Abstract
In elderly patients, ocular toxoplasmosis is one of the most common etiologies of uveitis, which should be differentially diagnosed from ocular lymphoma, another common pathology of uveitis in older adults. The high level of interleukin (IL)-10 and an IL-10/IL-6 ratio higher than 1 (>1.0) are helpful parameters to diagnose ocular lymphoma. In this study, we used aqueous humor samples to detect 4 cases of ocular toxoplasmosis in patients with high levels of IL-10 and an IL-10/IL-6 ratio higher than 1. Our results show that ocular toxoplasmosis may be associated with increased cytokine levels in aqueous humor.
-
Key words: Toxoplasma gondii, ocular toxoplasmosis, uveitis, intraocular lymphoma, Interleukin-10, cytokines
Toxoplasma gondii is an obligate intracellular protozoan parasite that can infect warm-blooded vertebrates, including humans, causing a disease known as toxoplasmosis [
1]. Approximately one third of the world’s population is estimated to be chronically infected with
T. gondii [
1]. Toxoplasmosis can be congenital or acquired [
2]. The prevalence of toxoplasmosis varies widely from 5% to 90%, depending on the region, and environment. This disease may be influenced by climate conditions, consumption behaviors (e.g., consuming raw meat or unwashed fruits and vegetables), and environmental hygiene standards [
3]. In general, disease prevalence is higher in warm and humid climates.
Toxoplasma gondii infection is a common cause of posterior uveitis in individuals with healthy immune systems [
4]. Toxoplasmic retinochoroiditis is a major determinant of visual impairment, accounting for 30–55% of cases of posterior uveitis in areas that show a high prevalence of
T. gondii infection, particularly in the United States and European countries [
5]. The frequency of ocular toxoplasmosis significantly increases with age, and therefore it should be considered when examining elderly patients with uveitis.
Patients over 35 years old and with a history of posterior uveitis should be considered at risk of suffering from primary intraocular lymphoma (PIOL). This malignant lymphoproliferation exhibits clinical features that resemble ocular infections, including ocular toxoplasmosis [
6]. The biochemical determination of an IL-10/IL-6 interleukin ratio higher than 1 has recently been helpful in diagnosing PIOL, particularly in cases where the predictive value of the cytological examination is poor.
During parasitic infection, lymphocytes produce specific cytokines that play critical roles against parasitic diseases. For instance, IL-6 promotes the production of antibodies and exerts a proinflammatory effect via the induction of acute-phase protein production. Moreover, IL-10 influences the type of immune response by inhibiting the production of proinflammatory cytokines and blocking the production of IL-6.
In this study, we reported 4 cases of patients with ocular toxoplasmosis whose cytokine levels in the aqueous humor were examined for differential diagnosis of PIOL. We assessed humoral and cellular responses to draw parallelisms between these clinical cases and patients with PIOL.
This study adheres to the tenets of the Declaration of Helsinki. All protocols were approved by the Institutional Review Board in our institution (KC23RISI0251). In this study, we reviewed the medical records of 4 patients (3 males and 1 female) with panuveitis. The study patients were treated after diagnosis at the Uveitis Service of the Ophthalmology Department, Seoul St. Mary’s Hospital.
Ocular toxoplasmosis was diagnosed based on the sudden onset of visual symptoms, the presence of specific inflammation and/or hyperpigmented scars in the retina and choroid, and laboratory test evidence of T. gondii infection. The enzyme-linked immunosorbent assay (ELISA) was carried out to determine serum IgG/IgM antibodies against T. gondii. The cut-offs for IgG antibodies against T. gondii were as follows: 1) negative, proportion <8 IU/ml; borderline, 11 IU/ml >proportion >8 IU/ml; 2) positive, proportion >11 IU/ml. The cut-offs for IgM antibodies against T. gondii were as follows: 1) negative, proportion <0.8 IU/ml; borderline, 1.1 IU/ml >proportion >0.8 IU/ml; 2) positive, proportion >1.1 IU/ml. The presence of T. gondii in aqueous humor and blood samples was detected by the polymerase chain reaction (PCR) analysis.
For differential diagnosis, we examined the cytokines levels in the aqueous humor of the study patients on their first visit to the medical center. A total of approximately 0.1 ml aqueous humor was drawn by a standard sterile procedure consisting of inserting a 30-gauge needle into the anterior chamber through the temporal limbus. The cytokine levels were quantified using the MILLIPLEX MAP Human Cytokine Magnetic Bead Panel (Merck Millipore, Billerica, MA, USA), according to the manufacturer’s instructions. We incubated samples containing standards and quality controls with magnetic beads coated with antibodies specific to each cytokine. To ensure consistency, the experiment was repeated twice. The samples were analyzed using the LUMINEX MAGPIX system (Luminex Corp., Austin, TX, USA). The levels of IFN-γ, IL-10, IL-12, IL-17, IL-2, IL-6, and TNF-α cytokines were determined and compared with the reference values specified by the manufacturer (48.4–127.6 pg/ml, 2.4–6.6 pg/ml, 8.6–27.2 pg/ml, 6.5–38.5 pg/ml, 1.1–9.2 pg/ml, 1.1–10.8 pg/ml and 14.2–61.7 pg/ml, respectively).
