Abstract
We retrospectively evaluated the clinical and imaging features of 6 patients with bone hydatid disease confirmed by surgery and pathological examination. Among the 6 patients, 2 were infected with Echinococcosis granulosus metacestode and 4 were infected with E. multilocularis metacestode. The 2 cases with cystic echinococcosis were diagnosed by computed tomographic (CT) examination, and other 4 cases were diagnosed by magnetic resonance (MR) imaging. On the initial evaluation, 1 case each was misdiagnosed as a giant cell tumor or neurogenic tumor, and 2 were misdiagnosed as tuberculosis. The imaging manifestations of bone hydatid disease are complex, but most common findings include expansive osteolytic bone destruction, which may be associated with sclerosing edges or dead bone formation, localized soft tissue masses, and vertebral lesions with wedge-shaped changes and spinal stenosis. Combining imaging findings with the patient’s epidemiological history and immunological examinations is of great help in improving the diagnosis and differential diagnosis of bone hydatid disease.
-
Key words: Echinococcosis, bone, computed tomography, magnetic resonance imaging, misdiagnosis
INTRODUCTION
Echinococcosis is a disease complex caused the larvae of
Echinococcus spp. mostly with
E. granulosus (cystic echinococcosis; CE) and/or
E. multilocularis (alveolar echinococcosis; AE). The disease is transmitted between humans and animals and seriously threatens human health [
1,
2]. The incidence of echinococcosis is low, but biological behavior of the parasites is quite complex. The clinical symptoms of bone echinococcoses are not typical, and it is easily misdiagnosed as tuberculosis, tumors, bone cysts, and other diseases. Echinococcosis threatens the quality of patients’ life with a high disability rate [
3,
4]. Imaging examinations are of great importance in the diagnosis of echinococcoses. Computed tomography (CT) and magnetic resonance (MR) scans are highly beneficial in diagnosing echinococcosis as these examinations clearly show the location, size, extent, and degree of the lesion(s) [
5]. However, the imaging manifestation of AE has a certain degree of similarity with the spinal tuberculosis and bone tumors, and it is frequently misdiagnosed. In this study, we retrospectively analyzed the clinical data and imaging manifestations of 6 patients with bone hydatid disease and analyzed the misdiagnosed cases to improve the understanding of echinococcosis and reduce the misdiagnosis, maltreatment, and adverse events.
CASE RECORD
Ethical statement
This study was approved by the Medical Ethics Committee of Qinghai Provincial People’s Hospital (2018-SF-114).
Patients
A total of 6 patients (4 males and 2 females) with bone hydatid disease confirmed by surgery and pathology in our hospital from 2014 to 2020 were enrolled (mean age, 43.2 years; standard deviation, 6.77 years; range, 37–55 years), with a medical history of 3 days to 20 years. All of them lived in pastoral areas. Three patients presented with chest pain, 2 patients presented with lower limb weakness and numbness, and 1 patient had liver, lung, and kidney echinococcosis at the same time.
Clinical and lesion characteristics of the patients
The general clinical features of the patients are shown in
Table 1. Patient 1, who complained of weakness of both lower limbs for 2 months, had a lesion located at the 8th thoracic vertebra. Patient 2 had lesions affecting the left femoral head-femoral neck-femoral trochanter. The clinical manifestations were left hip pain for 20 years and aggravated by trauma for 1 day. Patient 3 showed lesions encompassing the 11th and 12th thoracic vertebrae, and the clinical manifestations were chest and back pain for 2 months, that had been aggravated for 1 month. In patient 4, the lesions were observed in the 3rd thoracic vertebra and vertebral arch plate. The patient complained of sudden numbness, weakness, and chest pain of the left lower limb for 3 days. The lesions of patient 5 were thoracic vertebra 3 to 5, and the clinical features included lumbar and back pain for 4 months. Patient 6 had lesions in the 12th thoracic vertebra and vertebral arch plate and complained of chest and back pain for 1 year that had been aggravated during the past 1 month.
