Abstract
This experiment was undertaken to screen the acaricidal effects of herb essential oils (pennyroyal, ylang ylang, citronella, lemon grass, tea tree, and rosemary) at different doses (0.1, 0.05, 0.025, 0.0125, and 0.00625 µl/cm2) and exposure times (5, 10, 20, 20, 30 and 60 min) on house dust mites Dermatophgoides farinae and D. pteronyssinus. The most effective acaricidal components of pennyroyal (Mentha pulegium) were analyzed using a gas chromatography-mass spectrometer (GC-MS). Of these essential oils, the most effective was pennyroyal, which is composed essentially of pulegone (> 99%), at a dose of 0.025 µl/cm2 which at an exposure time of 5 min killed more than 98% of house dust mites. In the pennyroyal fumigation test, the closed method was more effective than the open method and maximum acaricidal effect was 100% at 0.025 µl/cm2, 60 min. The results show that herb essential oils, in particular, pennyroyal was proved to have potent acaricidal activity.
-
Key words: Dermatophagoides farinae, Dermatophagoides pteronyssinus, house dust mites, acaricidal effects, herb essential oils, fumigation method, GC-Mass
INTRODUCTION
The most important pyroglyphid mites include
Dermatophagoides farinae and
D. pteronyssinus, because of their cosmopolitan occurrence and abundance in homes (
Pollart et al., 1987;
Arlian, 1989). They are a major source of multiple potent allergens (
Fain et al., 1990), and are causally associated with sudden infant death syndrome (
Helson, 1971). Changes in the living environment such as an increase in the number of apartment households with central heating, space heating, tighter windows, and fitted carpets have provided better conditions for mite growth (
Pollart et al., 1987).
Control of these mite populations has been principally achieved by using chemicals such as γ-benzene hexachloride (γ-BHC), pirimiphos-methyl, benzyl benzoate, N,N-diethyl-m-toluamide (DEET), and dibutyl phthalate (
Pollart et al., 1987). Although effective, their repeated use results in the development of acaricidal resistance, and they have undesirable effects on non-target organisms, and have fostered environmental and human health concerns (
Hayes and Laws, 1991). These problems have highlighted the need for the development of new strategies for selective house dust mite control.
Plants may provide an alternative means of dust mite control because they constitute a rich source of bioactive chemicals. Because of this, much effort has been focused on plant extracts or phytochemicals as potential sources of commercial pest control agents (
Isman, 2000,
2001). The essential oils of herbs have been popularly applied commercially. In this experiment, 6 different herb essential oils were used, which were chosen to use as repellents and to get easily near around, namely, pennyroyal, ylang ylang, citronella, lemon grass, tea tree, and rosemary. These are generally known for different properties, e.g., as repellents, antiseptics, and anti-stress agents. This paper describes a laboratory study on the acaricidal activities, and presents the results of chromatography/mass spectroscopy (GC-MS) analyses of pennyroyal essential oil.
MATERIALS AND METHODS
Chemicals and herb essential oils
In total, 6 herb essential oils were purchased from Jin-Ah Aromatics (Anyang, Kyunggi Province, Korea); pennyroyal (Mentha pulegium), ylang ylang (Cananga odorata), citronella (Cymbopogon nardus), lemon grass (Cymbopogon citratus), tea tree (Melaleuca alternifolia), and rosemary (Rosmarinus officinalis). Negative control was applied ethanol only and positive control used was a commercial acaricidal product. The major active components of the positive control are permethrin 0.98 g/100 g, pthalthrin 0.294 g/100 g, and fragrance.
Mites
The mites D. farinae and D. pteronyssinus, which have been maintained in our veterinary parasitology laboratory for 5 years, without exposure to any known acaricide, were used. Mites were reared in plastic containers (12.5 × 10.5 × 5.0 cm) at 25.0 ± 1.0℃ and 75 % relative humidity in dark chamber and fed a laboratory diet (powdered rat feed plus Ebioze® powder 1:1 by weight).
