Abstract
Cockroaches can cause allergic sensitization in humans via contact with their feces or frass. Antibiotics can affect concentration of major allergen and total bacteria production in German cockroaches (Blattella germanica). This study examined the ability of antibiotic-treated German cockroaches to induce allergic airway inflammation and the effect of antibiotics on their lipopolysaccharide and Bla g1, 2, and 5 expression levels. Specifically, we measured the ability of German cockroach extract (with or without prior antibiotic exposure) to induce allergic inflammation in human bronchial epithelial cells and a mouse model of asthma. Bacterial 16S rRNA and lipopolysaccharide levels were lower in ampicillin-treated cockroaches than in the control group. The Bla g1, Bla g2, and Bla g5 expression in ampicillin-treated cockroaches decreased at both the protein and RNA levels. In human bronchial epithelial cell lines BEAS-2B exposed to the ampicillin-treated extract, expression levels of interleukin-6 and interleukin-8 were lower than that in the control group. The total cell count and eosinophil count in bronchoalveolar lavage fluid was also lower in mice exposed to the ampicillin-treated extract than in those exposed to normal cockroach extract. Mouse lung histopathology showed reduced immune cell infiltration and mucus production in the ampicillin group. Our results showed that ampicillin treatment reduced the symbiont bacterial population and major allergen levels in German cockroaches, leading to reduced airway inflammation in mice. These results can facilitate the preparation of protein extracts for immunotherapy or diagnostics applications.
-
Key words: Cockroach, antibiotic, allergen, mouse, airway inflammation
Introduction
Cockroach infestations are associated with human allergic diseases such as asthma, which can be induced by cockroaches spreading pathogenic bacteria through their feces or frass in homes, shops, and hospitals [
1,
2]. Treatment of German cockroaches with antibiotics has been shown to change their microbiome [
3]. The microbiome composition and allergen levels of cockroaches were also altered according to their environment or food [
4]. Specifically, dietary changes can influence the expression of
Bla g1, a digestive protein found in German cockroaches [
5].
Bla g1 can bind to various lipids and has a digestive function related to the nonspecific transport of lipid molecules [
6]. Similar to
Bla g1, Bla g2 is present at high concentrations in the digestive organs of German cockroaches, where it functions as a digestive enzyme [
7].
Bla g5 is a sigma-class glutathione transferase and one of the major allergens produced by German cockroaches as it induces a high IgE response [
8]. Although
Bla g5 concentrations in German cockroaches are 100-time lower than
Bla g1 and
Bla g2 concentrations, some patients respond strongly to
Bla g5 [
9].
Active pharmaceutical ingredient (API), the active ingredients of pharmaceutical drugs, exert beneficial health effects on consumers [
10]. However, APIs are also released into the natural environment during their manufacture, use, and disposal [
11]. Exposure to APIs can lead to problems such as antibiotic-resistant bacteria and feminization in fish [
12]. As cockroaches tend to prefer warm and humid areas [
13], they are likely to be exposed to APIs discharged into rivers or catchments. This process may alter the microbiome or allergens of cockroaches from those generated by exposure to normal food or water.
Cockroach extracts for immunotherapy or diagnostics applications have not yet been standardized, and the composition varies considerably depending on the breeding environment of the cockroach and the extraction method [
9]. Furthermore, the effect of cockroach extracts on T cell potency or IgE reactivity varies according to a patient’s allergen profile [
9]. Therefore, suitable methods are required to develop a standardized cockroach extract, as well as diverse extracts for various patient profiles.
In a previous study, German cockroaches treated with ampicillin contained few bacteria and exhibited an altered microbiome composition from the control cockroaches [
4]. Their gene expression was also altered, with reduced levels of
Bla g1 and
Bla g2, which are major allergens in the German cockroach [
4]. However, the ability of ampicillin-treated German cockroaches to cause allergic inflammation in humans remains unclear. This study aimed to examine airway inflammation in human bronchial cells and a mouse model resulting from exposure to ampicillin-treated German cockroach extracts.
Materials and Methods
Ethics statement
All experiments were approved by the Institutional Review Board of Yonsei University College of Medicine (IACUA no. 2021-0319).
Rearing conditions
German cockroaches (
Blattella germanica) were reared for several generations under the same laboratory conditions to minimize the potential influence of environmental factors and diet. All cockroaches were reared in plastic boxes (27×34×19 cm) incubated at 25°C under 50% relative humidity.
B. germanica were fed sterilized fish food and provided autoclaved antibiotic untreated tap water or tap water containing ampicillin (autoclaved before the addition of 0.03% ampicillin)
ad libitum (
Fig. 1A).
Total DNA was extracted using the NucleoSpin DNA Insect Kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s instructions. The DNA extracted from each sample was eluted in 20 μl of elution buffer. All procedures were conducted on a clean bench under a sterilized hood and in a DNA-free room. The DNA concentration was quantified using an ND-1000 NanoDrop system (Thermo Fisher Scientific, Waltham, MA, USA).
Protein extraction
Total protein was extracted by adding 2 ml of phosphate buffered saline (PBS) to each sample. Protein extract was extracted from the whole cockroach bodies. The samples were then sonicated (QSonica Q500, Fullerton, CA, USA) and centrifuged at 10,000×g for 30 min at 4°C. The resulting supernatants were filtered using a 0.22-μm membrane filter (Tullagreen, Carrigtwohill, Cork, Ireland).
Enzyme-linked immunosorbent assay (ELISA)
Cockroach protein extracts (2 mg/ml) were either diluted 100-fold to measure Bla g1 and Bla g2 levels or diluted 10-fold to measure Bla g5 levels using the corresponding ELISA kits (Indoor Biotechnologies, Charlottesville, VA, USA) according to the manufacturer’s instructions. Briefly, the detection antibody and conjugate mix were used for the immunoassay, and color development was obtained using the substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich, St. Louis, MO, USA).
RNA extraction and cDNA synthesis
Total RNA was extracted by adding 1 ml of TRIzol reagent (GeneAll, Seoul, Korea) to each sample and purified using isopropanol. RNA extracted from each sample was eluted in 20 μl of elution buffer. A master-mix, comprising 5×cDNA synthesis mix and 20×reverse transcriptase, was added to mRNA samples in PCR tubes for cDNA synthesis.
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was performed to quantify
Bla g1, Bla g2, Bla g5, and bacterial 16S rRNA levels in the cockroaches. Actin 5C was used as an internal control, and primers specific to this gene (ActinF and ActinR) were designed for the experiment (
Table 1) [
14]. All bacterial 16S rRNA was amplified using the forward primer BACT1369 and the reverse primer PROK1492R (
Table 1) from XenoTech with the AMPIGENE qPCR Mix (ENZO, USA) [
3].