All 6 patients were examined with a multidetector CT (Revolution; GE Healthcare, Fairfield, Connecticut, USA) with a tube voltage of 120 kVp and tube current of 250 mA. The images were transferred to the Advantage Workstation (AW) 4.6 workstation for reconstruction. The reconstructed slice thickness was 0.625 mm and the slice distance was 0.625 mm. All 6 patients were scanned with a 1.5T MR (Symphony; SIEMENS, Berlin, Germany) with an echo time (TE) of 2.5 ms, repetition time (TR) of 8.00 ms, turning angle of 20°, layer thickness of 1.5 mm, field of view (FOV) of 50×50 mm (Matrix=256×256), and 4 excitation times (NEX). The patients were placed in a supine position and the fast recovery spin echo sequence was used for the plain scan: T1-weighted imaging (T1WI) axial view, T2-weighted imaging (T2WI) axial view, fat-suppressed T2WI, T2WI coronal, T2WI sagittal, fast spin echo sequence T1WI sagittal scans, and gadolinium contrast enhanced T1WI. The layer thickness was 4 mm, and the layer spacing was 1 mm.
Imaging features
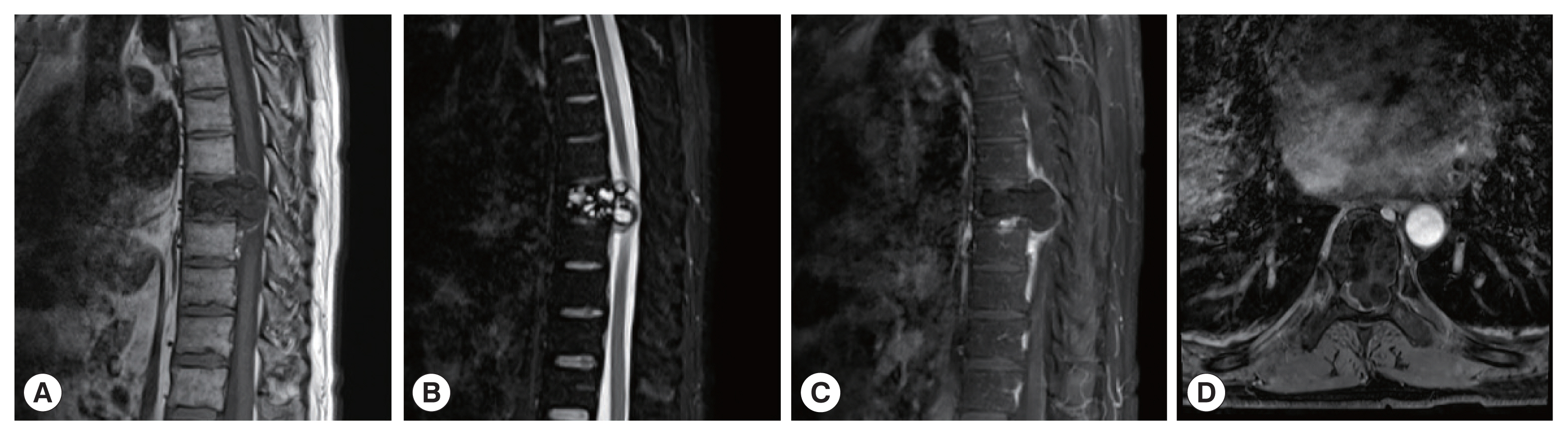
The CT images of patient 1 showed the uneven bone density and low-density bone destruction of the infected vertebral body. The boundary of the lesion was clear, but the edge was sclerotic. Multiple round low-density shadows and separation were seen, and the spinal canal was compressed and narrowed. The MRI scans also showed a low signal on T1WI, a high signal on T2WI, and a “grape bunch-like” cluster. The edge of the lesion was slightly enhanced on the enhanced scan, while no obvious enhancement was found in the interior portion. The lesion protruded into the spinal canal, causing spinal cord compression (
