Abstract
Exposure to storage mite (SM) and house dust mite (HDM) allergens is a risk factor for sensitization and asthma development; however, the related immune responses and their pathology have not been fully investigated. The HDMs Dermatophagoides farinae and Dermatophagoides pteronyssinus and SM Tyrophagus putrescentiae are potent allergens that induce asthma. Most SM-related studies have focused on the allergic reactions of individuals by measuring their immunoglobulin (Ig)E expression. Considering the limited research on this topic, the present study aims to investigate the differences in the immune responses induced by HDMs and SMs and histologically analyze lung tissues in a mouse asthma model to understand the differential effects of HDM and SM. The results revealed that all mite species induced airway inflammation. Mice challenged with T. putrescentiae had the highest airway resistance and total cell, eosinophil, and neutrophil counts in the bronchoalveolar lavage fluid (BALF). The SM-sensitized groups showed more severe lesions and mucus hypersecretions than the HDM-sensitized groups. Although the degree of HDM and SM exposure was the same, the damage to the respiratory lung tissue was more severe in SM-exposed mice, which resulted in excessive mucin secretion and increased fibrosis. Furthermore, these findings suggest that SM sensitization induces a more significant hypersensitivity response in mucosal immunity than HDM sensitization in asthma models.
-
Key words: Dermatophagoides farinae, Dermatophagoides pteronyssinus, Tyrophagus putrescentiae, house dust mite, storage mite, asthma, lung inflammation, mucin 5AC
Introduction
Asthma affects over 300 million individuals; its global prevalence has doubled over the last 3 decades and continues to rise in emerging economies [
1,
2]. Allergic asthma involves chronic inflammation of the airways, which is characterized by heterogeneous inflammatory lung disease, airway hyperresponsiveness (AHR), and mucus hypersecretion. It is accompanied by a robust type 2 helper T (Th2) cell response, with elevated levels of interleukin (IL)-4, IL-5, and IL-13, resulting in increased immunoglobulin (Ig)E and IgG1 production, cell recruitment to allergen-exposed sites, and subsequent airway remodeling, including eosinophilic infiltration, cell proliferation, and collagen deposition [
3,
4].
House dust mite (HDM) and storage mite (SM) cause allergic rhinitis and bronchial asthma [
5–
7]. Currently, HDMs, which belong to the Pyroglyphidae family, represent the main source of indoor allergens. Other mite species belonging primarily to the Glycyphagidae and Acaridae families are collectively known as SMs and are primarily found in wheat, wheat germ, mushrooms, cheese, and cereals. They predominantly affect occupationally exposed individuals, such as farmers and grain workers [
8,
9]. Allergic reactions to HDM exposure contribute to approximately 50% of asthma cases [
10]. The 2 most common species of HDMs found in Korean households are
D. farinae (Df) and
D. pteronyssinus (Dp) [
10–
13]. HDMs are present in over 90% of households in the Republic of Korea (Korea), and exposure to these mites is medically significant [
10]. Respiratory allergies tend to affect approximately 40–60% of the population in Korea, with the majority of allergies arising from HDM exposure [
10,
13]. Meanwhile, the SM
T. putrescentiae (Tp) is the third most common house mite in Korea [
10–
12].
The prevalence of SMs is greater than that of HDMs, especially in the households of individuals with allergies [
2,
7,
14–
16]. Previous studies have used ovalbumin, a common egg allergen, as a sensitizer in traditional animal asthma models [
15,
16]. Alternatively, other asthma models primarily incorporate respiratory allergens, such as Dp and cockroaches [
2,
15]. Despite the dominance of SMs and HDMs, no study has directly compared the immune response to their antigens.
Although airway mucus hypersecretion is an important pathophysiological feature of asthma, it can be initiated by multiple intracellular signaling pathways. However, the specific mechanism regulating airway mucus hypersecretion has not been determined [
17–
19]. This study investigated the differences in the immune responses induced by HDMs and SMs in a mouse asthma model.
Materials and Methods
Ethics statement
The animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Yonsei University Health System, Seoul, Korea (2015-0414), in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH publication no. 85–23, 1985, revised 1996). The laboratory was monitored and inspected regularly by the Ministry and the IACUC of the Yonsei University Health System. The animals were housed at a temperature of 21°C±2°C and humidity of 60% under a 12:12-h light–dark cycle, with free access to water, and acclimated for 7 days before the start of the study. All experiments were conducted to minimize the number of animals used.