The demographic and general characteristics of the study patients are listed in
Table 1. All of them received a comprehensive eye examination, including measurement of the best corrected visual acuity (BCVA), slit-lamp biomicroscopy, tonometry, fundus evaluation with a Goldmann 3-mirror lens and indirect ophthalmoscopy, and fundus fluorescein angiography.
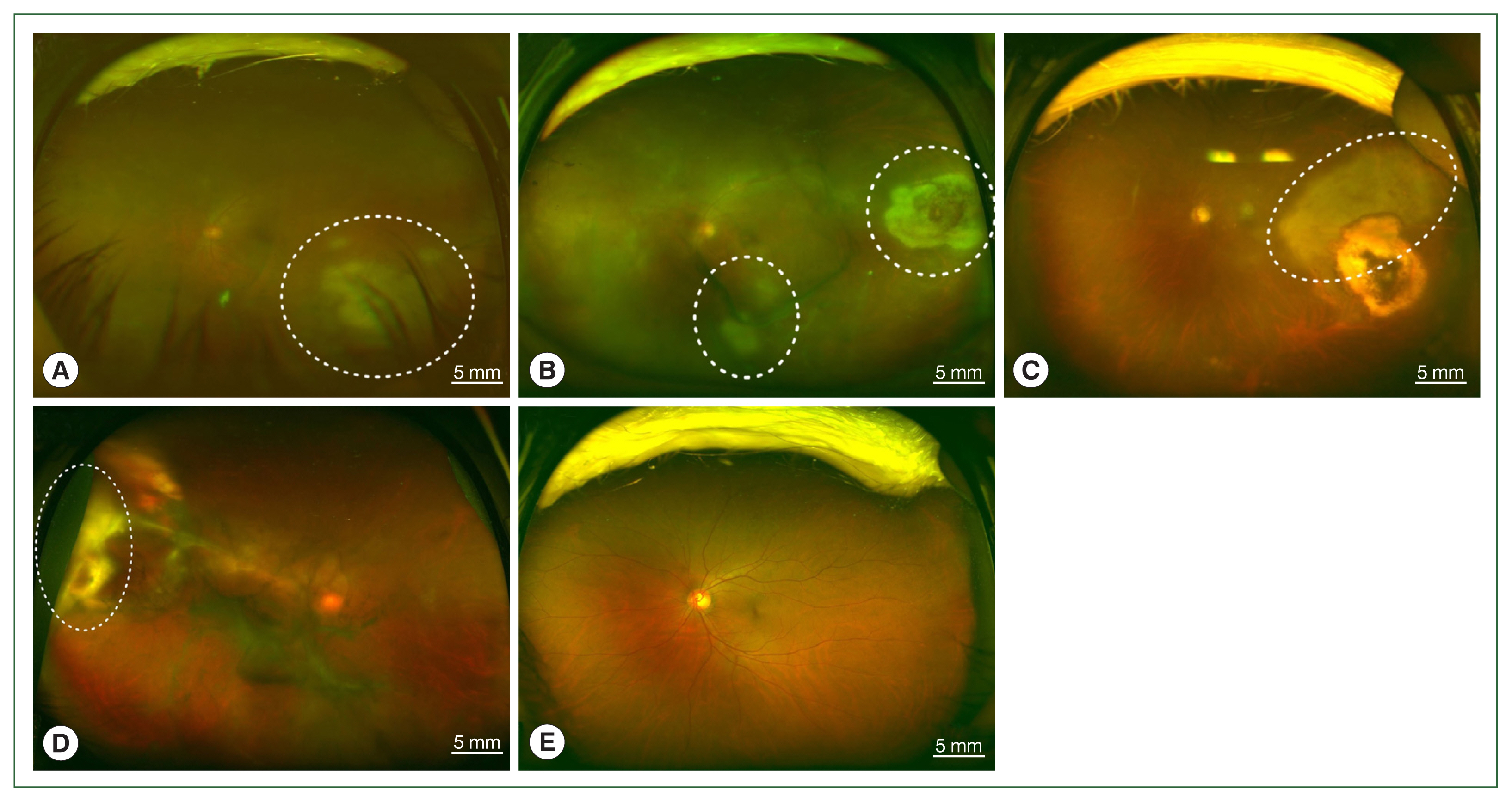
The 4 patients presented persistent unilateral visual disturbance 2–3 months before assessment. The ocular examination revealed that all study patients also exhibited unilateral panuveitis. Two patients (cases 1 and 2) did not show signs of chorioretinal scars, while the other 2 patients (cases 3 and 4) had preexisting scars on the retina and choroid. All of them showed an initial decimal visual acuity between 0.1 and 0.5. The ophthalmic analysis also revealed active retinochoroiditis in the 4 patients (
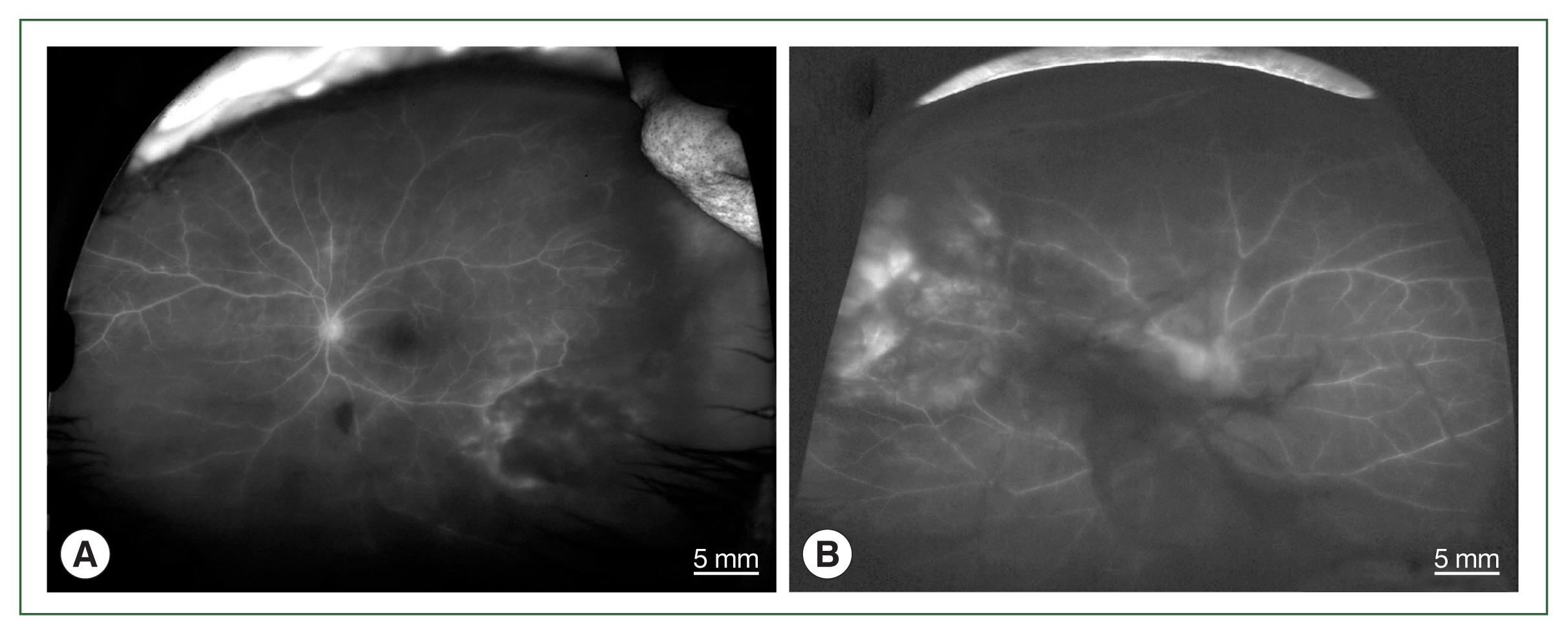
Fig. 1) and papillitis and periphlebitis in cases 1 and 4, which were more evident by fundus fluorescein angiography (
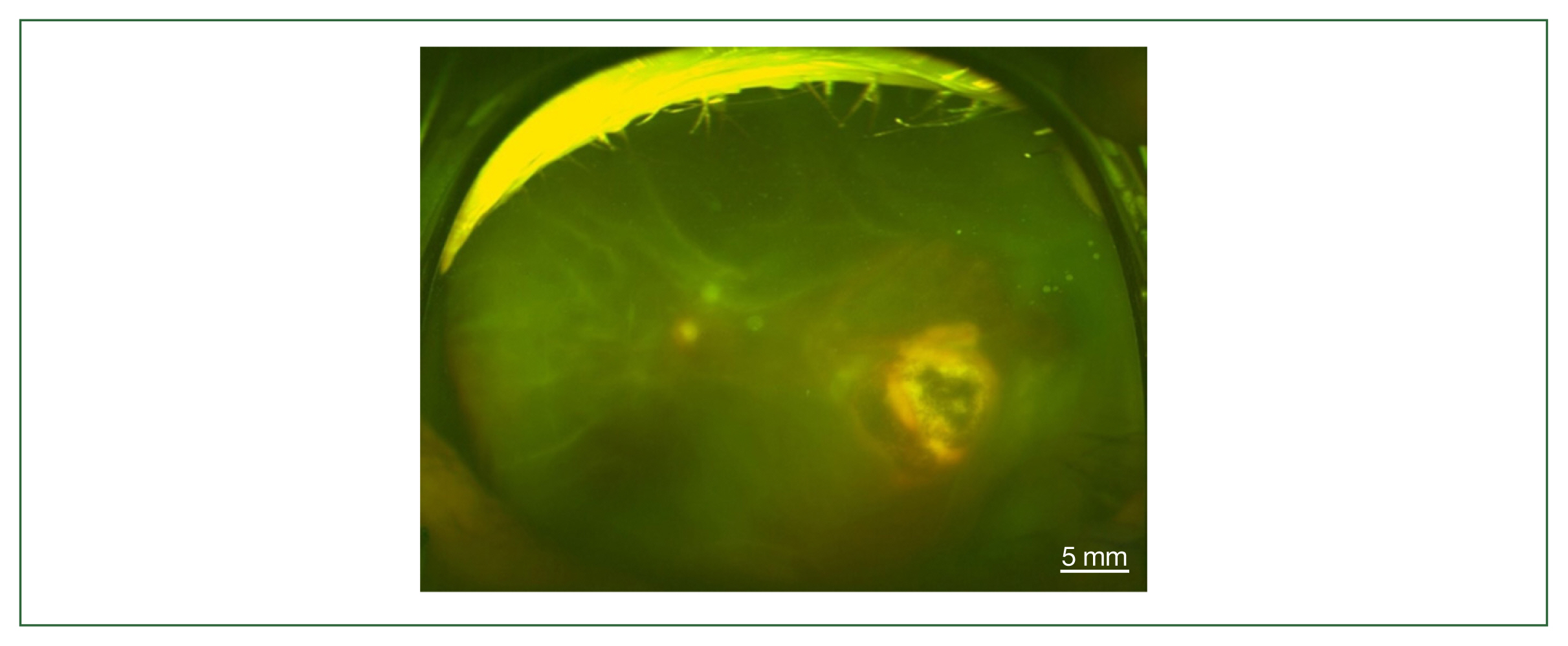
Fig. 2). All study patients also exhibited vitritis and anterior uveitis. Three patients (cases 1, 3, and 4) who underwent vitrectomy for diagnostic and therapeutic reasons had negative cytology for lymphoma cells. The remaining patient (case 3) had re-vitrectomy combined with scleral encircling for total retinal detachments developed later (