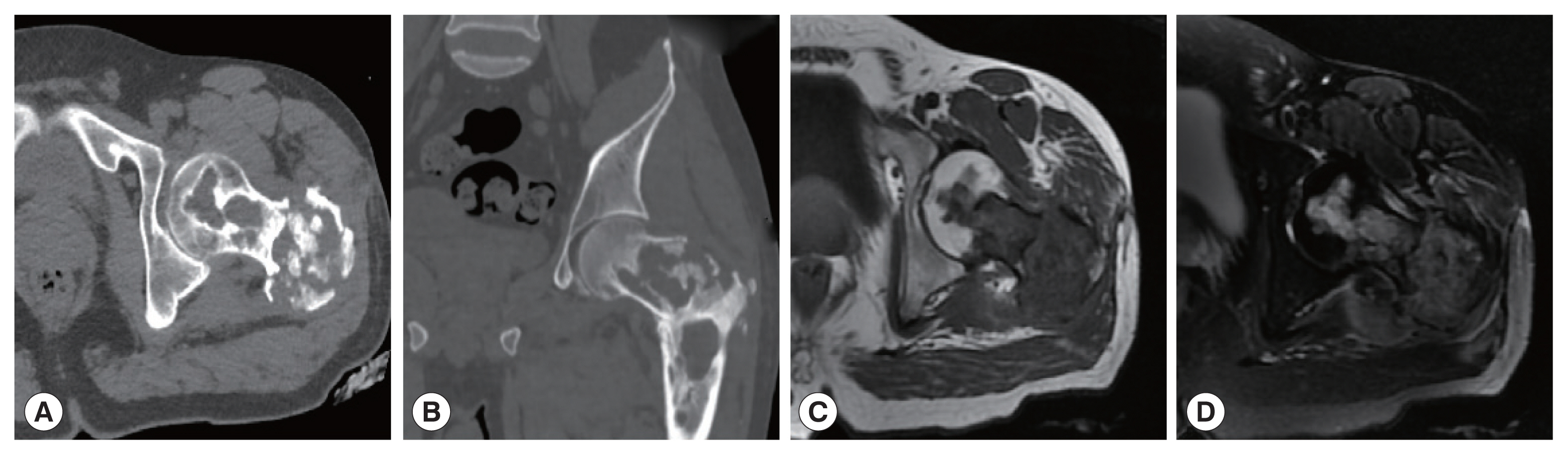
Fig. 1). In patient 2, the lesion involving the left femoral head-femoral neck-femoral trochanter was seen on CT scan. The left femoral head, neck, and trochanter showed cystic expansive bone destruction, accompanied by sclerotic edge and dead bone formation. Local soft tissue masses were also recognized. The MRI showed an irregular low signal on T1WI, a high signal on T2WI, and a patchy high signal on T2WI in the vastus lateralis muscle (
Fig. 2). Patient 3 showed osteolytic destruction of the vertebral body with wedge-shaped compression. The formation of sequestrum, intervertebral space stenosis, and swelling of the surrounding soft tissue were also noticed. In the other 3 patients (patients 4, 5, and 6), osteolytic destruction was observed, and some vertebral bodies showed wedge-shaped compression. Spinal canal stenosis and soft tissue masses were also seen locally.
One CE patient (patient 1) showed expansile bone destruction in the infected vertebral body on CT images, and polycystic changes on MR images. This patient was misdiagnosed with giant cell bone tumor. Two patients (patients 3 and 6) were misdiagnosed as tuberculosis on preoperative CT examinations because the infected vertebral body showed osteolytic bone destruction, together with sequestrum formation and soft tissue masses. Another patient (patient 4) revealed abnormal signal shadows with oval low signal on T1WI and high signal on T2WI. The patient was initially diagnosed with a neurogenic tumor due to edge enhancement on the enhanced scan.
