Abstract
We report a species of diplostomid fluke recovered from 3 carcasses of wild Korean raccoon dog, Nyctereutes procyonoides koreensis, in Korea. A total of 107 diplostomid flukes were recovered from the small intestines of Korean raccoon dogs, which were obtained from the Gangwon Wildlife Medical Rescue Center. Worms fixed with 10% neutral formalin were subjected to microscopic observation and those fixed in 70% ethanol were used for molecular genomic analysis. The worm was divided into 2 separate parts, forebody and hindbody, with a total length of 3,020–4,090 (3,855) μm and a width of 1,210–1,770 (1,562) μm. The boat-shaped forebody has a pair of characteristic tentacular appendage, 2 suckers, holdfast organ, and vitelline follicles. The oval to cylindrical hindbody has reproductive organs. The ovary was round or elliptical and located in the anterior of the testes. Two large testes were slightly segmented and tandemly arranged, occupying almost half of hindbody. The short uterus contained a relatively small number of unembryonated eggs sized 130–140×85–96 μm. The partial sequence of 18S rRNA of this fluke was consistent with Alaria alata. Based on the morphological and molecular characteristics, the diplostomid flukes recovered from the small intestine of Korean raccoon dogs were identified as A. alata (Digenea: Diplostomidae).
-
Key words: Alaria alata, Nyctereutes procyonoides koreensis, Korea
The trematode
Alaria alata (Goeze, 1782) is cosmopolitan parasite and commonly found in various wild carnivores that feed in water-rich areas such as ponds and lakes [
1]. Their life cycle requires 2 intermediate hosts. The first intermediate host is a freshwater snail such as
Helistoma, Planorbis, Lymnea, and
Anisus spp., and the second is an amphibian such as a frog [
2]. In addition, many species of omnivorous and carnivorous mammals can also act as paratenic hosts [
3,
4]. In particular, the wild boar is known as the most common paratenic host [
4].
A. alata is considered a potential cause of a human disease called alariosis, which is spread through game meat infected with the infective mesocercariae. Alariosis is a food-borne zoonotic helminthiasis [
5].
The typical definitive hosts for
A. alata would be its wild predators; raccoon dog (
Nyctereutes procyonoides) is one of main species. Raccoon dogs have successfully settled in Europe, and their numbers are increasing. As a result, they have the potential to pose a threat to human and animal health [
6]. There are 5 subspecies of raccoon dogs, among which Korean raccoon dog (
Nyctereutes procyonoides koreensis) is widely distributed in this country. As in European countries, in Korea, raccoon dogs can pose a threat to public health as they have many opportunities to encounter humans.
The prevalence of
A. alata in raccoon dog has been reported in Belarus, Estonia, Lithuania, Denmark, and Latvia, with prevalence rates ranging from 32.9% to 91.6% in each country [
6–
10]. However, only 1 study has been done on
A. alata in Korea.
A. alata was detected in 1 out of 30 Korean raccoon dogs, but morphological and molecular characteristics were lacking [
11].
From December 2019 to February 2020, 3 deceased Korean raccoon dogs were found in 3 sites in Gangwon-do (province): Manjong-ri (village) (37°20′59.8″N, 127°54′13.1″E), Hojeo-myeon (township), Wonju-si (city); Jung-ri (37°22′22.5″N, 128°24′27.1″E), Pyeongchang-eup (town), Pyeongchang-gun (county), Uiam-ri (37°49′57.0″N, 127°40′45.3″E), Sindong-myeon, Chuncheon-si. They were transferred to the Gangwon Wildlife Medical Rescue Center in Kangwon National University. The raccoon dogs were autopsied, and a total of 107 diplostomid flukes (18, 31, and 58 per raccoon dog) were recovered from the contents of the small intestine. Before the fixation of flukes, we took photograph of flukes stored in saline (
Fig. 1A). A part of the flukes was fixed in 10% neutral formaldehyde without flattening procedure, after which stained with Semichon’s acetocarmine. The 20 flukes were fixed in 70% ethyl alcohol for the molecular study.