Bla g1, Bla g2, and
Bla g5 gene expression was determined to measure the major allergen content. qPCR was performed using the 2×SensiFAST SYBR Hi-ROX kit (Bioline Meridian Bioscience, London, UK) with SYBR Green as the fluorescent reporter, H
2O, the corresponding primers, and either genomic or complementary DNA. At the end of each reaction, a melting curve was generated to check the specificity of amplification and confirm the absence of primer dimers. All reactions, including the negative controls (containing water instead of DNA), were run in duplicate in 96-well plates.
Human bronchial epithelial cells (BEAS-2B cells) were maintained in DMEM/F40 medium at 37°C with 5% CO2. Cells were seeded at a concentration of 1×106 cells/well in 6-well plates (SPL Life Sciences, Pocheon, Korea), then treated with each German cockroach extract (GCE) sample. Cells were sampled 24 h after a single exposure to 100 μg/ml of each GCE and compared with the PBS-treated controls. Four independent samples were examined for each GCE and PBS control.
Cytokine measurement
The concentrations of cytokines secreted from BEAS-2B cells were measured from the supernatants using the DuoSet ELISA human IL (Interleukin)-6 and IL-8 (R&D Systems, Minneapolis, MN, USA).
Mouse model of allergic airway inflammation
Wild-type BALB/c mice (6–8 weeks old) were purchased from Orient Bio 13 (Seongnam, Korea). A mouse model of allergic airway inflammation was used to investigate the effect of allergen-reduced German cockroaches on allergic disease. GCE from each group (120 μg) was administered intranasally twice per week for 3 weeks [
14]. The 3 treatments were as follows: PBS treatment (PBS group), normal GCE treatment (control group), and ampicillin-treated GCE treatment (ampicillin group). Mice were sacrificed on day 21, and bronchoalveolar lavage (BAL) was performed with 1 ml of PBS. Blood from the cardiac tissue was stored at 4°C. Lungs were dissected then frozen or fixed in formalin.
The procedure for BALF cell collection was identical to that described previously [
14]. After elimination of red blood cells, centrifugation (23×g for 15 min) was performed, and the total BALF cell count was determined using a hemocytometer. All slides were subjected to DiffQuik staining (Sysmex Corporation, Kobe, Japan). Eosinophil, macrophage, lymphocyte, and neutrophil cell counts were determined in 400 BALF cells.
Lung tissue samples were sectioned (5 μm-thickness) and subjected to hematoxylin and eosin (H&E) staining, along with periodic acid-Schiff (PAS) staining, using standard histological protocols to detect mucus-containing cells [
15]. The pathological change index of H&E-stained slides was assigned numerical values according to inflammatory cell infiltration and thickness around the airway and blood vessels (0, normal or no cells; 1, thickness of ≤3 cells; 2, thickness of 4–6 cells; 3, thickness of 7–9 cells; and 4, thickness of ≥10 cells). Similarly, numerical values were assigned according to the proportion of airways and blood vessels in each section surrounded by inflammatory cells (0, normal or no airways or blood vessels; 1, <25% of airways or blood vessels; 2, 25–50% of airways or blood vessels; 3, 51–75% of airways or blood vessels; and 4, ≥75% of airways or blood vessels). The exponent was calculated by multiplying the severity by the range, with a maximum possible score of 9. The number of mucus-containing cells/mm
2 in the basement membrane and the intensity of bronchial and perivascular inflammation were also measured. Furthermore, airway epithelial cells were scored on the degree of goblet cell hyperplasia on a percentage scale of PAS+ cytoplasm. PAS+ cells in the epithelial region were counted 6 times per section in 2 tissue sections per mouse (
n=8 mice/group) [
14,
15]. Each value was expressed as the mean ±SD.
The lungs were harvested after BAL fluid collection and homogenized using the T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific). After homogenization, the suspensions were incubated at 4°C for 30 min and centrifuged at 1,400×g for 10 min. Supernatants were filtered through a 0.45-μm filter to analyze the cytokine levels [
14].
ELISAs for IL-4, IL-5, IL-13, and Interferon gamma (IFN-γ) were performed using the respective commercial kits (Peprotech, Rocky Hill, NJ, USA) according to the manufacturer’s instructions.
Quantification of German cockroach-specific immunoglobulins IgE, IgG1, and IgG2a levels in serum
German cockroach-specific IgE, IgG1, and IgG2a levels in mouse sera were assessed using antigen-capture ELISA. Briefly, 96-well plates were coated with 20 μg of GCE in 100 μl of coating buffer and incubated overnight at 4°C. The plates were blocked with 200 μl/well of the assay diluent. Diluted serum samples (1: 10 dilution) were added to each well and incubated. The wells were washed, and biotin-anti-mouse IgG1, IgG2a, or IgE (BioLegend, San Diego, CA, USA) was added and incubated for 2 h. This was followed by 30 min of incubation with avidin-goat peroxidase (BioLegend). TMB substrate solution (100 μl) was added to each well and incubated in the dark for 20 min. The reaction was stopped using 2N sulfuric acid (H2SO4). Optical densities were measured at 450 nm using a spectrophotometer.
Statistical analysis
Student’s t-test and analysis of variance (ANOVA) with Bonferroni correction as a post-hoc analysis were used for data analysis. Differences with P-values of 0.05 or less were considered statistically significant.