Fig. 3). Only 1 patient (case 1), who was previously diagnosed with acute retinal necrosis (ARN) in a local ophthalmology clinic, received a systemic steroid treatment (60 mg per day) for posterior uveitis before visiting the Eye Center at the Seoul St. Mary Hospital, Catholic University. None had received any medication (including antibiotics) before the examination.
All patients exhibited blood cell counts, erythrocyte sedimentation rate, liver, and kidney function tests, chest X-rays, and serum angiotensin-converting enzyme levels within normal values. The serological tests for syphilis and human immunodeficiency virus were negative, while test results for IgG antibodies against T. gondii were positive in all sera. Also, we consulted other specialists for differential diagnosis, whole-body evaluation, and proper management. Brain Magnetic Resonance Imaging suggested meningitis in 1 patient (case 2), but it was discarded after the clinical examination by the neurologist. Thus, we did not find signs of infection with T. gondii in other body parts of the 4 study patients.
The PCR analysis detected infection with
T. gondii but was negative for different viruses and other microbes. In all study cases, cytokine profiling revealed high IL-10 levels and IL-10/IL-6 ratios higher than 1 in aqueous humor samples. In all cases of vitrectomy, the analysis of vitreous samples showed positive PCR detection for
T. gondii and 1 patient (case 1) with high IL-10 production and an IL-10/IL-6 rate >1 (
Table 2).
Ocular toxoplasmosis is a disease caused by
T. gondii, which induces lesions in the posterior portion of the eye [