Genomic DNA was extracted from the parasite using the G-spin Total DNA Extraction Mini Kit (INTRON Biotechnology, Seoul, Korea) according to the manufacturer’s instructions. Based on the 18S ribosomal RNA gene sequences of
A. alata (AY222091 and JF769484), primers were designed using the online tool Primer3Plus (
http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). The oligonucleotide sequences of primers were as follows: 5′-CCTGTTGCACTCTGTGATG-3′ (forward primer for AY222091), 5′-GCTAAAACAGCACCAATCC-3′ (reverse primer for AY222091), and 5′-TAGGTGAACCTGCGGAAG-3′ (forward primer for JF769484), 5′-ACCAAAGAGGAACATGACG-3′ (reverse primer for JF769484). The primer sets were designed to yield products of 1,628 bp and 985 bp, respectively. PCR was performed using a MyCycler Personal Thermal Cycler (Bio-Rad Laboratories, Hercules, California, USA) and EmeraldAmp GT PCR Master Mix (Takara, Kusatsu, Shiga, Japan) with 1 μl of DNA aliquot under standard conditions of 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 1 min. The PCR products were visualized via electrophoresis on 1.2% agarose gel, and then purified using the QIAquick PCR Purification Kit (Qiagen, Alameda, California, USA). PCR amplicons were directly sequenced using the ABI Prism Big Dye terminator ver. 3.0 ready reaction cycle sequencing kits (Applied Biosystems, Foster City, California, USA) with the use of the same primers used in PCR. After trimming the nonspecific sequencing data, we obtained 1,559 bp and 901 bp sequences, respectively. A phylogenetic tree was constructed by the Neighbor-Joining (NJ) method using the BLAST tree. The NJ method was based on a guide tree for pairwise and multiple alignment parameters, including 0.75 Max sequence difference [
12]. Different statistical method (Bootstrap consensus tree) was conducted in MEGA 11 [
13]. In bootstrap consensus tree, branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test 1,000 replicates are shown next to the branches.
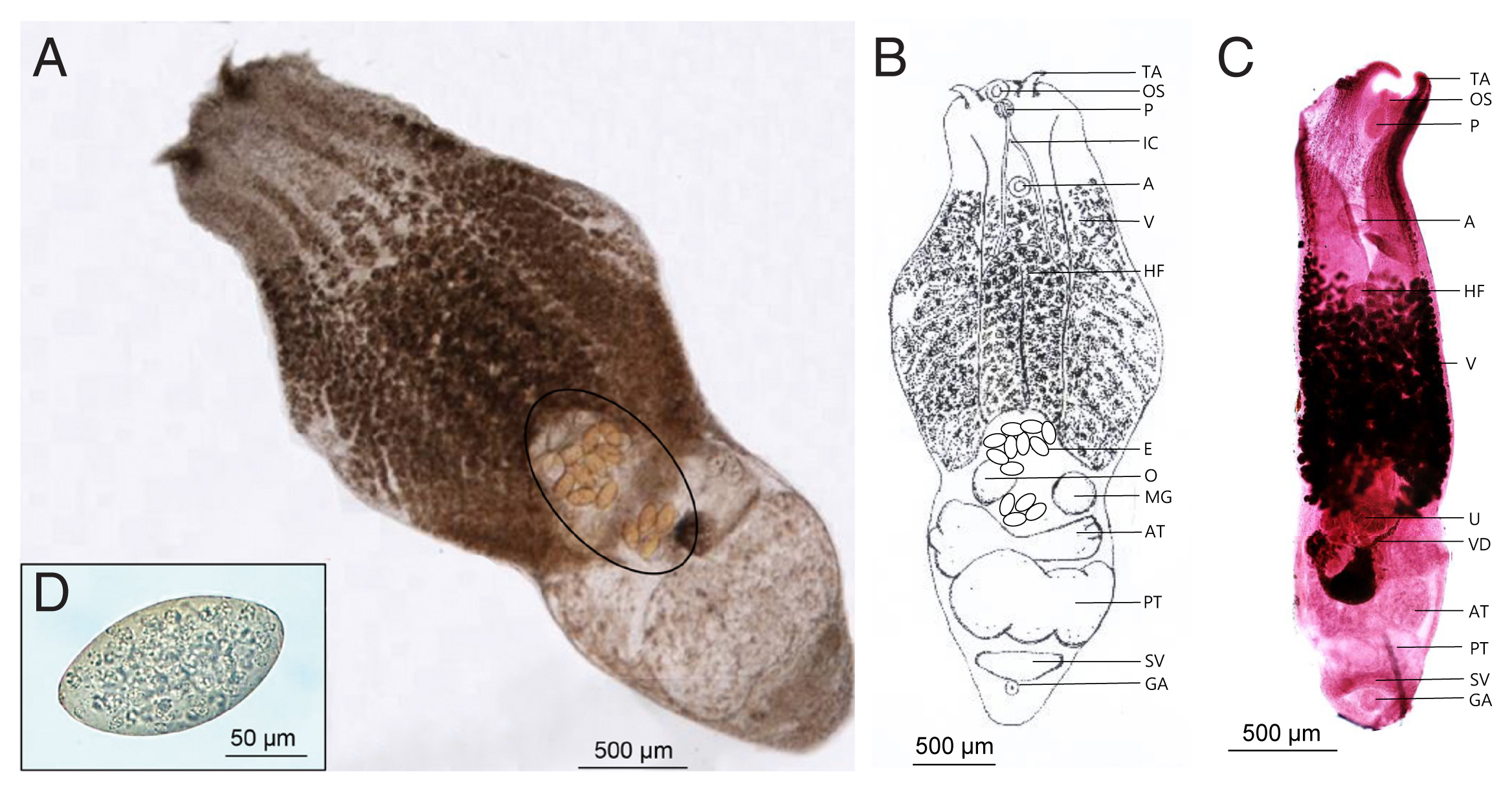
Fig. 1B, C showed the morphological characteristics of the flukes. The worms were distinctly bisegmented with a long boat-shaped forebody and a short cylindrical hindbody with an overall length of 3,020–4,090 (3,855)×1,210–1,770 (1,562) μm. The oral sucker located at the anterior end of the body measured 90–107 (104.5)×91–98 (95.7) μm, almost identical to that of the ventral sucker, 95–109 (103.2)×95–103 (98.8) μm. A pair of distinctive tentacular