Results
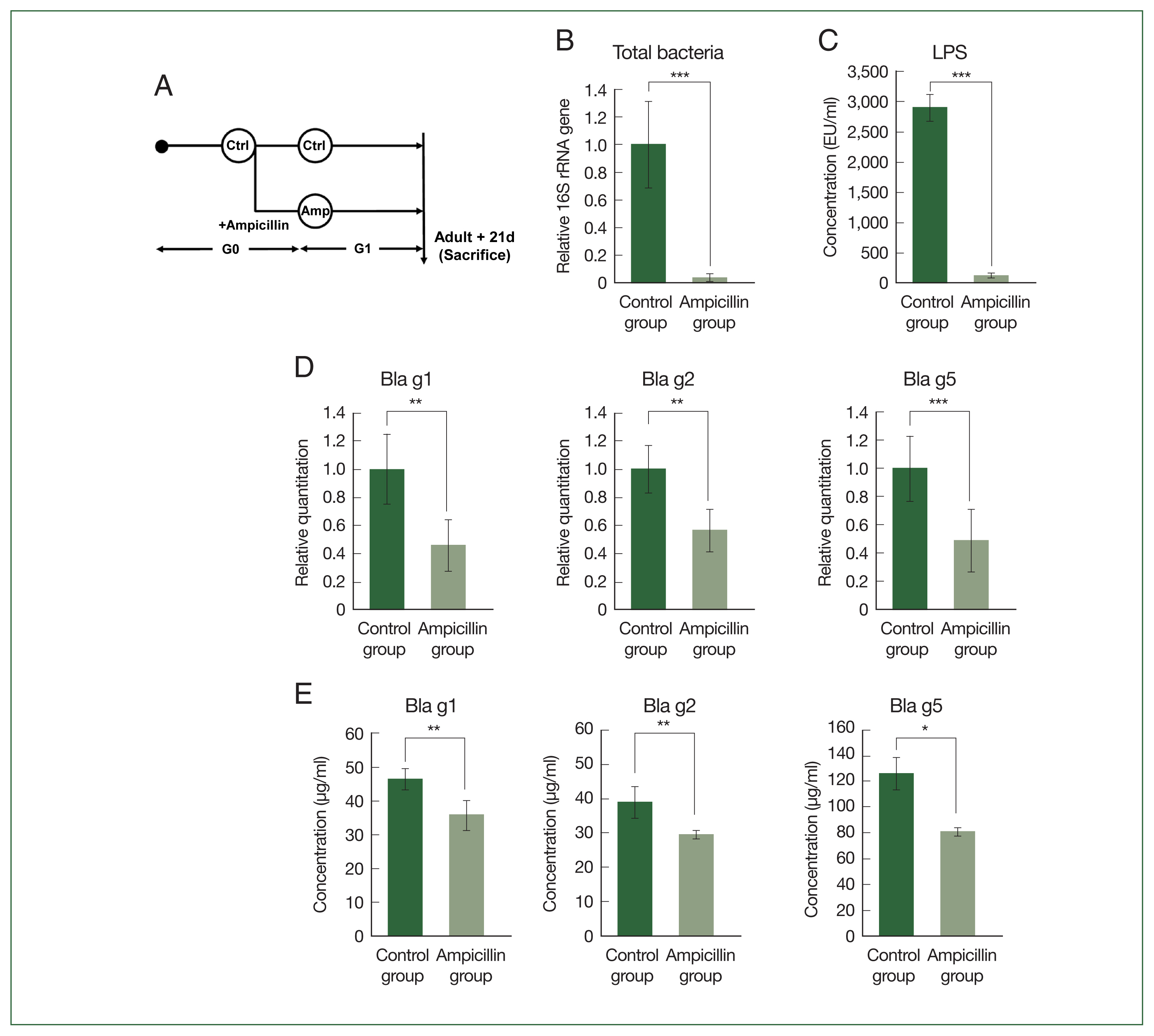
First, we checked whether the total bacterial count of the cockroaches was reduced by antibiotic treatment during rearing. As shown in
Fig. 1B, qPCR analysis demonstrated that the total number of bacteria in the cockroaches was 20 times higher in the control group than in the ampicillin group. We also observed a significant decrease in lipopolysaccharide, a bacteria-derived substance, in the ampicillin group (
Fig. 1C). The gene expression levels of the 3 major allergens (
Bla g1, Bla g2, and
Bla g5) were measured using qPCR. The expression levels of all 3 allergens were reduced in the ampicillin group (
Fig. 1D). When we measured the levels of
Bla g1, Bla g2, and
Bla g5 at the protein level (
Fig. 1E), the results were similar to those obtained from the gene expression analyses.
Bla g1, Bla g2, and
Bla g5 gene expression levels were significantly lower in the ampicillin group than in the control group.
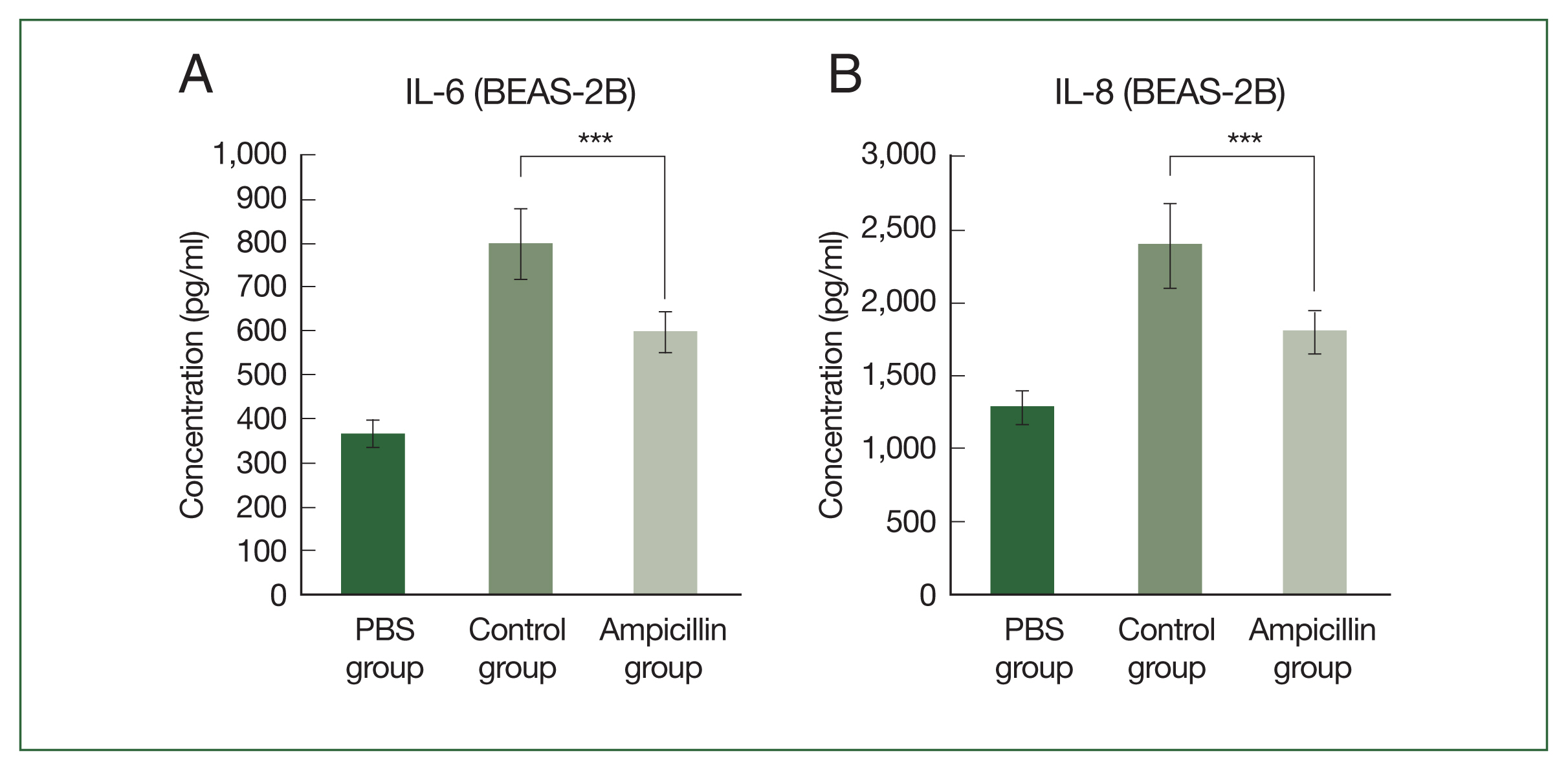
We next examine the effect of extracts from antibiotic-treated cockroaches on airway inflammation. Cytokine secretion was measured by treating airway epithelial cells with the cockroach extracts. When human bronchial epithelial cells (BEAS-2B) were treated with ampicillin-treated cockroach extracts, the secretion of proinflammatory cytokines, such as IL-6 and IL-8, was significantly lower than that in cells treated with the control (
Fig. 2A, B). In the allergen-dose equivalent condition in which the
Bla g5 concentrations of the 2 groups were the same, the difference in cytokine production between groups was reduced, although cytokine production was still lower in the antibiotic-treated group (
Supplementary Fig. S1).