Mite extract preparation
Two HDM species, Df and Dp, and one SM species, Tp, were maintained at the Arthropods of Medical Importance Resource Bank, Yonsei University College of Medicine, Seoul, Korea. Mite extracts were prepared as described previously [
11]. The mite bodies were isolated in saturated salt water, whereas the allergens were extracted in phosphate-buffered saline (PBS) (1×PBS; pH 7.4). The extract was then dialyzed extensively against distilled water, lyophilized, reconstituted with PBS, aliquoted, and freeze-dried for future use. The dry powder was suspended in PBS prior to administration to the animals. Furthermore, the protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA).
Five-week-old male BALB/c mice were purchased from Orient Bio (Seongnam, Korea). The mice were randomly divided into 4 groups (
n=10 per group) according to the experimental extracts they were administered: Df, Dp, Tp, and PBS (control). The mice were then administered with mite extract proteins (100 μg/ml) in 10 ml PBS or 10 ml of PBS as a negative control for 10 min through an air-compressing nebulizer in an acrylic dome-shaped chamber with a diameter of 25 cm. The extracts were administered on Days 7, 14, 21, 28, 35, 42, 60, and 61, and the mice were euthanized on Day 63 (
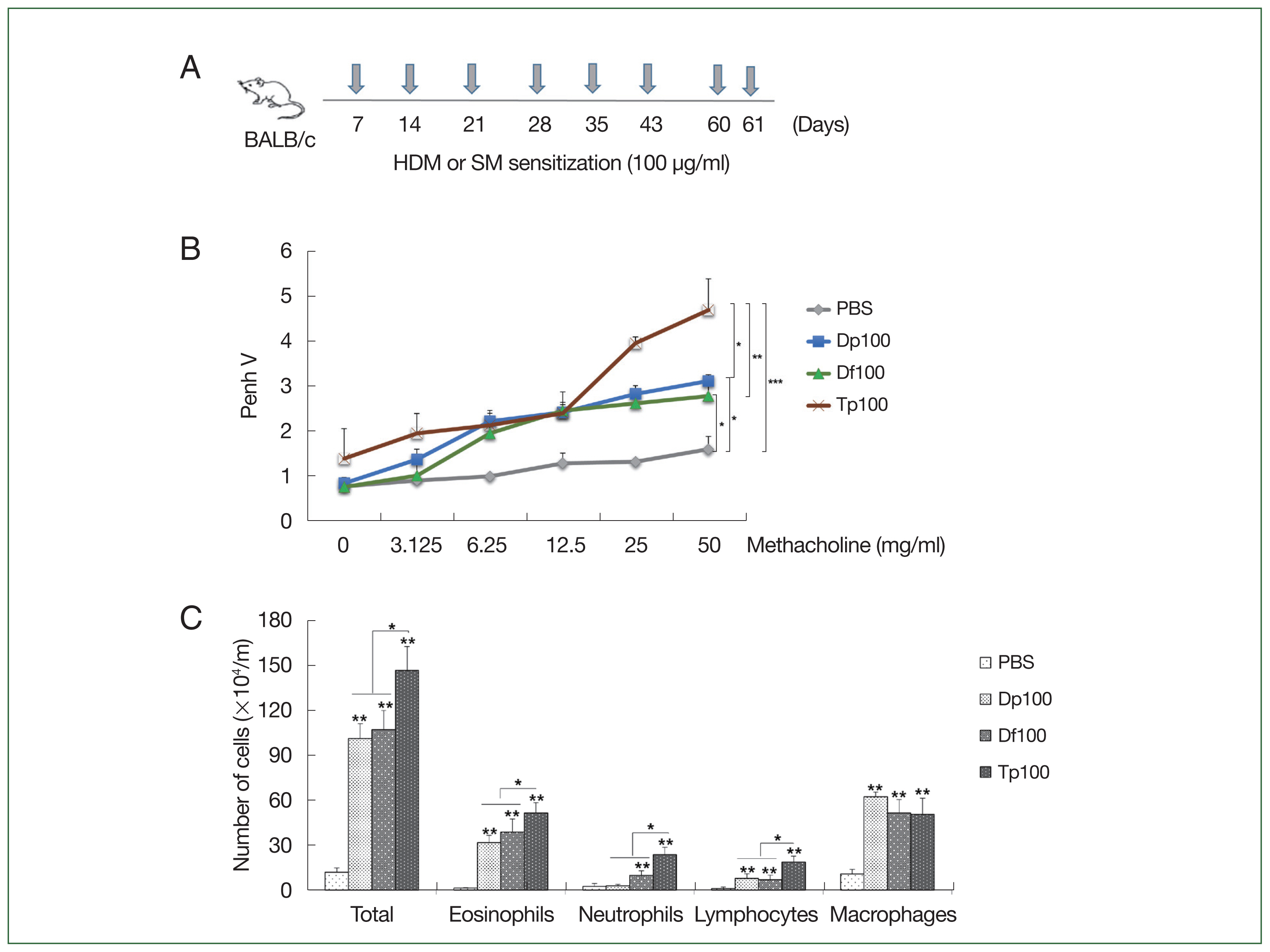
Fig. 1A). Moreover, the mice were euthanized using a standard dose of anesthetics, which consists of a mixture of xylazine (10 mg/kg; Bayer, Seoul, Korea) and Zoletil-50 (30 mg/kg; Virbac, France).
AHR was determined using a flexiVent 5.1 small animal ventilator (SCIREQ, QC, Canada) 24 h after the final mite extract administration. The mice were administered with a saline control aerosol, followed by increasing concentrations of methacholine (3.1, 6.25, 12.5, 25, and 50 mg/ml; Sigma–Aldrich, St. Louis, MO, USA). Moreover, an aerosol was generated using an ultrasonic atomizer and delivered to the inspiratory line of the flexiVent 5.1 small animal ventilator using the bias current of the medical air. Each aerosol was delivered for 10 sec to maintain regular ventilation and was measured twice at 1-min intervals [