Pathological manifestations
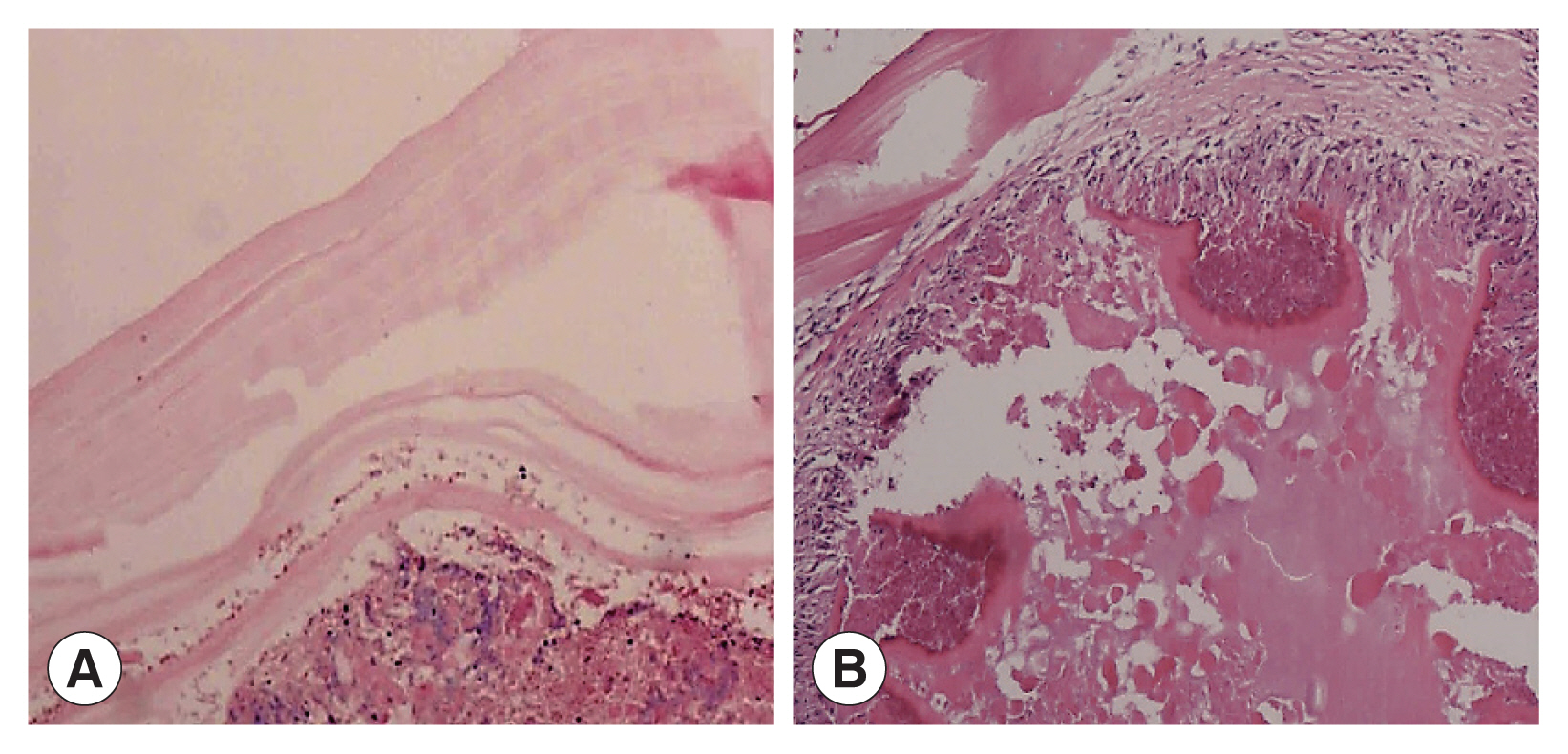
Two cases showed powdery parallel lamellar stratum corneum hydatid cysts under the microscope and were diagnosed definitively as CE (
Fig. 3A). In the other 4 cases, large sheets of necrotic tissue, small cystic structures, homogeneous layers and sequestrum formations were seen microscopically. Peripheral infiltration of numerous inflammatory cells were also observed (
Fig. 3B). Their final diagnosis was AE.
DISCUSSION
Bone hydatid disease refers to a series of clinical symptoms and signs produced by the metacestodes of
E. granulosus or
E. multilocularis, and these cases account for 0.5% to 4.0% of all hydatid diseases [
6]. CE is more common, while AE is relatively rare. Most cases comes from the hematogenous spread of hepatic cysts, and its metastasis rate approximated to be 1.3% [
7,
8]. The systemic circulation of the metacestodes and subsequent invasion of the bone might depend on the activity of the local blood supply and the growth of the metacestodes. The incidence of bone echinococcosis is the highest in the pelvis and spine, followed by the metaphysis of the long bones of the limbs, scapula, and ribs. The expansion and growth of the larvae is restricted because the bone tissue is dense and the trabecular space is narrow. Therefore, the onset of hydatid disease is frequently subclinical, which is difficult to detect during the early stage. Due to extensive destruction of bone trabeculae and bone cortex, hydatid disease can result in pathological compression fractures and disc destruction.