7]. The most common manifestation of ocular toxoplasmosis is a sight-threatening condition known as posterior uveitis, and it particularly affects individuals with a normal immune system [
4]. Ocular toxoplasmosis is diagnosed by ophthalmic examination and the presence of clinical symptoms that are indicative of infection with
T. gondii in the retina. Ocular toxoplasmosis typically manifests as progressive and recurrent focal necrotizing retinitis and can lead to vision-threatening complications, including retinal detachment, choroidal neovascularization, and glaucoma. Generally, this disease is associated with vitritis and anterior uveitis. Ocular toxoplasmosis may also manifest as an optic nerve inflammation (papillitis). Elevated
T. gondii antibody titers in ocular fluids or detection by PCR can be used to confirm ocular toxoplasmosis in cases where fundoscopic examination fails to establish a definitive diagnosis [
8]. However, seropositivity itself cannot confirm the diagnosis.
We received 4 confusing cases of panuveitis in immunocompetent elderly person that did not exhibit any underlying disease. Two of them (cases 1 and 2) had already been diagnosed with ARN and cytomegalovirus (CMV) retinitis in a local ophthalmology clinic, and these patients were referred to our department due to their unresponsiveness to treatment. We performed a panel of laboratory tests for differential diagnosis and detected anti-Toxoplasma IgG antibodies in sera and T. gondii DNA in the aqueous humor of all study patients. We also found high IL-10 levels and IL-10/IL-6 ratios higher than 1 in the 4 patients, which are outcomes indicative of PIOL.