appendages were present on either side of the oral sucker. They had a well-developed pharynx, 910–1,120 (1,098)×530–630 (583) μm-long holdfast organ, and a short esophagus. Vitellaria extended slightly from the acetabulum to the hindbody and was distributed in the ventral median line. The cylindrical hind body contained reproductive organs. Two testes, much wider than long, were tandem and slightly segmented. The anterior testis was 353–732 (471.2)×245–295 (273.4) μm in size, smaller than the posterior testis sized 366–918 (601.9)×265–310 (297.5) μm. Ovary was located anterior to the testis and measured 95–115 (107.7)×85–110 (101.8) μm. The uterus was short and contained a small number of relatively large non-embryonated eggs (130–140 μm in length and 85–96 μm in width). The seminal vesicles were located posterior to the testes. The genital pore and atrium were located behind the seminal vesicle and were covered with gonotyle.
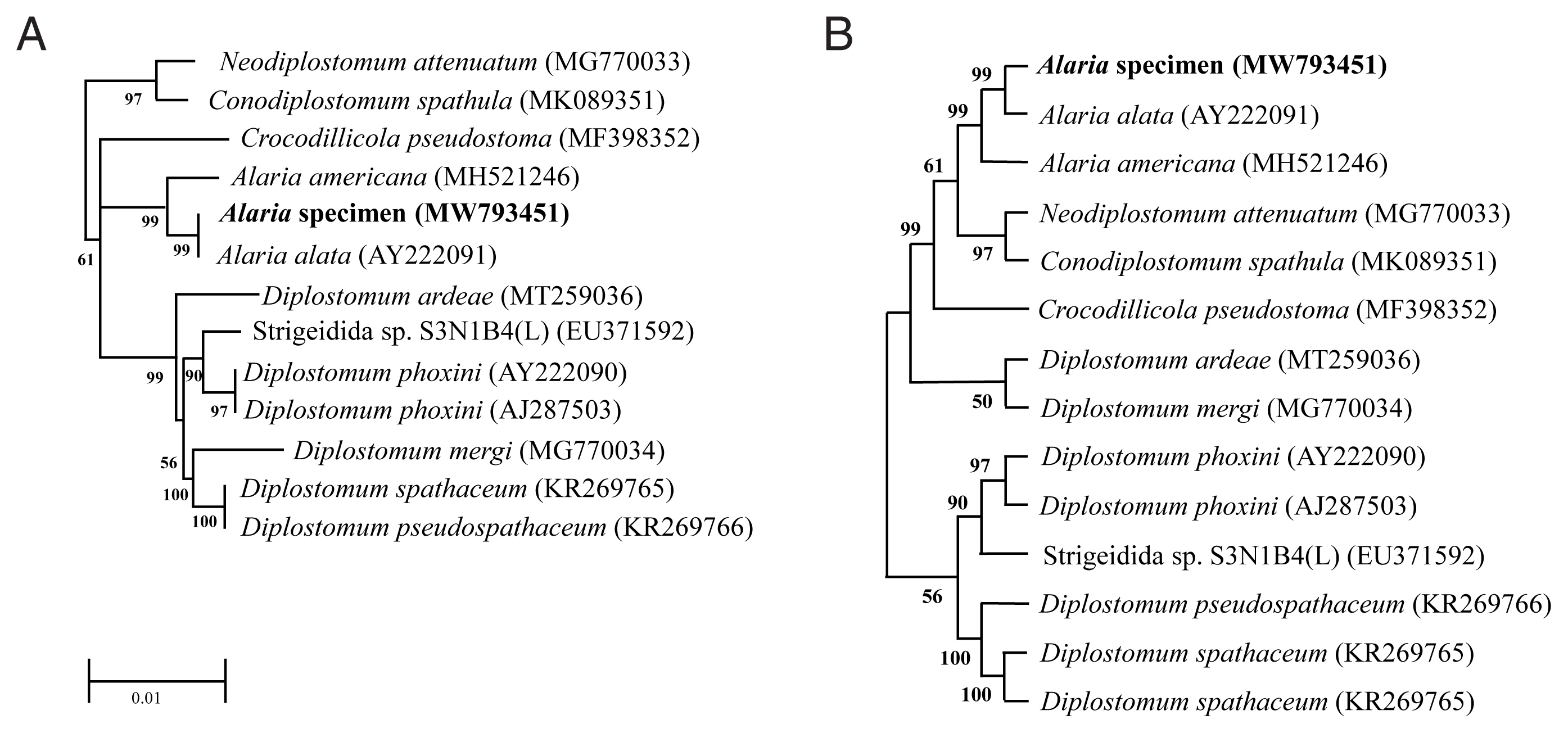
Genotype and phylogenetic relationships of this fluke with
A. alata or Diplostomidae species were determined using the molecular characteristics of the 18S rRNA gene. We registered the sequence information into GenBank under accession numbers MW793451 and MZ057614. As shown in
Fig. 2, the 18S rRNA sequence of
Alaria specimen was compared to those of other parasites, including
A. alata (AY222091) and
A. americana (MH521246). The sequence of our specimen (MW793451) was identical to that of
A. alata (AY222091) (
Fig. 2A). Phylogenetic relationships were presented in the topology as distance matrix (scale bar). Furthermore, another sequence of our specimen (MZ057614) contained 24 variable sites of sequences that belonged to the same clade as that of
A. americana (MH521246) (
Fig. 2B). The heuristic search tree was obtained automatically by applying NJ and BioNJ algorithms to a matrix of pairwise distances estimated by the Maximum Composite Likelihood (MCL) (
Fig. 2B). We analyzed the topology with superior log likelihood value using 14 nucleotide sequences. Branches corresponding to partitions reproduced from less than 50% bootstrap replicates were collapsed. In the bootstrap test 1,000 replicates, the percentage of replicate trees in which the associated taxa clustered together was shown next to the branch (
Fig. 2B). We eliminated all positions containing gaps and missing data for evolutionary analyses and used 8,300 positions in the final dataset. Based on the evolutionary analysis, adults in the genus