In this study, 2 cases suffered from CE and 4 cases were from AE. The main lesions were spinal cords, which is consistent with a previous report [
9]. Two CE cases were characterized by cystic expansile bone destruction, and 4 AE manifested with osteolytic bone destruction. On CT scans, both AE and CE manifest as round or oval low-density bone destruction of varying sizes, with clear lesion edges and calcification in the cyst or on the cyst wall [
10,
11]. MRI shows better soft tissue imaging ability than CT. For intraspinal and paravertebral hydatid disease, MRI clearly displays the number and location of hydatid cysts, which is of great value in diagnosing the disease. In this study, a low signal on T1WI, a high signal on T2WI, a high signal in the cyst contents, and a “grape bunch-like” cluster [
12,
13] were visible on MRI scan.
However, it is sometimes difficult to differentiate echinococcosis from bone tuberculosis on imaging findings. Bone tuberculosis is characterized by irregular bone destruction or pseudoparavertebral abscess. Some patients show intervertebral space stenosis, but mostly intervertebral disc is intact [
14]. In addition, echinococcosis should be differentiated from metastatic tumors, giant cell tumors, and neurogenic tumors. When echinococcosis invades adjacent soft tissues, nodules and “gritty”-like calcifications can be seen in the soft tissue mass. These findings can be helpful for the identification of spinal metastases. Metastatic paravertebral soft tissue invasion often lacks calcification and small high-intensity vesicles. Multivertebral metastasis often shows characteristics of “jumping” [
15,
16].
Some patients with echinococcosis present with polycystic bone destruction with visible bone ridges, which can easily be misdiagnosed as giant cell tumor of the bone, which usually develops at the end of long tubular bones with osteolysis and swelling on imaging studies. The identification points include soap-bubble-like changes of bone, with septations or bone ridges, without sclerotic edges. Neurogenic tumors, which are often found in young adults and children, are often located next to the posterior mediastinal spine, appear as oval or fusiform soft tissue or low-density masses and are often asymptomatic.
Notes
-
We have no conflict of interest related to this work.
ACKNOWLEDGEMENTS
The work was supported by the Qinghai Provincial Health Committee Guiding Program Project (2020-wjzdx-04) and the Qinghai Provincial Department of Science and Technology Basic Research Project (2021-ZJ-732).
Fig. 1A 41-year-old man with vertebral echinococcosis. MR sagittal scan show low signals on T1WI at T8 vertebral body (A). The contents of the fat-pressed sac on T2 show high signal (B). The clusters were a “grape bunch-like”; and the edge of the lesions on the enhanced scan show slight enhancement without obvious internal enhancement (C). The lesions protruding into the spinal canal resulting in spinal cord compression, which is seen with multiple small cystic lesions with a low signal on axial T1WI images (D).