PIOL manifests with symptoms that resemble various types of ocular infections, including ocular toxoplasmosis [
6]. This pathological condition (PIOL) was first defined as a subtype of primary central nervous system lymphoma (PCNSL), and it is associated with cancerous lymphoma cells present only in the eyes, without any evidence of the disease in the brain or the cerebrospinal fluid [
9]. PIOL often also affects different parts of the central nervous system, including the brain, or the cerebrospinal fluid, at later stages of the disease [
11], thereby diagnosis requires a multidisciplinary approach. If neuroimaging does not reveal any PCNSL lesions and the evaluation of cerebrospinal fluid (CSF) is negative, biopsy remains one of the hallmark procedures in the diagnosis of PIOL [
12]. Tissue biopsy, particularly of the vitreous, is performed through a diagnostic vitrectomy with cytology. But it can be challenging to make a pathologic diagnosis of PIOL [
13]. As a result, researchers have been working to develop additional methods to aid in the diagnosis of PIOL. The levels of certain cytokines may be useful in differentiating PIOL from uveitis. A high level of IL-6 secreted by inflammatory cells is characteristic of uveitis, while elevated IL-10 production by malignant B lymphocytes is a hallmark of intraocular and central nervous system lymphoma [
14]. In addition, an IL-10/IL-6 ratio higher than 1 has strongly been associated with PIOL [
14]; however, the use of the IL-10/IL-6 ratio to diagnose PIOL is controversial because elevated IL-10 and IL-6 levels have also been reported in eyes with non-neoplastic uveitis [
15,
16].
Cytokines secreted by lymphocytes play crucial roles in the development and progression of parasitic diseases. For instance, IL-6 levels increase approximately twofold in individuals infected with
T. gondii compared to healthy individuals, indicating the presence of an inflammatory state caused by the parasite [
17,
18]. Moreover, it has been shown that the IL-10 level is 5 times higher in individuals with toxoplasmosis than those without [