Alaria Schrank (1788) discovered in the small intestine of Korean raccoon dogs were identified to be
A. alata.
Family Diplostomidae Poirier (1886) could be characterized by a foliate, spatulate or calyciform forebody and a cylindrical or coniform hindbody [
14]. This family Diplostomidae is divided into 2 subfamilies; mammalian parasites Alariinae and avian parasites Diplostominae [
14]. The subfamily Alariinae is morphologically characterized by restricted distribution of vitellaria in the forebody and tandem arrangement of testes in opposite directions [
14]. Considering the definitive host, distribution of vitellaria, and arrangement of testes, this fluke was identified as belong to the Alariinae.
Subfamily Alariinae Hall and Wigdor, 1918 includes
Alaria,
Cynodiplostomum,
Pharyngostomoides, and
Procyotrema [
14]. Among them, genus
Alaria has 2 distinct features from other 3 genera. Flukes of the genus
Alaria have tandem testes on the hindbody and pseudosuckers forming tentacular appendages. Conversely, flukes in the other 3 genera have pseudosucker without unique characteristics and opposite testes on both sides [
14]. The flukes in this study have pseudosucker forming tentacular appendages and tandem, multi-lobed testes in the hindbody. We concluded that this fluke belonged to the genus
Alaria.
Adults of the genus
Alaria have a scoop shaped forebody and a cylindrical hindbody. Trematodes of the genus
Alaria are distributed globally. However, despite several morphological studies of flukes in the genus
Alaria, the validity of each species remains obscure and debatable. The genus
Alaria includes
A. alata,
A. arisaemoides (Augustine and Uribe 1927),
A. canis (La Rue and Fallis 1936),
A. intermedia (Olivier and Odlaug 1938),
A. marcianae (La Rue 1917),
A. mustelae (Bosma 1931), and
A. taxideae (Swanson and Erickson 1946) [
1,
3].
A. alata is mainly found in Europe including Russia, while the rest of the
Alaria species are found mainly in North and South America [
1]. Three species, i.e.,
A. alata,
A. canis, and
A. marcinanae, have a pair of tentacular appendages on the both sides of oral suckers [
15–
17]. The worms detected in this study have tentacular appendages on both sides of the oral suckers (
Table 1). However, the remaining 4 species, i.e.,
A. arisaemoides,
A. intermedia,
A. mustelae, and
A. taxideae, have a pair of pseudosuckers instead of tentacular appendages on the both sides of oral suckers [
18–
21].