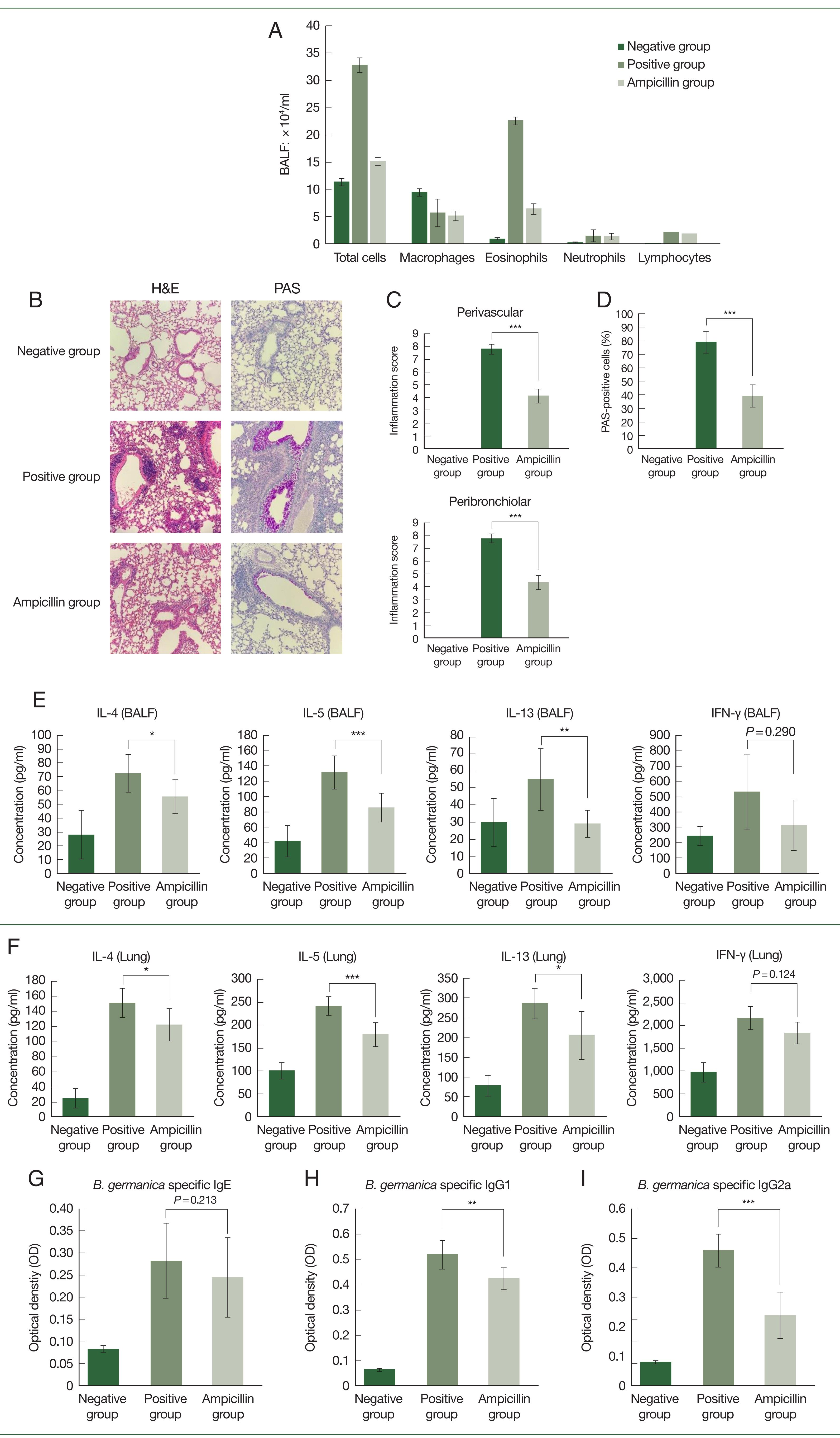
Thereafter, a mouse asthma model was established using GCEs. In BALF, eosinophil levels were lower in the ampicillin group than in the control group (
Fig. 3A). Moreover, lung histopathology showed infiltration of less immune cells in the ampicillin group compared to the control group (
Fig. 3B–D). The ampicillin group also had lower immune cell infiltration and inflammation scores than the control group (
Fig. 3C, D). Furthermore, the concentrations of IL-4, IL-5, and IL-13 in the BALF and lung tissue were lower in the ampicillin group than in the control group (
Fig. 3E, F). However, we observed no significant difference in IFN-γ levels between the 2 groups (
Fig. 3E, F). Serum immunoglobulin levels were also measured.
B. germanica-specific IgE levels increased in both asthma groups, with no significant difference between these groups (
Fig. 3G). Serum IgG1 and IgG2a levels were lower in the ampicillin group than in the control group (
Fig. 3H, I).
Discussion
Previous research confirmed a reduction in bacterial abundance and diversity in German cockroaches treated with ampicillin [
4]. Similarly, we found that cockroaches reared under ampicillin treatment showed reduced amounts of total bacteria in their bodies. Further, ampicillin-treated cockroaches showed reduced amounts of lipopolysaccharides (LPS), an immunomodulatory molecule derived from bacteria. Although not measured in this study, we expect that several microbe-associated molecules other than LPS also decreased in the extract of ampicillin-treated cockroaches. Similar to a previous study, levels of the major allergens such as
Bla g1, Bla g2, and
Bla g5 were significantly decreased at both the protein and mRNA levels.
Bla g1 is a strong risk factor for sensitization [
16].
Bla g2 is the most important allergen in German cockroaches and typically has the highest sensitization rate of 54–71% [
17]. In a previous study, out of 23 sera from a cockroach-sensitized cohort, 12 (37.5%) showed positive IgE reactivity against recombinant
Bla g5 [
18]. This study confirmed a reduction in total bacteria caused by antibiotic treatment, as well as changes in gene expression, which led to a reduction of allergen levels [
4].
Moreover, IL-6 and IL-8 levels were decreased in airway epithelial cells (BEAS-2B) treated with ampicillin-treated cockroach extract. IL-6 is a major proinflammatory cytokine responsible for immune response activation [
19]. IL-8 is also a proinflammatory cytokine with proangiogenic, proliferative, and promotility activities [
20].