17,
18]. During acute
T. gondii infection, IL-10 plays a crucial role by inhibiting cellular (IL-12, TNF-α) and inflammatory (IL-6) responses [
17,
18]. IL-10 can help mitigate the negative effects of the inflammatory response and suppresses the immune system, which is beneficial for both the host and the parasite [
19].
In conclusion, we examined the cytokines levels in the aqueous humor of 4 patients diagnosed with ocular toxoplasmosis. Interestingly, we found results well known to suggest PIOL. Patients infected with T. gondii can show similar cytokine levels, likely due to an equivalent humoral response. Therefore, if we do not have definite pathological evidence of lymphoma, then we cannot easily conclude PIOL just with cytokine profiles results, especially when ocular toxoplasmosis has not yet been ruled out.
Notes
-
Author contributions
Conceptualization: Kwak JH, Park YH
Data curation: Kim GH, Kwak JH
Formal analysis: Kim GH, Kwak JH
Funding acquisition: Park YH
Investigation: Park YH
Project administration: Park YH
Resources: Kim GH, Kwak JH
Software: Kim GH, Kwak JH
Supervision: Park YH
Validation: Park YH
Visualization: Kim GH, Kwak JH
Writing – original draft: Kim GH
Writing – review & editing: Park YH
-
None of the authors have any conflicting interests to disclose.
Acknowledgment
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2020R1F1A1074898).
Fig. 1Fundus photographs of vitritis (headlight in the fog) with active lesions seen as whitish foci of retinochoroiditis which is marked by white dotted circles; (A) left eye of case 1, (B) left eye of case 2, (C) left eye of case 3, (D) right eye of case 4, and (E) unaffected left eye of case 4.
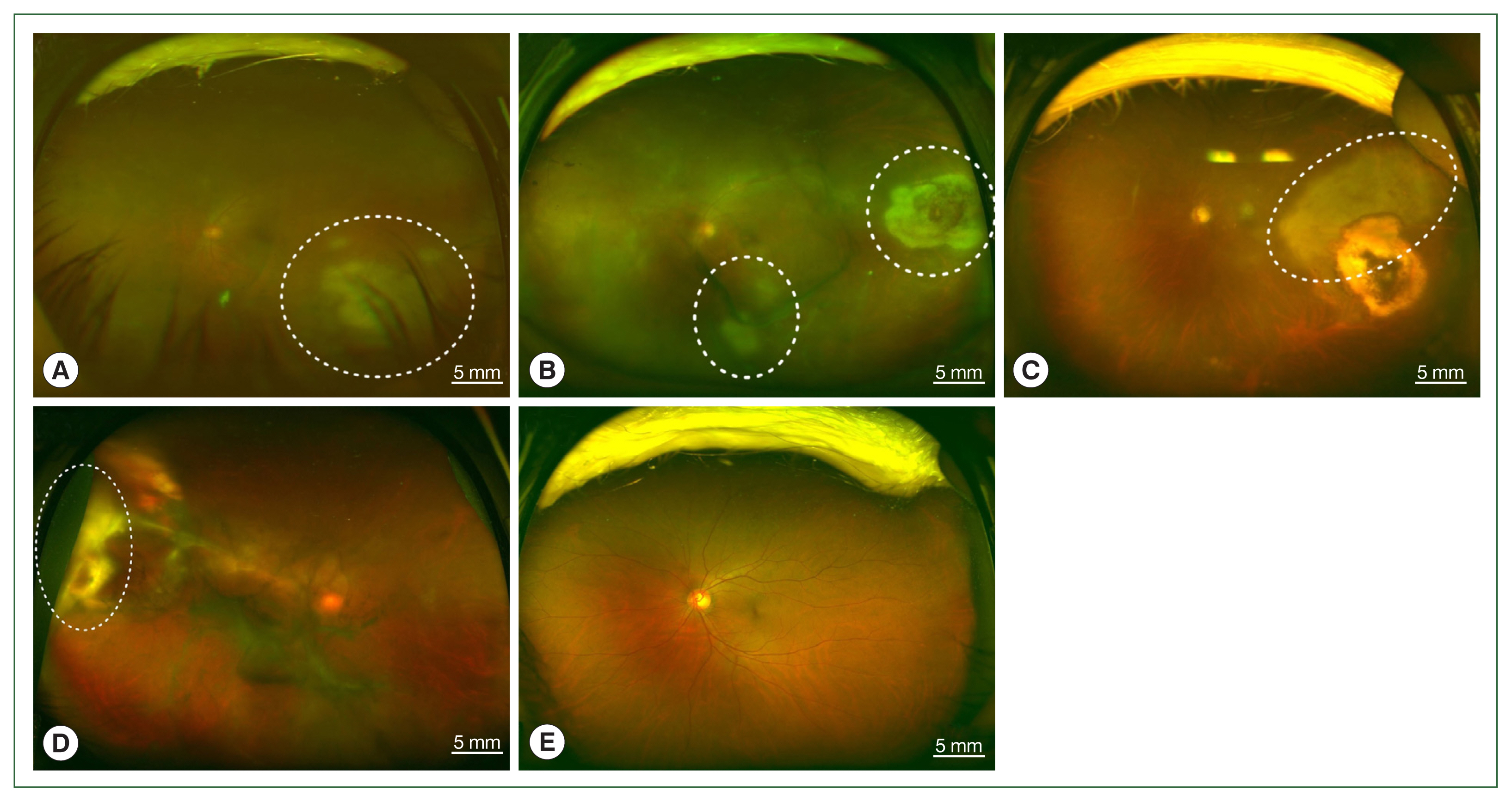
Fig. 2Fundus fluorescein angiographs of papillitis and vasculitis in an ocular toxoplasmosis patient; (A) left eye of case 1, (B) right eye of case 4.
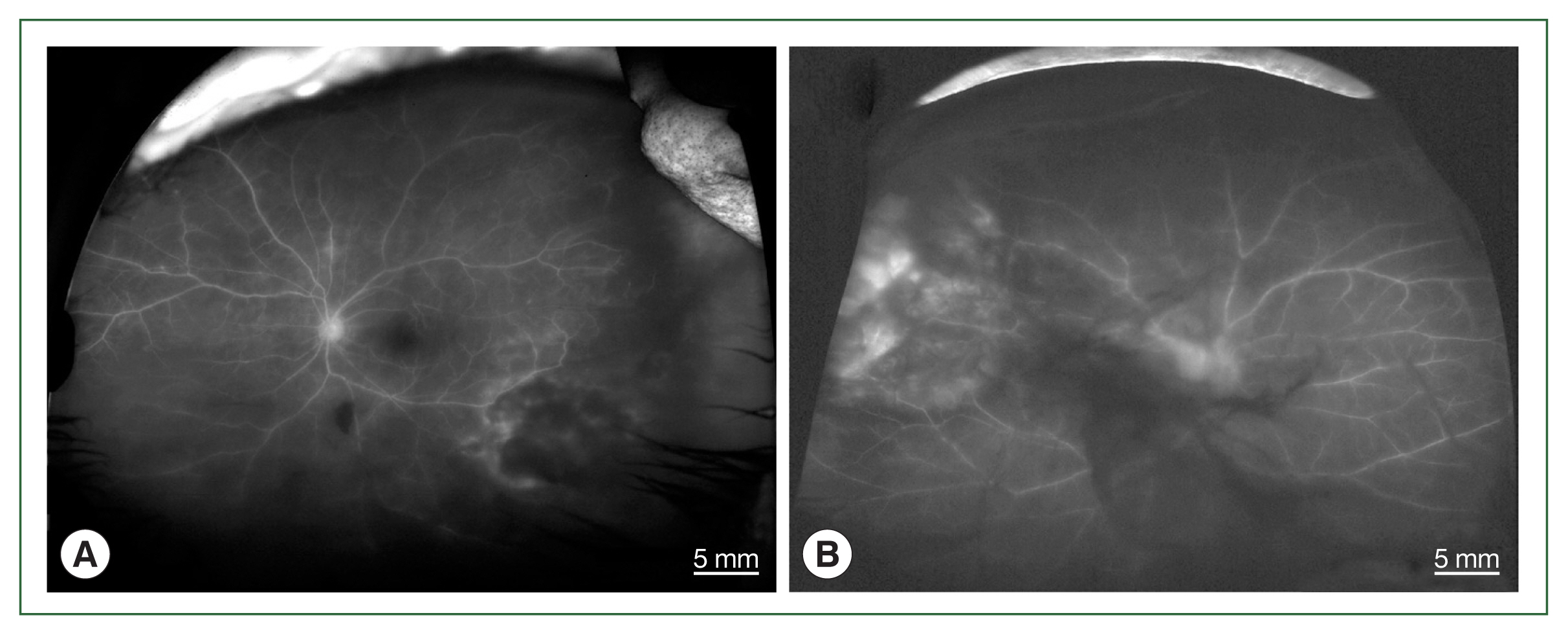
Fig. 3Fundus photography of total retinal detachment associated with active ocular toxoplasmosis (left eye of case 3).