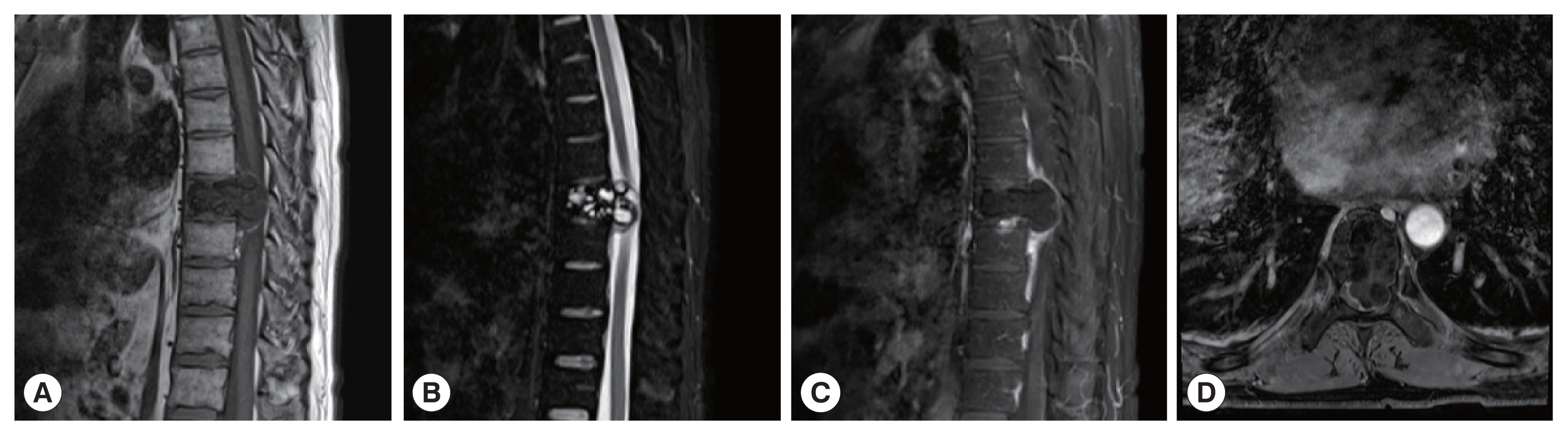
Fig. 2A 38-year-old man with bone hydatid disease in left femoral head-neck-trochanteric zone. Cross-sectional (A) and coronal CT scans (B) show that the lesion involves cystic expansile bone destruction with sclerotic edges and sequestrum formation. A soft tissue mass is also seen locally. MR cross-section scan shows irregular low signal on T1WI (C) and high signal on T2WI (D), with patchy high signals in the vastus lateralis muscle.

Fig. 3(A) Microscopic examination shows powdery parallel lamellar cuticle CE cysts and dermoid tissue (HE×100). (B) Microscopic examination shows large sheets of necrotic tissue and small cystic structures, with a homogeneous layer and a sequestrum surrounded by a large amount of inflammatory cell infiltration (HE×100).
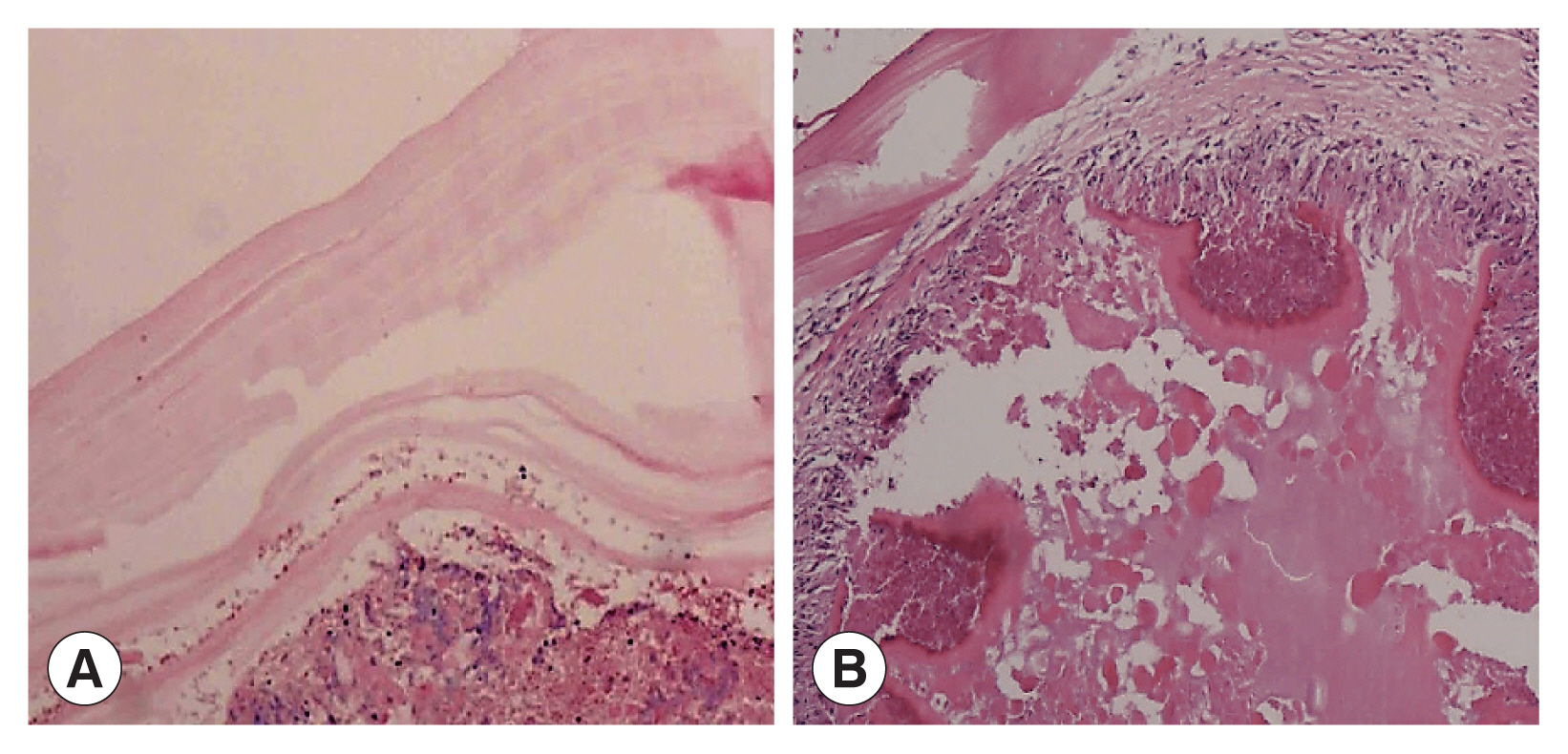
Table 1General clinical data and imaging features of different parts
Table 1
|
Patient code |
1 |
2 |
3 |
4 |
5 |
6 |
|
Age (year)/sex |
41/Male |
38/Male |
41/Female |
37/Male |
47/Male |
55/Female |
|
Clinical manifestations |
Weakness of both lower limbs for 2 months |
Left hip pain for 20 years, aggravated by trauma for 1 day |
Chest and back pain for 2 months, aggravated for 1 month |
Sudden left lower limb numbness, weakness, chest pain for 3 days |
Low back pain for 4 months |
Chest and back pain for 1 year, aggravated for 1 month |
|
Lesion |
T8 vertebral body |
Left femoral head- femoral neck- femoral tuberosity |
T11, T12 vertebral bodies |
T3 vertebral body and vertebral arch plate |
T3-T5 vertebral bodies |
T12-L1 vertebral bodies and vertebral arch plate |
|
Cystic expansion bone destruction |
+ |
+ |
− |
− |
− |
− |
|
Polycystic changes |
+ |
− |
− |
+ |
− |
− |
|
Osteolytic destruction |
− |
− |
+ |
+ |
+ |
+ |
|
Hardened edge |
+ |
+ |
− |
− |
− |
− |
|
Separate |
+ |
− |
− |
+ |
− |
− |
|
Calcified or sequestered bone |
+ |
+ |
+ |
− |
− |
− |
|
Wedge deformation |
− |
− |
+ |
− |
+ |
+ |
|
Spinal stenosis |
+ |
− |
+ |
+ |
+ |
+ |
|
Soft tissue mass |