Direct contact testing of herb essential oils
A filter paper contact bioassay was used to evaluate toxicities of the herb essential oils and acaricides to D. farinae and D. pteronyssinus. In preliminary experiments with pennyroyal, ylang ylang, citronella, lemon grass, tea tree, and rosemary essential oils and the positive control, 0.1 µl/cm2 appeared an appropriate starting dose for primary screening. If an herb essential oil had similar or better activity than the positive control further bioassays were conducted. First, 40 µl of ethanol were applied to all testing filter papers. After drying under a fume hood for 1 min, each filter paper was placed at the bottom of a Petri dish (5 cm in diameter, 1.2 cm in height). Batches of about 300 D. farinae and D. pteronyssinus were placed in each Petri dish. The negative control filter papers received 40 µl of ethanol and about 300 mites only. Amounts (0.1, 0.05, 0.025, 0.0125, and 0.00625 µl/cm2) of each herb essential oil and the positive control were applied to filter papers (Whatman No.2, 4.25 cm in diameter) and lids were then fitted.
Fumigation test of pennyroyal
In a separate experiment, vapor phase toxicity against
D. farinae and
D. pteronyssinus was investigated according to the method described by Kwon and Ahn (
2002). Briefly, batches of about 300
D. farinae and
D. pteronyssinus were placed on the bottom of Petri dishes (5 cm diameter × 1.2 cm) and covered with a lid containing a fine wire sieve (200 mesh, 4 cm diameter) attached to the middle part of the Petri dish. Filter papers (4 cm in diameter), treated with pennyroyal oil concentrations (0.1, 0.05, 0.025, 0.0125, and 0.00625 µl/cm
2) were placed over the wire sieve, which prevented direct contact between mites and test oils. Each Petri dish was then covered with a lid (method B) to investigate the vapor phase toxicities of the test compounds or left uncovered (method A). Control Petri dishes were exposed to filter paper containing 40 µl of ethanol only. Mortalities were determined at 5, 10, 20, 30, and 60 min after treatment under a binocular microscope. Mites were considered dead if appendages did not move when the mite was prodded with a pin.
Chromatographic analyses were performed using a Hewlett-Packard 5890II (Palo Alto, California, USA) series gas chromatograph, equipped with an injector and a flame ionization detection (FID) system. Separation and quantitation were performed using a DB-WAX column, 30 m × 0.252 mm ID, with a film thickness 0.25 µm (J & W Scientific Inc., Folsom, California, USA).
The GC temperature program used was as follows: initial temperature 40℃, held for 5 min then ramped at 6℃/min to 240℃, and held for 5 min. Helium was used as the carrier (l ml/min). The FID gases were hydrogen and air at flow-rates of 500 and 45 ml/min, respectively. The detector temperature was set at 270℃ and the injector block temperature at 240℃.
GC-MS was performed using a GC (HP 5890II) - Mass spectrometer (JMS-600W, JEOL, Tokyo, Japan), using the GC column and temperature conditions described above. Helium was used as the carrier gas at a flow of 1 ml/min. The GC/MS interface was held at 230℃ and mass spectra were obtained at 70 eV. The effluent of the capillary column was introduced directly into the MS ion source. The mass analyzer was set to scan from 50 to 550 amu every 0.7 sec. Constituents of pennyroyal oil were identified by comparing of MS data with data in a MS library (Wiley Registry of Mass Spectral Data, 6th ed.) and these findings were confirmed by comparing GC retention times with those of authentic samples.
RESULTS
Direct contact testing of herb essential oils
Direct contact testing showed that pennyroyal at a dose of 0.025 µl/cm
2 for 5 min had the greatest acaricidal effect (98.6% mortality) followed by ylang ylang (50.4%), lemon grass (9.8%), tea tree (0%), and rosemary (0%). Mortality depended on exposure time and dose (
Table 1). The negative control had a mortality of 0% at this test dose and exposure time and the positive control had a mortality of 0% at a dose of 0.1 µl/cm
2 for 5 min.