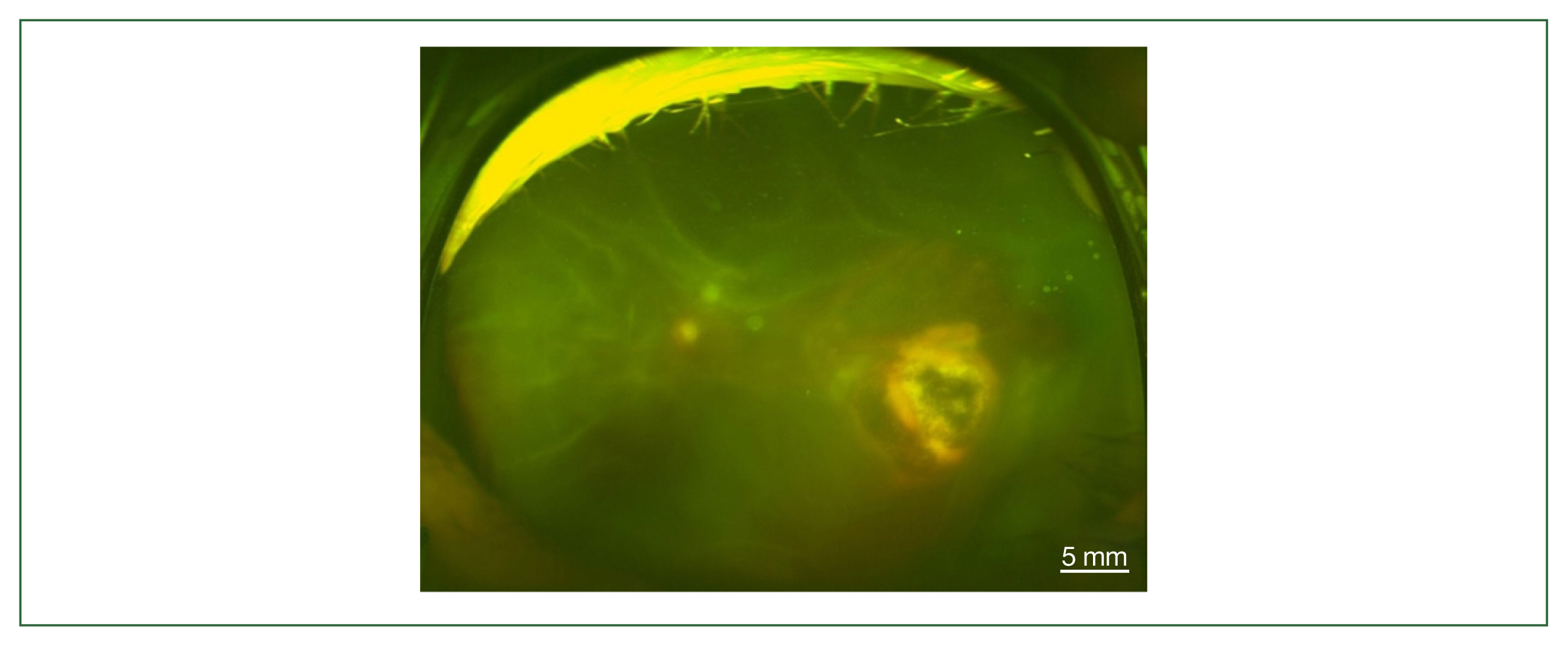
Table 1Demographic and general characteristics of 4 patients of ocular toxoplasmosis
Table 1
|
Case 1 |
Case 2 |
Case 3 |
Case 4 |
|
Age (yr) |
64 |
65 |
71 |
53 |
|
Sex |
Male |
Female |
Male |
Male |
|
Underlying disease |
Hypertension |
None |
None |
Diabetes, Hypertension |
|
Manifestation |
Periphlebitis, Papillitis, Vitritis, Anterior uveitis |
Vitritis, Anterior uveitis |
Chorioretinal scars, Vitritis, Anterior uveitis, Retinal detachment |
Chorioretinal scars, Periphlebitis, Papillitis, Vitritis, Anterior uveitis, Retinal detachment |
|
Initial BCVAa
|
0.25 |
0.32 |
0.4 |
0.1 |
|
Final BCVA |
0.8 |
0.32 |
CF |
1.0 |
|
Intervention |
Diagnostic vitrectomy |
None |
Diagnostic vitrectomy
Re-vitrectomy with scleral encircle |
Diagnostic vitrectomy with scleral encircle |
Table 2Laboratory findings of the 4 patients
Table 2
|
Case 1 |
Case 2 |
Case 3 |
Case 4 |
|
Serum (ELISA) |
T. gondii IgG (+) |
T. gondii IgG (+) |
T. gondii IgG (+) |
T. gondii IgG (+) |
|
Vitreous cytology for lymphoma |
Negative |
N/Aa
|
Negative |
Negative |
|
Aqueous humor |
|
|
|
|
|
PCR |
T. gondii DNA (+) |
T. gondii DNA (+) |
T. gondii DNA (+) |
T. gondii DNA (+) |
|
Cytokines (pg/ml) |
IL-10=568.8 |
IL-10=33.2 |
IL-10=1479.0 |
IL-10=185.4 |
|
IL-6=88.5 |
IL-6=9.2 |
IL-6=894.4 |
IL-6=143.0 |
|
IFN-γ=40.6 |
IFN-γ=6.1 |
IFN-γ=85.2 |
IFN-γ < 0.1 |
|
Vitreous |
|
|
|
|
|
PCR |
T. gondii DNA (+) |
N/A |
T. gondii DNA (+) |
T. gondii DNA (+) |
|
Cytokines (pg/ml) |
IL-10=829.4 |
N/A |
N/A |
N/A |
|
IL-6=731.4 |
|
|
|
|
IFN-γ=62.2 |
|
|
|
References
- 1. Subauste CS, Ajzenberg D, Kijlstra A. Review of the series “Disease of the year 2011: toxoplasmosis” pathophysiology of toxoplasmosis. Ocul Immunol Inflamm 2011;19(5):297-306.