The mechanism underlying cockroach allergen-induced sensitization involves disturbance of airway epithelial integrity by cockroach-derived proteases, which leads to increased cockroach allergen penetration. Proteases can modulate airway epithelial cell responses: i.e., cockroach-derived and other proteases can induce airway epithelial cell responses through G protein-coupled proteinase-activated receptors (PARs) [
21]. Stimulation of PAR2 can induce airway epithelial cells to release Granulocyte-macrophage colony-stimulating factor (GM-CSF), a neutrophil and eosinophil chemoattractant and a survival factor [
22]. A previous study reported that house dust mite group 1 allergens and endotoxins are recognized by PAR2 and Toll-like receptor 4, thus stimulate the secretion of IL-6 and IL-8 in airway epithelial cells. In addition, LPS inhibitor treatment reduces the amount of IL-6 and IL-8 released from BEAS-2B cells after treatment with house dust mite extract. This indicates that bacterial components, such as endotoxins, act synergistically on airway epithelial cells to induce an inflammatory response [
23]. Similar to these findings, reduced endotoxin levels in GCE owing to antibiotic usage are likely associated with a decrease in allergic airway inflammation in bronchial epithelial cells.
We confirmed that our asthma model was sensitized to intranasal injections with cockroach extract. We observed that eosinophil count in the BALF was reduced in the ampicillin group. Moreover, eosinophilic airway inflammation, a key feature of allergic airway diseases including allergic asthma, was significantly reduced in the ampicillin group. Similarly, in lung sections, perivascular and peribronchiolar immune cell infiltration scores were significantly reduced. Further, PAS staining showed a significant decrease in the number of mucus-producing cells. In the serum, IgE levels were not significantly different but showed a decreasing trend from that in the control group. The levels of eosinophils, PAS, IL-4, and IL-5 in the ampicillin-treated group were significantly lower than those in the positive group; however, the IgE level did not show any significant difference. As IgE is already produced during the sensitization phase, there may be no significant difference in serum after mouse sacrifice. IgG1 and IgG2a levels were significantly lower in the ampicillin group than in the control group. IgG1 and IgG2a are immunoglobulins associated with T helper 2 (Th2) and T helper 1 (Th1) responses, respectively. Levels of the cytokines such as IL-4, IL-5, IL-13, and IFN-γ were measured in both BALF and lung homogenates. Although there was no significant difference in IFN-γ levels between the ampicillin treated group and control group, IL-4, IL-5, and IL-13 levels were significantly lower in the ampicillin group than in the control group. Thus, we confirmed decreases in the Th2 related cytokines and immunoglobulin (IgG1), which is correlated with reduced airway inflammation in ampicillin group.
A reduction in airway inflammation was also confirmed in BEAS-2B cells and in the mouse asthma model induced by cockroach extracts. As total bacteria in the cockroaches were greatly reduced by ampicillin treatment, it is expected that their extract contained less LPS and other bacteria-derived substances. During protein extraction, bacteria were removed using a filter. However, bacteria-derived substances cannot be filtered out. Further, extracts from ampicillin-treated German cockroaches showed lower levels of the major allergens Bla g1, 2, and 5. Thus, we thought that the reduced airway inflammation might be caused by a decrease in major allergen levels and the absence of bacteria-derived substances followed by the decrease in total bacteria.
In the allergen-dose equivalent condition, the difference in cytokine production was smaller than that shown in
Fig. 2, which highlighted the role of allergens on cytokine production. Nevertheless, cockroaches in the ampicillin-treated group produced less cytokine than those in the control group (although the difference was not statistically significant). The reduction in cytokine production may be attributable to the decreased bacterial load following antibiotic treatment.
GCEs have not yet been standardized for immunotherapy or diagnosis. A previous study reported that the potency of the allergic response in patients varies according to the level of allergens present in German cockroaches [
18]. Further, it is important to prepare several extracts because the T cell potency of each extract differs depending on the most dominant allergen for the patient [
9]. Although the levels of major allergens were lower in the protein extract of cockroaches treated with ampicillin, the levels of all proteins did not decrease, but the pattern changed. Therefore, the use of extracts from ampicillin-treated German cockroaches is considered a suitable approach for preparing various extracts for immunotherapy and diagnosis.
The amount of LPS or major allergens in the extract differs depending on the extraction method or rearing environment of the cockroaches [
4]. From an environmental perspective, large amounts of antibiotics are discharged into rivers and lakes [
24]. As cockroaches are omnivores and inhabit sewers, they are expected to consume water containing antibiotics. In this experiment, we only used ampicillin-treated German cockroaches to assess their allergen production and ability to induce airway inflammation. However, wild cockroaches consume multiple types of antibiotics [
24]. Wild cockroaches are expected to exhibit variable allergen ability and airway inflammation in humans. Further research is required to determine the changes in the ability of cockroaches to cause allergies in different countries or regions, owing to exposure to different APIs.
This study has limitations. Cytokines were evaluated only in BALF and lung homogenates but not in lymph nodes or spleen. In future research, we will evaluate cytokines in lymph nodes and spleen. Depending on the rearing season of cockroaches or the amount of water and food intake, differences may occur in. The degree of reduction of bacteria and allergens in cockroach protein extract may vary, depending on the season in which the cockroaches are reared, and the amount of water and food that they consume.
In conclusion, German cockroaches treated with ampicillin caused reduced airway inflammation in human epithelial cells and mice. It is expected that the extract developed in this study can contribute to research into the standardization of extracts for immunotherapy and diagnosis, as well as the development of various extracts for future clinical trials.
Notes
-
Author contributions
Conceptualization: Lee S, Yi MH, Yong TS, Kim JY
Data curation: Lee S, Kim SL, Yong TS
Formal analysis: Lee S
Resources: Jang YS
Software: Choi JH, Kim SL
Writing – original draft: Lee S, Kim JY
Writing – review & editing: Yong TS, Kim JY
-
The authors declare no conflicts of interest.
Supplementary Information
Supplementary Fig. S1
Concentrations of (A) IL-6 and (B) IL-8 secreted from human bronchial epithelial cells (BEAS-2B) exposed to cockroach extracts in a dose-dependent manner, with allergen-dose equivalent condition. The extract was adjusted to contain the same amount of Bla g5 in both groups.
Acknowledgment
This study was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (NRF–2020R1I1A2074562).
Fig. 1Total bacteria and major allergens of German cockroaches treated with ampicillin. (A) Experimental design depicting ampicillin treatment in B. germanica. The cockroaches were divided into 2 groups, and individuals were either treated with ampicillin (ampicillin group: A) or left untreated as control specimens (control group: C). Ampicillin was administered to cockroaches from the G1 (i.e., offspring from G0) generation, 21 days after they reached the adult stage. (B) Relative levels of bacterial 16S rRNA genes in the control group and ampicillin group. (C) Concentration of lipopolysaccharides (LPS) in 1 mg/ml of B. germanica extract. (D) Quantitative PCR (qPCR) analysis showing Bla g1, Bla g2, and Bla g5 gene expression levels in German cockroaches. (E) Allergen levels in extracts from the 2 German cockroach groups. *P<0.05, **P<0.01, ***P<0.001.