The measurements of
Alaria species are summarized in
Table 1. In the total body length of
Alaria species,
A. alata 2,800–4,000 μm [
15],
A. canis 2,500–4,200 μm [
16], and
A. marcianae 1,560–1,840 μm [
17]. In this study, body length (3,020–4,090 μm) is larger than that of
A. marcianae, but similar to
A. alata and
A. canis. And the vitellaria of
A. canis is distributed beyond the forebody to the hindbody, while the vitellaria of
A. alata is distributed only in the forebody [
17]. In this study, vitelline follicles are distributed in the forebody, which are similar to those observed in
A. alata [
15].
The flukes of the genus
Alaria detected in this study had almost no morphological differences from
A. alata except for very small differences. The
Alaria species identified in this study had smaller ovary and posterior testis but larger eggs than the species identified in the previous studies [
15]. Differences in these measurements may be influenced not only by the size of the individual host, but also by the maturation status of the host and parasite. There can also be significant differences between individuals in each population.
The ribosomal 18S rRNA gene was used to study inter- and intra-specific relationships. We confirmed that the 18S rRNA sequences of Alaria specimens were separated from A. canis and Alaria sp., but were identical to that of A. alata. Our diplostomid flukes were identified as A. alata based on their morphologic and molecular characteristics.
The
Alaria spp. from North America is zoonotic; 7 cases of human alariosis have been reported [
1]. The pathogenicity of
A. alata has been poorly studied. However, there may have a zoonotic potential because it is closely related to North American
A. canis.
A. canis can cause severe disease in humans, including one fatal case of eating inadequately cooked frog legs [
1]. Portier et al., [