The method A was less effective than the method B (
Table 2). For the method A at a dose of 0.1 µl/cm
2, mortalities for pennyroyal oil ranged from 0% to 42.6% (time from 5 to 60 min did not much affect these results), whereas for the method B, mortalities of pennyroyal ranged from 2.1% to 100% and these were time- (from 5 to 60 min) and dose-dependent (from 0.1 to 0.025 µl/cm
2). For the method B, after exposure for 30 and 60 min, mortalities ranged from 90.4% to 100% at 0.1 µl/cm
2. The minimum dose required to kill house dust mites was 0.025 µl/cm
2 for 60 min (
Table 2). The other doses and time had no acaricidal effects.
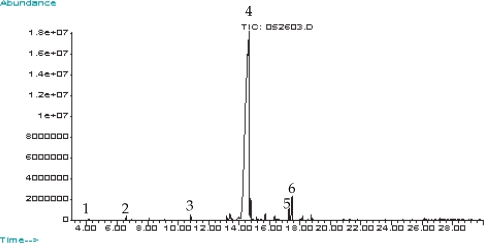
GC-MS showed that pennyroyal essential oil composed almost entirely of pulegone component (99%) (
Fig. 1). Five other constituents were identified, i.e., cyclohexanone, 8-hydroxy-δ-4(5)-p-methen-3-one, 3-octanol, dl-limonene, and β-pinene. Pulegone had a retention time on our system of 14.55 min, and β-pinene was the first eluted at 4.15 sec.
DISCUSSION
Plant essential oils have potential as dust mite control products because many are pest selective, and have no or few harmful effects on non-target organisms or the environment (
Isman, 2000,
2001). Moreover, they can be applied in conventional ways to beds, sofas, furniture, and carpets (
Pollart et al., 1987). Many plant essential oils and phytochemicals are known to possess acaricidal activity, which is attributed in part, to their lipophilic natures and high vapor pressures (
Kim et al., 2004). Those of naturally grown acaricidal herbs reported are as follows; butylidenephthalide from the rhizomes
Cnidium officinale Makino (
Kwon and Ahn, 2002); O-anisaldehyde, citronellal, and perillaldehyde derived from perilla oil (
Watanabe et al., 1989); isosericenine, acryophyllene oxide, and α-cadinol from the essential oil of the leaves of
Neolitsea sericea Blume (
Furuno et al., 1994); pisiferic acid from the leaves of
Chamaecyparis pisiferta Sieb. et Zucc. (
Yatagai and Nakatani, 1994); sericealactone from the heartwood of
Neolitsea sericea (
Sharma et al., 1993); and cinnamaldehyde, cinnamyl alcohol, and salicylaldehyde from the bark of
Cinnamomum cassia Blume (
Kim, 2001).
In direct contact bioassay, we found that pennyroyal had a great acaricidal effect in adult
D. farinae and
D. pteronyssinus and it is believed because of the components of pulegone in the pennyroyal.
Fig. 1 showed that pennyroyal essential oil had 99% of pulegone component through GC-MS. Based on the results, we made a hypothesis that the acaricidal effects of pennyroyal were because of the pulegone. About this result, a further study has to be proven in extraction of pulegone, as a single element from the pennyroyal as well as safety matters for the component.
In the fumigation test, acaricidal activity was greater by the method B than by the method A because of its greater effect on the mite's respiratory system (
Ahn et al., 2003). The results indicate that these compounds are largely delivered in the vapor phase, and suggest that their toxicities depend on access via the respiratory system. However, the precise natures of their modes of action remain to be further clarified.