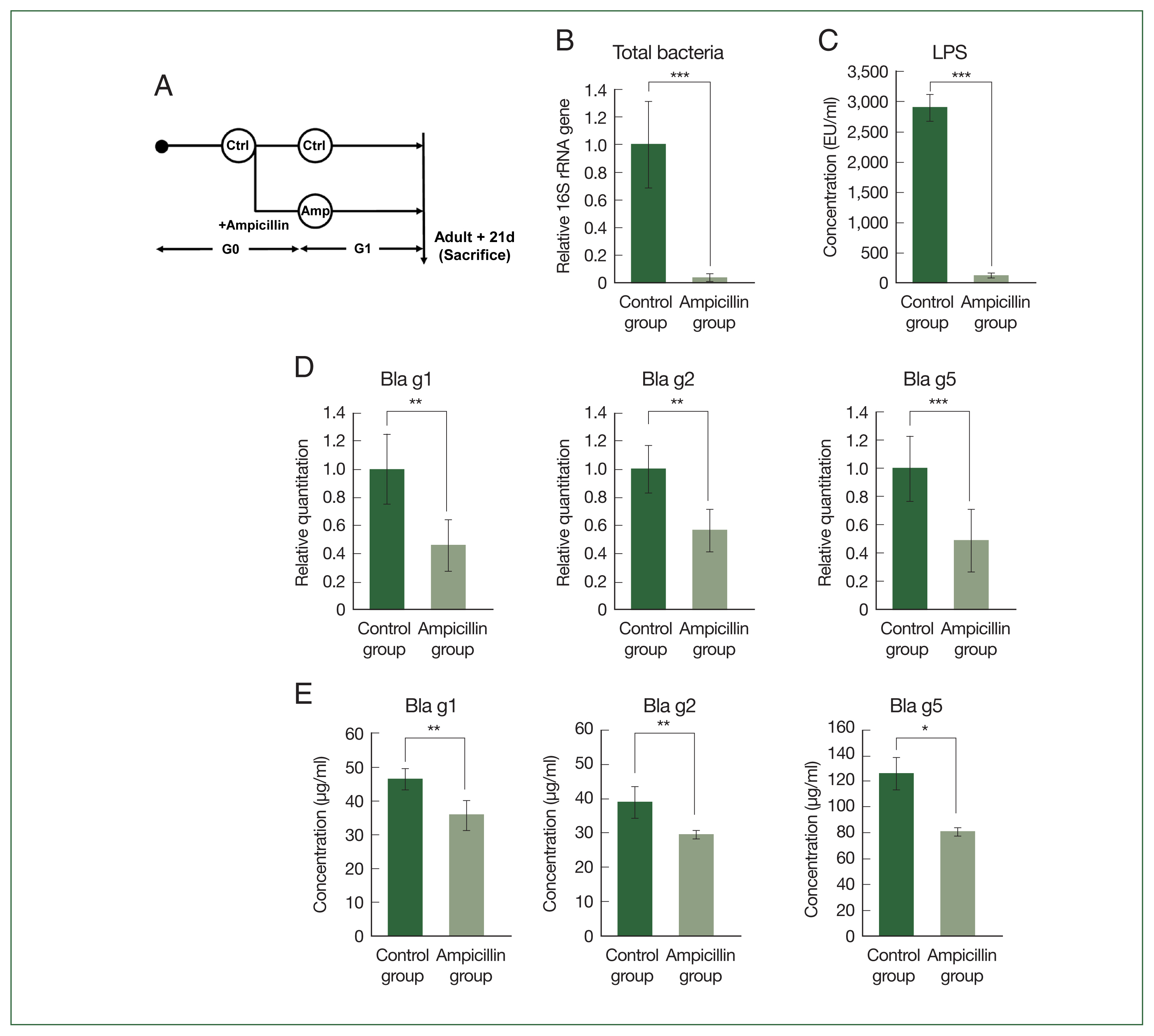
Fig. 2Effect of exposure to extracts of antibiotic-treated cockroaches on cytokine expression in bronchial epithelium in vitro. Concentrations of (A) IL-6 and (B) IL-8 secreted from human bronchial epithelial cells (BEAS-2B) exposed to the extract of German cockroaches treated with 0.03% ampicillin (ampicillin group). ***P<0.001.
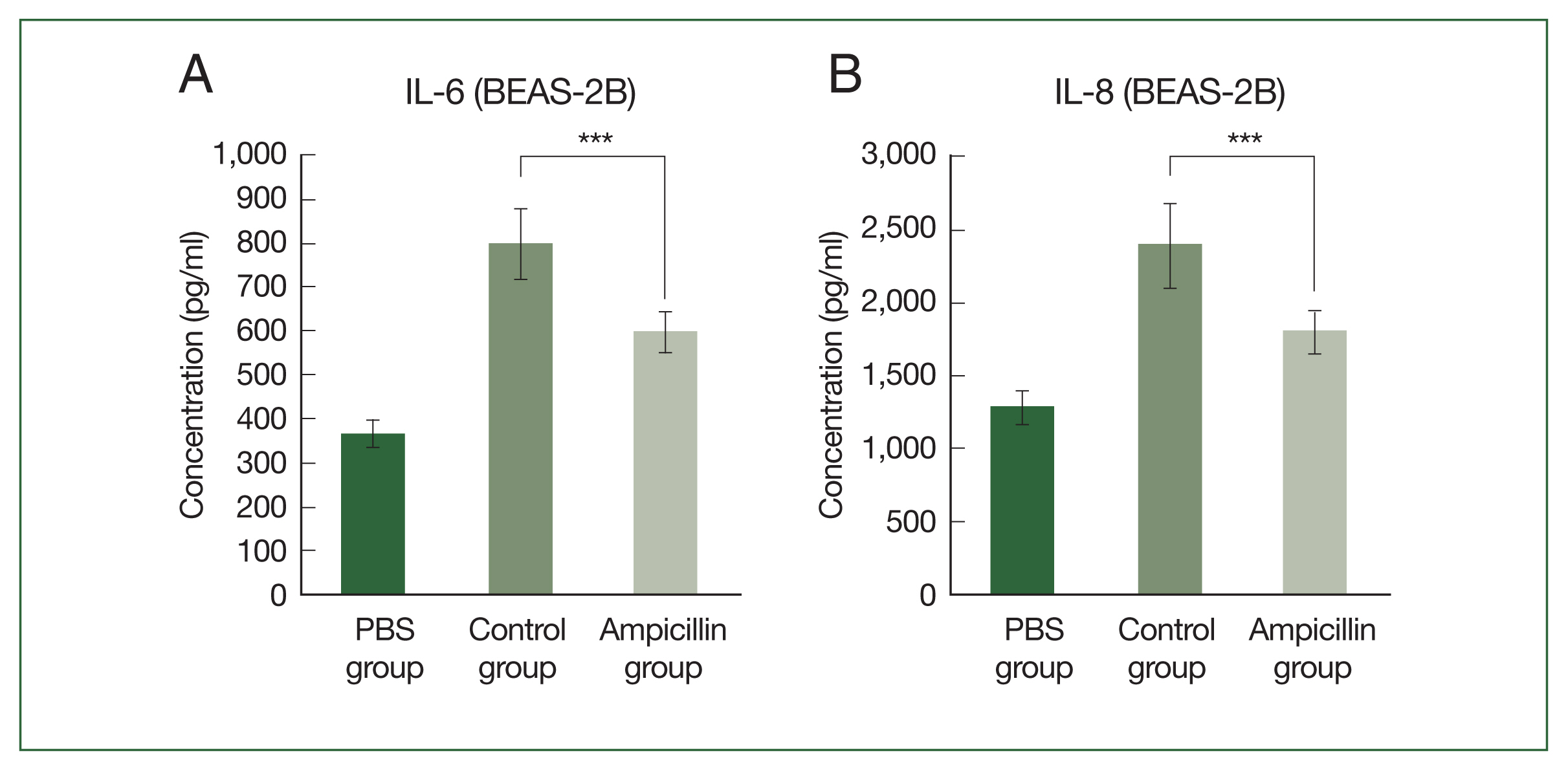
Fig. 3Effect of exposure to extracts of antibiotic-treated cockroaches in a mouse model of asthma. (A) Number of total cells, macrophages, eosinophils, neutrophils, and lymphocytes in the bronchoalveolar lavage (BAL) fluid of asthma model mice. (B–D) Lung histology results in the mouse model of allergic airway inflammation. (B) Histologic results with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining of lung tissues. (C, D) Immune cell infiltration scores and inflammation scores including mucus production scores. Data are reported as mean±SE (n=8/group). (E, F) Supernatants of BAL fluid were collected after centrifugation, and the production of IL-4, IL-5, IL-13, and IFN-γ was measured. Concentrations of IL-4, IL-5, IL-13, and IFN-γ in lung tissues were analyzed using the respective ELISA kits. Comparison of serum B. germanica-specific (G) IgE, (H) IgG1, and (I) IgG2a levels among B. germanica-induced asthmatic mice groups. Optical density values measured during ELISA are also presented. Data are reported as mean±SE (n=8/group). One-way ANOVA was conducted, and the Bonferroni-corrected p-values are presented. PBS group: PBS-treated group; Control group: group exposed to normal German cockroach extract; Ampicillin group: group exposed to ampicillin-treated German cockroach extract. *P<0.05, **P<0.01, ***P<0.001.
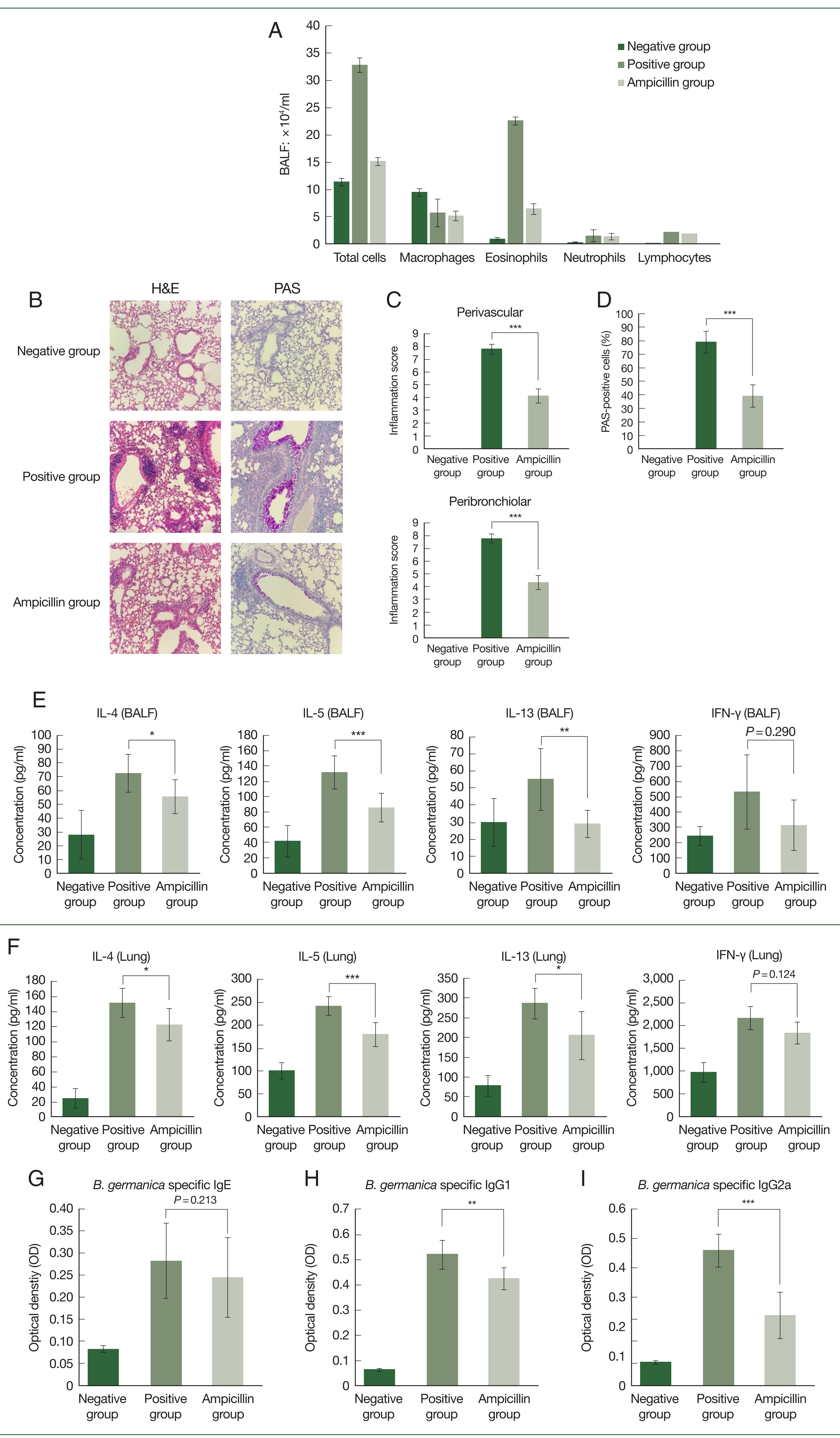
Table 1Primers used in this study
Table 1
|
Primer name |
Primer sequence (5′ → 3′) |
|
ActinF |
CACATACAACTCCATTATGAAGTGCGA |
|
ActinR |
TGTCGGCAATTCCGGTACATG |
|
BACT1369 |
CGGTGAATACGTTCYCGG |
|
PROK1492R |
GGWTACCTTGTTACGACTT |
|
Blag1F |
CTATATGACGCCATCCGTTCTC |
|
Blag1R |
CACATCAACTCCCTTGTCCTT |
|
Blag2F |
TGATGGGAATGTACAGGTGAAA |
|
Blag2R |
TGTTGAGATGTCGTGAGGTTAG |
|
Blag5F |
GATTGATGGGAAGCAAACACAC |
|
Blag5R |
CGATCTCCAAGTTCTCCCAATC |
References
- 1. Do DC, Zhao Y, Gao P. Cockroach allergen exposure and risk of asthma. Allergy 2016;71(4):463-474. https://doi.org/10.1111/all.12827