4] indicated that human cases of alariosis have not reported in Europe, but the zoonotic risk can be presumed The potential danger of this parasite should be taken into consideration. The likelihood of an outbreak due to
A. alata mesocercaria is also increasing in Korea.
In conclusion, based on the morphological and molecular finding, the diplostomatid flukes recovered from the small intestine of Korean raccoon dog were identified as A. alata. Since there is no information on the first and second intermediate hosts of A. alata in Korea, further studies on intermediate hosts should be continued.
Notes
-
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2017 RIDIAIB06031728).
Fig. 1
Alaria alata recovered from the intestine of Korean raccoon dog. (A) Unstained adult worm. Note a few eggs (circle). (B) Schematic drawing of the “A” specimen. (C) Early- stage adult worm stained with Semichon’s acetocarmine. (D) Egg from feces. A, acetabulum; AT, anterior testis; E, egg; GA, genital atrium; HF, Holdfast organ; IC, intestinal crura; MG, Mehlis’s gland; O, ovary; OS, oral sucker; P, pharynx; PT, posterior testis; SV, seminal vesicle; TA, tentacular appendage; U, uterus; V, vitelline follicles; VD, vitelline duct.
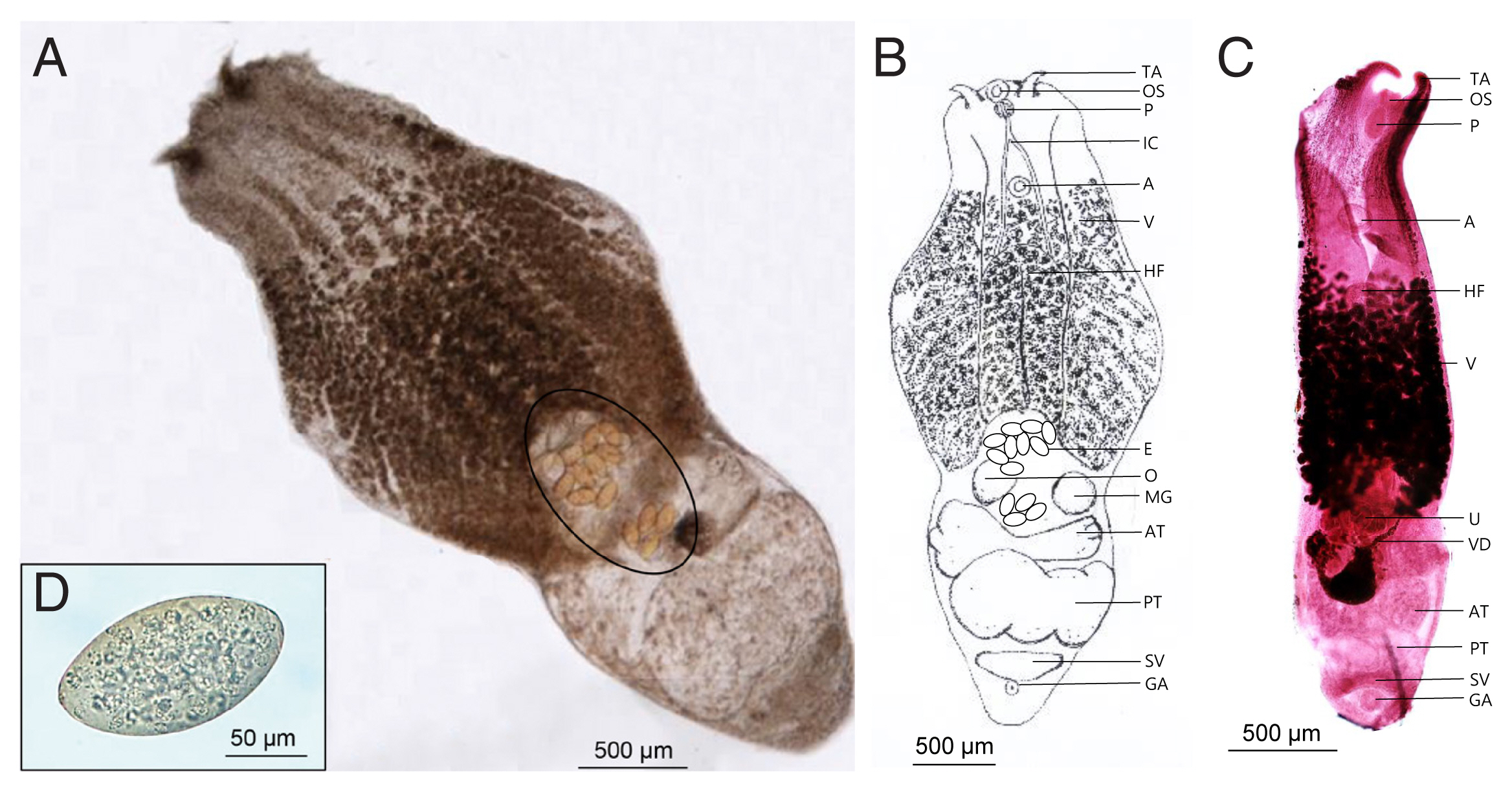
Fig. 2Phylogenetic tree (Neighbor-Joining) based on A. alata sequence analysis. (A) Phylogenetic tree using partial sequence of 18S ribosomal RNA gene. (B) The bootstrap consensus tree inferred from 1,000 replicates.
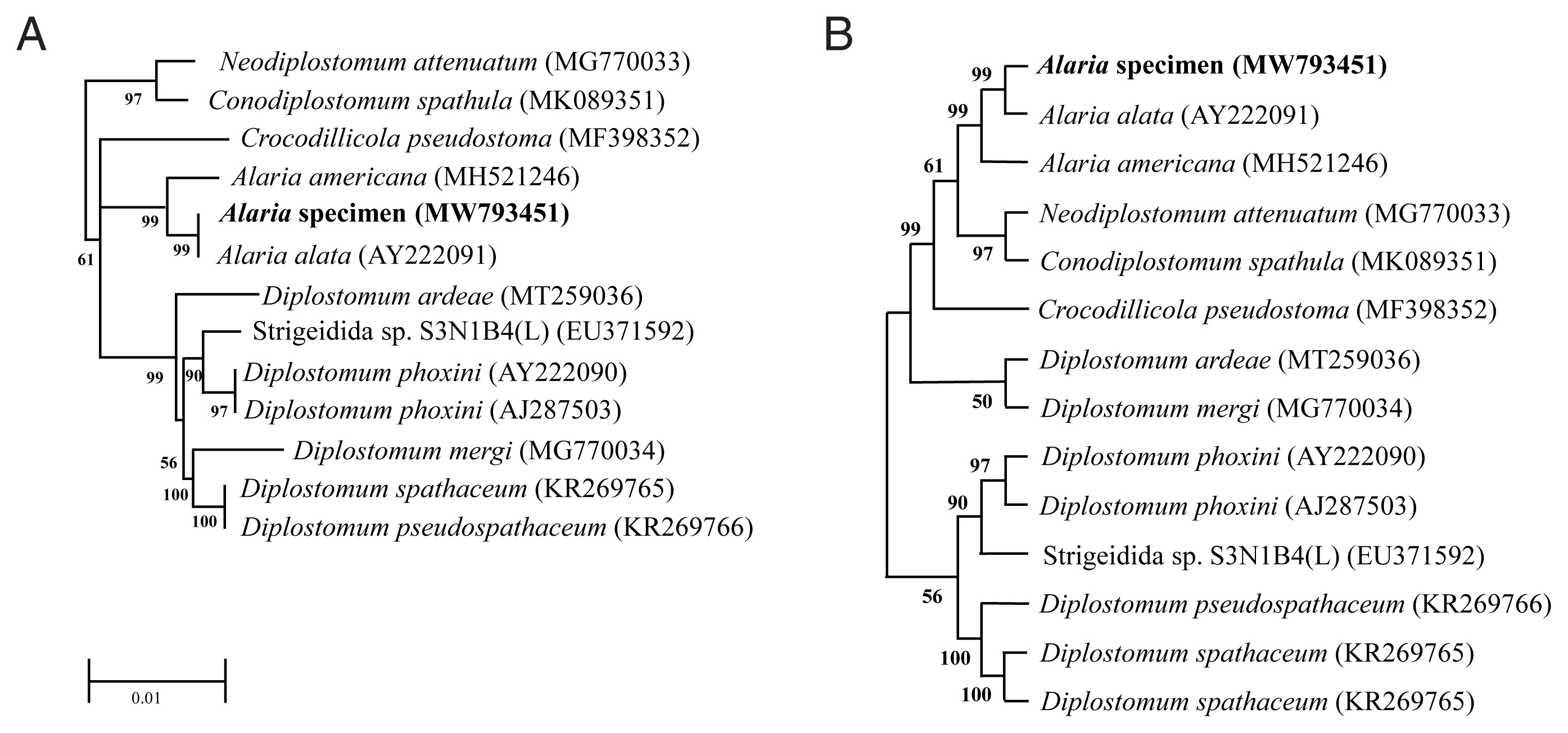
Table 1Comparison of the measurements of Alaria spp
Table 1
|
Types |
Species with tentacular appendage |
Species with pseudosucker |
|
|
|
|
Species |
A. alata
|
A. alata
|
A. canis
|
A. marcianae
|
A. arisaemoides
|
A. intermedia
|
A. mustalae
|
A. taxideae
|
|
Host |
Korean raccoon dog |
Red fox |
Dog |
Striped skunk |
Red fox |
Dog |
Mink, weasel, cat, dog, ferret |
Badger |
|
|
Total length (in mm)a
|
3.0–4.1 |
2.8–4.0 |
2.5–4.2 |
1.56–1.84 |
7.0–10.6 |
0.86–1.6 |
0.82–1.74 |
1.10–2.27 |
|
|
Oral sucker (L)b
|