− |
+ |
+ |
+ |
− |
+ |
|
Final diagnosis |
CE |
CE |
AE |
AE |
AE |
AE |
References
- 1. Kaya H, Karahan G, Sabah D. Is hydatid cyst with musculoskeletal involvement a problem that causes morbidity? long-term follow-up and functional results. Indian J Orthop 2021;56:680-688. https://doi.org/10.1007/s43465-021-00556-6
- 2. Boussaid S, Daldoul C, Hassayoun M, Rekik S, Jammali S, Sahli H, Elleuch M. Primitive pelvic bone hydatidosis: What an amazing extension. Clin Case Rep 2021;9:e05054. https://doi.org/10.1002/ccr3.5054
- 3. Xu W, Xilinbaoleri , Liu H, Wang R, Bai J. Spinal cord biological safety of image-guided radiation therapy versus conventional radiation therapy. Neural Regen Res 2012;7:2755-2760. https://doi.org/10.3969/j.issn.1673-5374.2012.35.002
- 4. Liang Q, Xiang H, Xu L, Wen H, Tian Z, Yunus A, Wang C, Jiang D, Abuduwaili M, Chen J, Song X. Treatment experiences of thoracic spinal hydatidosis: a single-center case-series study. Int J Infect Dis 2019;89:163-168. https://doi.org/10.1016/j.ijid.2019.09.024
- 5. Luan H, Liu K, Deng Q, Sheng W, Maimaiti M, Guo H, Li H. Multiple debridement of cavity lesions combined with antiparasitic chemotherapy in the treatment of mid or advanced spinal echinococcosis: a retrospective study of 33 patients. Int J Infect Dis 2022;114:261-267. https://doi.org/10.1016/j.ijid.2021.11.014
- 6. Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics 2000;20:795-817. https://doi.org/10.1148/radiographics.20.3.g00ma06795
- 7. Meinel TR, Gottstein B, Geib V, Keel MJ, Biral R, Mohaupt M, Brügger J. Vertebral alveolar echinococcosis-a case report, systematic analysis, and review of the literature. Lancet Infect Dis 2018;18:e87-e98. https://doi.org/10.1016/S1473-3099(17)30335-3
- 8. Barth TFE, Casulli A. Morphological characteristics of alveolar and cystic echinococcosis lesions in human liver and bone. Pathogens 2021;10:1326. https://doi.org/10.3390/pathogens10101326