https://doi.org/10.3109/09273948.2010.605198
- 2. Evering T, Weiss LM. The immunology of parasite infections in immunocompromised hosts. Parasite Immunol 2006;28(11):549-565.
https://doi.org/10.1111/j.1365-3024.2006.00886.x
- 3. Dodds EM. Toxoplasmosis. Curr Opin Ophthalmol 2006;17(6):557-561.
https://doi.org/10.1097/ICU.0b013e32801094ca
- 4. Gilbert RE, Dunn DT, Lightman S, Murray PI, Pavesio CE, et al. Incidence of symptomatic toxoplasma eye disease: aetiology and public health implications. Epidemiol Infect 1999;123(2):283-289.
https://doi.org/10.1017/s0950268899002800
- 5. McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol 1996;121(1):35-46.
https://doi.org/10.1016/s0002-9394(14)70532-x
- 6. Ridley ME, McDonald HR, Sternberg P Jr, Blumenkranz MS, Zarbin MA, et al. Retinal manifestations of ocular lymphoma (reticulum cell sarcoma). Ophthalmology 1992;99(7):1153-1161.
https://doi.org/10.1016/s0161-6420(92)31834-2
- 7. Rattray KM, Cole MD, Smith SR. Systemic non-Hodgkin’s lymphoma presenting as a serpiginous choroidopathy: report of a case and review of the literature. Eye (Lond) 2000;14:706-710.
https://doi.org/10.1038/eye.2000.188
- 8. Garweg JG, Jacquier P, Boehnke M. Early aqueous humor analysis in patients with human ocular toxoplasmosis. J Clin Microbiol 2000;38(3):996-1001.
https://doi.org/10.1128/JCM.38.3.996-1001.2000
- 9. Buggage RR, Chan CC, Nussenblatt RB. Ocular manifestations of central nervous system lymphoma. Curr Opin Oncol 2001;13(3):137-142.
https://doi.org/10.1097/00001622-200105000-00001
- 10. Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol 2002;13(6):411-418.
https://doi.org/10.1097/00055735-200212000-00012
- 11. Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol 2004;242(11):901-913.
https://doi.org/10.1007/s00417-004-0973-0
- 12. Karma A, von Willebrand EO, Tommila PV, Paetau AE, Oskala PS, et al. Primary intraocular lymphoma: improving the diagnostic procedure. Ophthalmology 2007;114(7):1372-1377.
https://doi.org/10.1016/j.ophtha.2006.11.009
- 13. Blumenkranz MS, Ward T, Murphy S, Mieler W, Williams GA, et al. Applications and limitations of vitreoretinal biopsy techniques in intraocular large cell lymphoma. Retina 1992;12(suppl):64-70.
https://doi.org/10.1097/00006982-199212031-00014
- 14. Cassoux N, Merle-Beral H, Lehoang P, Herbort C, Chan CC. Interleukin-10 and intraocular-central nervous system lymphoma. Ophthalmology 2001;108(3):426-427.
https://doi.org/10.1016/s0161-6420(00)00401-2
- 15. Akpek EK, Maca SM, Christen WG, Foster CS. Elevated vitreous interleukin-10 level is not diagnostic of intraocular-central nervous system lymphoma. Ophthalmology 1999;106(12):2291-2295.
https://doi.org/10.1016/s0161-6420(99)90528-6
- 16. Muhaya M, Calder VL, Towler HM, Jolly G, McLauchlan M, et al. Characterization of phenotype and cytokine profiles of T cell lines derived from vitreous humour in ocular inflammation in man. Clin Exp Immunol 1999;116(3):410-414.
https://doi.org/10.1046/j.1365-2249.1999.00921.x
- 17. Nickdel MB, Lyons RE, Roberts F, Brombacher F, Hunter CA, et al. Intestinal pathology during acute toxoplasmosis is IL-4 dependent and unrelated to parasite burden. Parasite Immunol 2004;26(2):75-82.
https://doi.org/10.1111/j.0141-9838.2004.00686.x
- 18. Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol 2005;165(1–2):63-74.
https://doi.org/10.1016/j.jneuroim.2005.04.018
- 19. Lang C, Gross U, Lüder CG. Subversion of innate and adaptive immune responses by Toxoplasma gondii.
. Parasitol Res 2007;100(2):191-203.
https://doi.org/10.1007/s00436-006-0306-9