90–107 |
51–122 |
75–140 |
95–122 |
100 |
76–110 |
62–116 |
78–178 |
|
|
Oral sucker (W)c
|
91–98 |
56–138 |
110–150 |
102–136 |
90 |
76–110 |
62–107 |
78–152 |
|
|
Acetabulum (L)b
|
95–109 |
72–117 |
90–120 |
75–109 |
100 |
76–86 |
53–89 |
65–106 |
|
|
Acetabulum (W)c
|
95–103 |
102–133 |
90–140 |
88–122 |
100 |
76–86 |
53–89 |
57–114 |
|
|
Pharynx (L)b
|
115–145 |
102–138 |
140–170 |
150–177 |
150 |
100–130 |
75–125 |
122–240 |
|
|
Pharynx (W)c
|
89–102 |
46–112 |
90–140 |
109–136 |
130 |
67–100 |
62–116 |
95–190 |
|
|
Ovary (L)b
|
85–110 |
150–320 |
- |
- |
350 |
100–160 |
107–214 |
102.6–140.6 |
|
|
Ovary (W)c
|
95–115 |
230–490 |
- |
- |
450 |
40–70 |
107–214 |
152–298 |
|
|
Holdfast organ (L)b
|
910–1,120 |
880–1,630 |
1,140 |
540–700 |
- |
280–420 |
250–580 |
- |
|
|
Holdfast organ (W)c
|
530–630 |
400–970 |
300 |
280–570 |
- |
170–320 |
116–340 |
- |
|
|
Anterior testis (L)b
|
245–295 |
300–500 |
- |
- |
700 |
170–300 |
- |
193.8–240 |
|
|
Anterior testis (W)c
|
353–732 |
400–700 |
- |
- |
1,040 |
85–170 |
152–320 |
247–447 |
|
|
Posterior testis (L)b
|
265–310 |
400–800 |
- |
- |
680 |
320–460 |
- |
212.8–308 |
|
|
Posterior testis (W)c
|
366–918 |
600–1,100 |
- |
- |
1,500 |
140–170 |
187–360 |
285–744 |
|
|
Eggs (L)b
|
130–140 |
103–124 |
107–133 |
112–129 |
124–148 |
125 |
89–134 |
106–143 |
|
|
Eggs (W)c
|
85–96 |
70–80 |
77–99 |
65–75 |
76–94 |
72 |
62–89 |
60–82 |
|
|
References |
This study |
[15] |
[16] |
[17] |
[18] |
[19] |
[20] |
[21] |
References
- 1. Möhl K, Große K, Hamedy A, Wüste T, Kabelitz P, Lücker E. Biology of Alaria spp. and human exposition risk to Alaria mesocercariae–a review. Parasitol Res 2009;105:1-15. http://dx.doi.org/10.1007/s00436-009-1444-7
- 2. Johnson EM, Nagamori Y, Duncan-Decocq RA, Whitley PN, Ramachandran A, Reichard MV. Prevalence of Alaria infection in companion animals in north central Oklahoma from 2006 through 2015 and detection in wildlife. J Am Vet Med Assoc 2017;250:881-886.
