKNOWLEDGMENT
This work was supported by the research fund of Hanyang University (HY-2017).
Fig. 1Effect of T. vaginalis ESP on apoptosis of mouse sperm. Mouse sperms were treated with ESP for 3 h, and then used for the following assays. (A) Annexin V-PI staining, CT (upper panel); ESP (lower panel). (B) Determination of mitochondrial membrane potentials using FITC-conjugated DiOC6. CT (upper panel); ESP (lower panel). (C) DNA fragmentation by TUNEL assays. Solid and dotted lines indicate CT and ESP, respectively. Data are representative of 3 independent experiments. (CT, control; ESP, T. vaginalis excretory-secretory products-treated group)
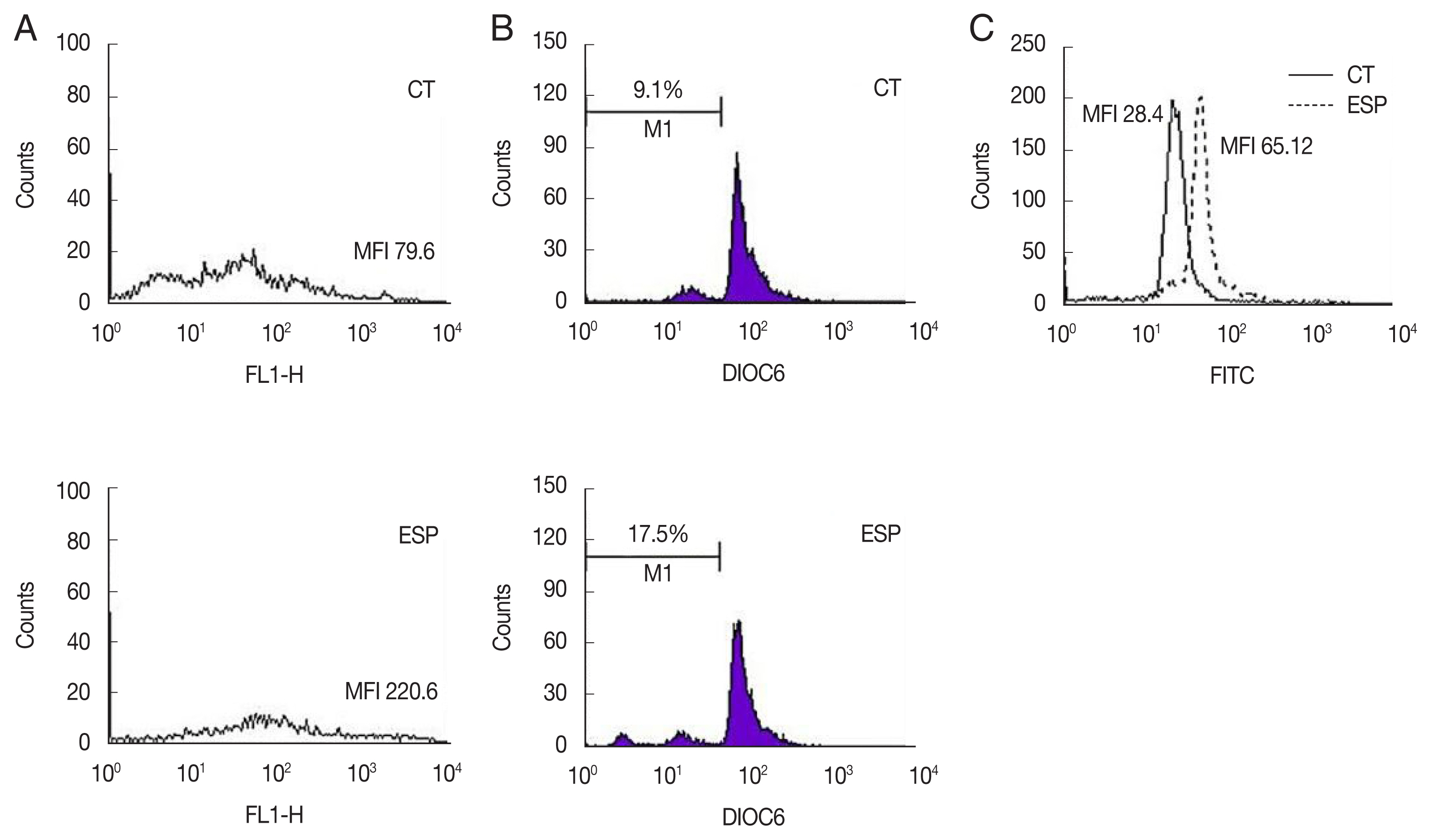
Fig. 2Effect of T. vaginalis ESP on ultrastructure of mouse sperm. Scanning electron micrographs of sperm (A) incubated in the absence of ESP (CT, left panel) or in the presence of ESP (ESP, right panel). Arrows indicate intact acrosome and arrowheads indicate injured head or neck. Transmission electron micrographs of sperm incubated (B) in culture medium (CT, left panel) or ESP (right panel). Arrows indicate acrosome and arrowheads depict acrosome exocytosis and head destruction. Data are representative of 3 biological replicates. (CT, control; ESP, T. vaginalis ESP-treated group)
References
- 1. Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect 2013;89:418-422. https://doi.org/10.1136/sextrans-2013-051075
- 2. El-Shazly AM, El-Naggar HM, Soliman M, El-Negeri M, El-Nemr HE, Handousa AE, Morsy TA. A study on Trichomoniasis vaginalis and female infertility. J Egypt Soc Parasitol 2001;31:545-553.