Observations of symptoms of poisoning are of practical importance for dust mite control. Five types of poisoning symptoms in mites have been reported, i.e., a knockdown-type death caused by
Neolitsea sericea leaf oil in adult
D. farinae and
D. pteronyssinus (
Furuno et al., 1994); death related with an uncoordinated behavior without knockdown by (E)-cinnamaldehyde, cinnamyl alchol, salicylaldehyde, benzyl benzoate, or DEET in adult
D. farinae and
D. pteronyssinus (
Kim, 2001); death associated with desiccation by monoterpenes, such as, fenchone, linalool, menthone, and pulegone in adult
Tyrophagus putrescentiae (
Sanches-Ramos and Castanera, 2001); death related with a characteristic depression of the dorsal surface of the idiosome by tricalcium phosphate and ferric phosphate in adult
Tyrophagus putrescentiae (
Ignatowicz, 1981), and death associated with lethargy by butylidenephthalide in adult
D. farinae and
D. pteronyssinus (
Kwon and Ahn, 2002).
In the present study, all tested compounds resulted in similar symptoms; forelegs were extended forward in unison without knockdown. Moreover, mite's external features had some changes compare to alive. Despite different initial intoxication symptoms (
Kim, 2001), mites had no change in cuticle glossiness.
Volatile compounds of many plant extracts and essential oils are composed of alcohols, aldehydes, alkanes, and terpenoids, especially monoterpenoids, and exbibit fumigant activity (
Coats et al., 1991;
Kim and Ahn, 2001;
Kwon and Ahn, 2002;
Park et al., 2003;
Kim et al., 2004). Results of the present study indicate that plant-derived essential oils could be used as fumigants for
D. farinae and
D. pteronyssinus. Further toxicity and safety studies are required on pulegone, but formulations are required that optimize its acaricidal potency and stability.
Notes
-
This work was supported by the research grant of the Chungbuk National University in 2005.
References
Fig. 1Total ion chromatogram of pennyroyal essential oil. 1: β-pinene; 2: dl-limonene; 3: 3-octanol; 4: pulegone; 5: 8-hydroxy-δ-4(5)-p-methene-3-one; 6: 2-cyclohexene-1-one, 3-methyl-6-(1-methylethylidene).

Table 1.Mortality of mites by direct contact bioassay
Table 1.
|
Treatment at 5 min |
Na)
|
Mortality of mites (%) according to dose of herb oils (μl/cm2)
|
|
0.1 |
0.05 |
0.025 |
0.0125 |
0.00625 |
|
Negative control |
306 |
0 |
0 |
0 |
0 |
0 |
|
Positive control |
287 |
0 |
0 |
0 |
0 |
0 |
|
Pennyroyal |
302 |
100 |
100 |
98.7 |
2.6 |
0 |
|
Ylang ylang |
312 |
98.4 |
98.7 |
95.5 |
5.1 |
0 |
|
Citronella |
297 |
0 |
0 |
0 |
0 |
0 |
|
Lemon grass |
309 |
60.5 |
10.4 |
9.4 |
10 |
5.2 |
|
Tea tree |
307 |
10.1 |
10.4 |
0 |
0 |
0 |
|
Rosemary |
309 |
0 |
0 |
0 |
0 |
0 |
Table 2.Mortality of mites in fumigation testing by pennyroyal
Table 2.
|
Time of exposure (min) |
Methodsa)
|
Mortality (%) of mites by different doses of pennyroyal (μl/cm2)
|
|
Control |
0.1 |
0.05 |
0.025 |
0.0125 |
0.00625 |
|
5 |
A |
0 |
0 |
0 |
0 |
0 |
0 |
|
B |
0 |
2.1 |
0 |
0 |
0 |
0 |
|
10 |
A |
0 |
41.2 |
0 |
0 |
0 |
0 |
|
B |
0 |
81.3 |
41.5 |
0 |
0 |
0 |
|
20 |
A |
0 |
40.5 |
0 |
0 |
0 |
0 |
|
B |
0 |
83.4 |
83.1 |
0 |
0 |
0 |
|
30 |
A |
0 |
42.1 |
0 |
0 |
0 |
0 |
|
B |
0 |
90.4 |
100 |
71.5 |
0 |
0 |
|
60 |
A |
0 |
42.6 |
0 |
0 |
0 |
0 |
|
B |
0 |
100 |
100 |
100 |
0 |
0 |