- 3. Tăbăran F, Sándor AD, Marinov M, Cătoi C, Mihalca AD. Alaria alata Infection in European mink. Emerg Infect Dis 2013;19:1547-1549. https://doi.org/10.3201/eid1909.130081
- 4. Portier J, Jouet D, Ferté H, Gibout O, Heckmann A, Boireau P, Vallée I. New data in France on the trematode Alaria alata (Goeze, 1792) obtained during Trichinella inspections. Parasite 2011;18:271-275. https://doi.org/10.1051/parasite/2011183271
- 5. Kramer MH, Eberhard ML, Blankenberg TA. Respiratory symptoms and subcutaneous granuloma caused by mesocercariae: a case report. Am J Trop Med Hyg 1996;55:447-448. https://doi.org/10.4269/ajtmh.1996.55.447
- 6. Kjær LJ, Jensen LM, Chriél M, Bødker R, Petersen HH. The raccoon dog (Nyctereutes procyonoides) as a reservoir of zoonotic diseases in Denmark. Int J Parasitol Parasites Wildl 2021;16:175-182. https://doi.org/10.1016/j.ijppaw.2021.09.008
- 7. Anisimova EI. The structure of helminths fauna of raccoon dog (Nectereutes procyonoides, Gray) nationalized in Belarus. IOZ 2008;2:80-82. (in Belarusian).
- 8. Laurimaa L, Süld K, Davison J, Moks E, Valdmann H, Saarma U. Alien species and their zoonotic parasites in native and introduced ranges: The raccoon dog example. Vet Parasitol 2016;219:24-33. https://doi.org/10.1016/j.vetpar.2016.01.020
- 9. Bružinskaitė-Schmidhalter R, Šarkūnas M, Malakauskas A, Mathis A, Torgerson PR, Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012;139:120-127. https://doi.org/10.1017/S0031182011001715
- 10. Ozoliņa Z, Bagrade G, Deksne G. The host age related occurrence of Alaria alata in wild canids in Latvia. Parasitol Res 2018;117:3743-3751. https://doi.org/10.1007/s00436-018-6074-5
- 11. Jung KS. Helminth fauna of stomach and intestine of wild animals in the Republic of Korea. PhD dissertation. Kangwon National University; Korea. 2010.
- 12. Hillis DM, Dixon MT. Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 1991;66:411-453.
- 13. Tamura K, Stecher G, Kumar S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol 2021;38:3022-3027. https://doi.org/10.1093/molbev/msab120.
- 14. Gibson DI, Jones A, Bray RA. Keys to the Trematoda. Volume I. 24. Family Diplostomidae Poirier 1886 The natural history museum. London, UR. 2002, pp 167-196.
- 15. Machida M, Kitamura Y, Kamiya H. Occurrence of Alaria alata (Diplostomidae: Digenea) from the red fox in Hokkaido, Japan. Jap J Parasitol 1975;24:144-147.
- 16. La Rue GR, Fallis AM. Morphological study of Alaria canis n. sp. (Trematoda: Alariidae), a trematode parasite of the dog. Tran Am Microsc Soc 1936;55:340-351. https://doi.org/10.2307/3222975
- 17. Johnson AD. Life history of Alaria marcianae (La Rue, 1917) Walton, 1949 (Trematoda: Diplostomatidae). J Parasitol 1968;54:324-332. https://doi.org/10.2307/3276944
- 18. Augustine DL, Uribe C. Alaria arisaemoides, n. sp. a trematode from Vulpes fulva. Parasitology 1927;19:236-244. https://doi.org/10.1017/S0031182000005631
- 19. Odlaug TO. Morphology and life history of the trematode, Alaria intermedia. Tran Am Microsc Soc 1940;59:490-510. https://doi.org/10.2307/3222994
- 20. Bosma NJ. The life history of the trematode Alaria mustelae, Bosma, 1931. Trans Am Microsc Soc 1934;53:116-153. https://doi.org/10.2307/3222088
- 21. Swanson G, Erickson AB. Alaria taxideae n. sp., from the badger and other mustelids. J Parasitol 1946;32:17-19. https://doi.org/10.2307/3272697