12].
After AHR assessment, the mice were euthanized via an intraperitoneal injection with 2% pentobarbital sodium (50 mg/kg, Sigma–Aldrich). The mice were then immediately dissected below the larynx, and a flexible polyurethane tube (BD Biosciences, CA, USA) connected to a 24-gauge needle was inserted into the trachea. The lung was flushed once with 1 ml of ice-cold PBS and the BALF was collected 3 times. The BALF was centrifuged at 500 g for 10 min at 4°C and resuspended in PBS. The cells were cytospun onto slides, fixed, and stained using a Diff-Quik stain kit (Sysmex, Kobe, Japan) [
13]. Differential counts of eosinophils, neutrophils, lymphocytes, and macrophages were determined in duplicate on coded slides of 200 cells from each sample.
An enzyme-linked immunosorbent assay (ELISA) kit (Peprotech, NY, USA) was used in accordance with the manufacturer’s instructions to determine IL-4, IL-5, IL-6, IL-10, IL-13, and IL-17 levels in the BALF and splenocytes. Splenocytes were ground in extraction buffer (RPMI-1640 medium supplemented with 5% fetal bovine serum) on cell strainers to obtain mononuclear cells. The sera were diluted (1:10 for IgE and 1:200 for IgG1 and IgG2a). Subsequently, the samples were probed with anti-mouse IgE-HRP, IgG1-HRP, or IgG2a-HRP conjugates (1:500; Novus Biologicals, Littleton, CO, USA). For color development, the samples were incubated with TMB for 10 min in the dark, and the reaction was terminated with the addition of 4 N H2SO4. The absorbance of the samples was then subsequently read at 450 nm on a Ceres 900 ELISA microtiter plate reader (BioTek, Winooski, VT, USA).
Hematoxylin and eosin (H&E) staining
The murine lung tissues were isolated and fixed in 10% buffered formalin for 24 h, dehydrated, embedded in paraffin, cut into 4-μm-thick sections, and stained with H&E for morphological analysis at 200×and 400×magnifications.