- 3. Soper D. Trichomoniasis: under control or undercontrolled? Am J Obstet Gynecol 2004;190:281-290. https://doi.org/10.1016/j.ajog.2003.08.023
- 4. Gopalkrishnan K, Hinduja IN, Anand Kumar T. Semen characteristics of asymptomatic males affected by Trichomonas vaginalis. J In Vitro Fert Embryo Transf 1990;7:165-167. https://doi.org/10.1007/BF01135682
- 5. Tuttle JP Jr, Holbrook TW, Derrick FC. Interference of human spermatozoal motility by Trichomonas vaginalis. J Urol 1977;118:1024-1025. https://doi.org/10.1016/s0022-5347(17)58285-3
- 6. Jarecki-Black JC, Lushbaugh WB, Golosov L, Glassman AB. Trichomonas vaginalis: preliminary characterization of a sperm motility inhibiting factor. Ann Clin Lab Sci 1988;18:484-489.
- 7. Hobbs MM, Kazembe P, Reed AW, Miller WC, Nkata E, Zimba D, Daly CC, Chakraborty H, Cohen MS, Hoffman I. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex Transm Dis 1999;26:381-387. https://doi.org/10.1097/00007435-199908000-00003
- 8. Roh J, Lim YS, Seo MY, Choi Y, Ryu JS. The secretory products of Trichomonas vaginalis decrease fertilizing capacity of mice sperm in vitro. Asian J Androl 2015;17:319-323. https://doi.org/10.4103/1008-682X.145070
- 9. Han Q, Liu J, Wang T, Xiao H, Fang Z. Influence of the metabolite produced by Trichomonas vaginalis on human sperm motility in vitro. National J Androl 2004;10:272-274. (in Chinese).
- 10. Kranjčić-Zec I, Džamić A, Mitrović S, Arsić-Arsenijević V, Radonjić I. The role of parasites and fungi in secondary infertility. Med Pregl 2004;57:30-32. (in Serbian). https://doi.org/10.2298/mpns0402030k
- 11. Grunewald S, Said T, Paasch U, Glander HJ, Agarwal A. Relationship between sperm apoptosis signalling and oocyte penetration capacity. Int J Androl 2008;31:325-330. https://doi.org/10.1111/j.1365-2605.2007.00768.x
- 12. Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril 2001;75:674-677. https://doi.org/10.1016/s0015-0282(00)01796-9
- 13. Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 2003;9:331-345. https://doi.org/10.1093/humupd/dmg027
- 14. Bellamy CO, Malcolmson RD, Harrison DJ, Wyllie AH. Cell death in health and disease: the biology and regulation of apoptosis. Semin Cancer Biol 1995;6:3-16. https://doi.org/10.1006/scbi.1995.0002
- 15. Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984;70:219-228. https://doi.org/10.1530/jrf.0.0700219
- 16. Mielczarek E, Blaszkowska J. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection 2016;44:447-458. https://doi.org/10.1007/s15010-015-0860-0
- 17. Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod 2000;15:1338-1344. https://doi.org/10.1093/humrep/15.6.1338
- 18. Sharma R, Masaki J, Agarwal A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol Biol 2013;927:121-136. https://doi.org/10.1007/978-1-62703-038-0_12
- 19. Sommer U, Costello CE, Hayes GR, Beach DH, Gilbert RO, Lucas JJ, Singh BN. Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J Biol Chem 2005;280:23853-23860. https://doi.org/10.1074/jbc.M501752200
- 20. Gong YH, Liu Y, Li P, Zhu ZJ, Hong Y, Fu GH, Xue YJ, Xu C, Li Z. A nonobstructive azoospermic patient with Trichomonas vaginalis infection in testes. Asian J Androl 2018;20:97-98. https://doi.org/10.4103/1008-682X.195561
Citations
Citations to this article as recorded by

- The possible pathogenic mechanisms of microorganisms in infertility: a narrative review
Zahra Chegini, Amin Khoshbayan, Milad Kashi, Raha Zare Shahraki, Mojtaba Didehdar, Aref Shariati
Archives of Microbiology.2025;[Epub] CrossRef