- 9. Neumayr A, Tamarozzi F, Goblirsch S, Blum J, Brunetti E. Spinal cystic echinococcosis--a systematic analysis and review of the literature: part 1. Epidemiology and anatomy. PLoS Negl Trop Dis 2013;7:e2450. https://doi.org/10.1371/journal.pntd.0002450
- 10. Wu J, Gui X, Jiang H, Liang X, Wang E, Xu X, Chen X, Wu X. Study on effect of echinococcus granulosus protoscolices on fibrosis of bone marrow mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2020;34:630-636. (in Chinese). https://doi.org/10.7507/1002-1892.201909050
- 11. Niazi A, Alibraheem A, Al-Mouakeh A, Abouzied MK, Basha SR, Suliman S, Hendawi Y, Ayoub K. Gluteal muscles primary hydatid cyst after cortical bone destruction in the sacrum. Ann Med Surg 2020;59:89-92. https://doi.org/10.1016/j.amsu.2020.09.019
- 12. Iken M, Lamkhantar A, Boussaidane M, Naoui H, Lmimouni B, Boussouga M. Multiple pelvic and thigh hydatid cyst: a case report. Ann Parasitol 2021;67:553-557. https://doi.org/10.17420/ap6703.371
- 13. Neumayr A, Tamarozzi F, Goblirsch S, Blum J, Brunetti E. Spinal cystic echinococcosis--a systematic analysis and review of the literature: part 2. Treatment, follow-up and outcome. PLoS Negl Trop Dis 2013;7:e2458. https://doi.org/10.1371/journal.pntd.0002458
- 14. Ma C, Luo X, Mahan W, Tang Y, Duan Y, Mao R, Xie Z. Outcomes of Radiotherapy for Osseous Echinococcosis of Meriones meridianus. Biomed Res Int 2020;2020:6457419. https://doi.org/10.1155/2020/6457419
- 15. Monge-Maillo B, Olmedo Samperio M, Pérez-Molina JA, Norman F, Mejía CR, Tojeiro SC, López-Vélez R. Osseous cystic echinococcosis: a case series study at a referral unit in Spain. PLoS Negl Trop Dis 2019;13:e0007006. https://doi.org/10.1371/journal.pntd.0007006
- 16. Cattaneo L, Manciulli T, Cretu CM, Giordani MT, Angheben A, Bartoloni A, Zammarchi L, Bartalesi F, Richter J, Chiodini P, Godbole G, Junghanss T, Stojkovic M, Sammarchi L, Dore R, Vercelli A, Benazzo F, Cuzzocrea F, Tamarozzi F, Brunetti E. Cystic echinococcosis of the bone: a European multicenter study. Am J Trop Med Hyg 2019;100:617-621. https://doi.org/10.4269/ajtmh.18-0758