- 2. Taylor D. The pharmaceutical industry and the future of drug development. In Hester RE, Harrison RM eds, Pharmaceuticals in the Environment; Royal Society of Chemistry; London, UK. : 2015. 1-33 https://doi.org/10.1039/9781782622345-00001
- 3. Rosas T, García-Ferris C, Domínguez-Santos R, Llop P, Latorre A, et al. Rifampicin treatment of Blattella germanica evidences a fecal transmission route of their gut microbiota. FEMS Microbiol Ecol 2018;94:https://doi.org/10.1093/femsec/fiy002
- 4. Lee S, Kim JY, Yi MH, Lee IY, Yong D, et al. Reduced production of the major allergens Bla g1 and Bla g2 in Blattella germanica after antibiotic treatment. PLoS One 2021;16(11):e0257114. https://doi.org/10.1371/journal.pone.0257114
- 5. Gore JC, Schal C. Gene expression and tissue distribution of the major human allergen Bla g1 in the German cockroach, Blattella germanica L. (Dictyoptera: Blattellidae). J Med Entomol 2004;41(5):953-960. https://doi.org/10.1603/0022-2585-41.5.953
- 6. Mueller GA, Pedersen LC, Lih FB, Glesner J, Moon AF, et al. The novel structure of the cockroach allergen Bla g1 has implications for allergenicity and exposure assessment. J Allergy Clin Immunol 2013;132(6):1420-1426. https://doi.org/10.1016/j.jaci.2013.06.014
- 7. Arruda LK, Vailes LD, Mann BJ, Shannon J, Fox JW, et al. Molecular cloning of a major cockroach (Blattella germanica) allergen, Bla g2. Sequence homology to the aspartic proteases. J BiolChem 1995;270(33):19563-19568. https://doi.org/10.1074/jbc.270.33.19563
- 8. Jeong KJ, Jeong KY, Kim CR, Yong TS. IgE-binding epitope analysis of Bla g5, the German cockroach allergen. Protein Pept Lett 2010;17:573-577. https://doi.org/10.2174/092986610791112765
- 9. Birrueta G, Frazier A, Pomés A, Glesner J, Filep S, et al. Variability in German cockroach extract composition greatly impacts T cell potency in cockroach-allergic donors. Front Immunol 2019;10:313. https://doi.org/10.3389/fimmu.2019.00313
- 10. Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl AcadSci U S A 2007;104(21):8897-8901. https://doi.org/10.1073/pnas.0609568104
- 11. Wellington EMH, Boxall AB, Cross P, Feil EJ, Gaze WH, et al. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect Dis 2013;13(2):155-165. https://doi.org/10.1016/S1473-3099(12)70317-1
- 12. Shahraki GH, Parhizkar S, Nejad ARS. Cockroach infestation and factors affecting the estimation of cockroach population in urban communities. Int J Zool 2013;2013:649089. https://doi.org/10.1155/2013/649089
- 13. Ordoñez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J RespirCrit Care Med 2000;161(4 pt 1):1185-1190. https://doi.org/10.1164/ajrccm.161.4.9812061
- 14. Lim S, Ho Sohn J, Koo JH, Park JW, Choi JM. dNP2-ctCTLA-4 inhibits German cockroach extract-induced allergic airway inflammation and hyper-responsiveness via inhibition of Th2 responses. ExpMol Med 2017;49(8):e362. https://doi.org/10.1038/emm.2017.107
- 15. Woo LN, Guo WY, Wang X, Young A, Salehi S, et al. A 4-week model of house dust mite (HDM) induced allergic airways inflammation with airway remodeling. Sci Rep 2018;8(1):6925. https://doi.org/10.1038/s41598-018-24574-x
- 16. Pomés A, Vailes LD, Helm RM, Chapman MD. IgE reactivity of tandem repeats derived from cockroach allergen, Bla g1. Eur J Biochem 2002;269(12):3086-3092. https://doi.org/10.1046/j.1432-1033.2002.02990.x
- 17. Sohn MH, Kim KE. The cockroach and allergic diseases. Allergy Asthma Immunol Res 2012;4(5):264-269. https://doi.org/10.4168/aair.2012.4.5.264
- 18. Glesner J, Filep S, Vailes LD, Wünschmann S, Chapman MD, et al. Allergen content in German cockroach extracts and sensitization profiles to a new expanded set of cockroach allergens determine in vitro extract potency for IgE reactivity. J Allergy Clin Immunol 2019;143(4):1474-1481e8. https://doi.org/10.1016/j.jaci.2018.07.03
- 19. Anton K, Glod J. Tumor-secreted factors that induce mesenchymal stromal cell chemotaxis. In Bolontrade MF, García MG eds, Mesenchymal Stromal Cells as Tumor Stromal Modulators. Academic Press; Cambridge USA. 2017, pp 193-214 https://doi.org/10.1016/B978-0-12-803102-5.00008-2
- 20. Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy 2003;33(1):35-42. https://doi.org/10.1046/j.1365-2222.2002.01481.x
- 21. Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol 2001;108(5):797-803. https://doi.org/10.1067/mai.2001.119025
- 22. Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol 2001;107(4):679-685. https://doi.org/10.1067/mai.2001.114245
- 23. Kim JY, Yi MH, Lee S, Lee IY, Yong D, et al. Microbiome and mycobiome interaction in house dust mites and impact on airway cells. Clin Exp Allergy 2021;51(12):1592-1602. https://doi.org/10.1111/cea.13962
- 24. Wilkinson JL, Boxall ABA, Kolpin DW, Leung KMY, Lai RWS, et al. Pharmaceutical pollution of the world’s rivers. Proc Natl AcadSci U S A 2022;119(8):e2113947119. https://doi.org/10.1073/pnas.2113947119
 , Myung-Hee Yi
, Myung-Hee Yi , Yun Soo Jang, Jun Ho Choi, Myungjun Kim, Soo Lim Kim, Tai-Soon Yong
, Yun Soo Jang, Jun Ho Choi, Myungjun Kim, Soo Lim Kim, Tai-Soon Yong , Ju Yeong Kim,*
, Ju Yeong Kim,*