Masson’s trichrome staining
To evaluate the peribronchial fibrosis, the sections were stained using Masson’s trichrome. The sections were dewaxed, stained with hematoxylin for 10 min, differentiated with 1% hydrochloric acid in alcohol, and stained with trypan blue. After washing, the sections were stained using Ponceau staining solution for 7 min, washed, stained with 1% phosphomolybdate for 4 min, and placed in aniline blue for 5 min for water purification. The sections were then dehydrated with 95% ethanol, dried in an oven at 60°C, subjected to xylene clearing, fixed, and observed (400×magnification) [
3]. Moreover, the percentage of collagen was measured using the ImageJ software (NIH, Bethesda, MD, USA), and the final score for each section was the average of the scores of different sites.
The degree of mucus production and goblet cell hyperplasia in the airway epithelium was determined by treating the oxidized sections with 10 g/L periodic acid, rinsing them with a ferrous sulfate solution and distilled water, drying them at 18°C–20°C, and treating them with Schiff solution for 1 h at 37°C. Finally, the sections were assessed under a microscope at 400×magnification [
14].
The lung tissues were homogenized in RIPA lysis buffer (Invitrogen) for western blot analysis. The lysates (20 μg) were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gels at 100 V for 2 h and transferred onto microporous polyvinylidene difluoride membranes at 100 mA for 2 h. The membranes were blotted with anti-mucin 5AC (MUC5AC) monoclonal antibodies (1:500; Abcam, Cambridge, UK) and GAPDH polyclonal antibodies (1:2,000; BioLegend, San Diego, CA, USA) for the detection of specific proteins. Subsequently, they were incubated with anti-rabbit IgG conjugated to HRP (Abcam, Cambridge, UK) for visualization using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, USA).
Reverse transcription-quantitative polymerase chain reaction
The total RNA was isolated from the right lung tissues of the mice using TRIzol reagent (Invitrogen). The isolated RNA (1 μg) was reverse-transcribed to cDNA using a SuperScript III First-Strand Synthesis kit (Invitrogen; Thermo Fisher Scientific Inc.) in accordance with the manufacturer’s protocol. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was conducted using SYBR GREEN PCR master mix (Applied Biosystems). The primers used were as follows: MUC5AC-FNM 5′-CAGGACTCTCTGAAATCGTACCA-3′ (forward) and MUC5AC-R 5′-GAAGGCTCGTACCACAGGG-3′ (reverse) (Invitrogen; Thermo Fisher Scientific). The expression of MUC5AC was analyzed using the Applied Biosystems 7700 Sequence Detection System (Applied Biosystems) in accordance with the manufacturer’s instructions. The PCR cycling conditions used for all reactions were as follows: 10 min at 95°C, followed by 35 cycles at 95°C for 15 sec and 58°C for 1 min. Each assay was performed in triplicate. The relative expression level of MUC5AC was normalized against that of GAPDH and analyzed using the 2−ΔΔCt method.
Statistical analysis
The values in each graph represent the mean±standard deviation of the results obtained from independent experiments. The significance of the differences among the groups was determined using the one-way analysis of variance with the Tukey post hoc test, while that between the groups was analyzed using an unpaired 2-tailed Student’s t-test. Statistical significance was set at a *P value of<0.05 and **P value of<0.01.
Results
Establishment of the murine asthma model
Fig. 1A schematically presents the protocol for mice-induced airway inflammation. The mice in the HDM-sensitized group were initially restless, running back and forth, showing increased nasal burning symptoms, followed by polypnea and gasping. AHR was characterized as hypersensitivity to methacholine in the mite-sensitized groups. The Tp-sensitized mice showed higher Penh values than the HDM-sensitized mice did (
Fig. 1B), whereas the HDM-sensitized mice showed significantly higher values than the saline-treated control group did (
Fig. 1B). These values were similar between the Df- and Dp-sensitized mice.
Inflammatory cells in the mouse BALF samples
The number of total cells, inflammatory cells, eosinophils, neutrophils, mononuclear macrophages, and lymphocytes was higher in the BALF of mite-sensitized mice than in the saline-treated mice (
P<0.01). Consistent with the results of the airway resistance experiment, the total number of cells was the highest in the Tp-sensitized group. Moreover, the number of neutrophils, eosinophils, and lymphocytes was significantly elevated in the Tp-sensitized group than in the Dp- and Df-sensitized groups (
P<0.05;
Fig. 1C).
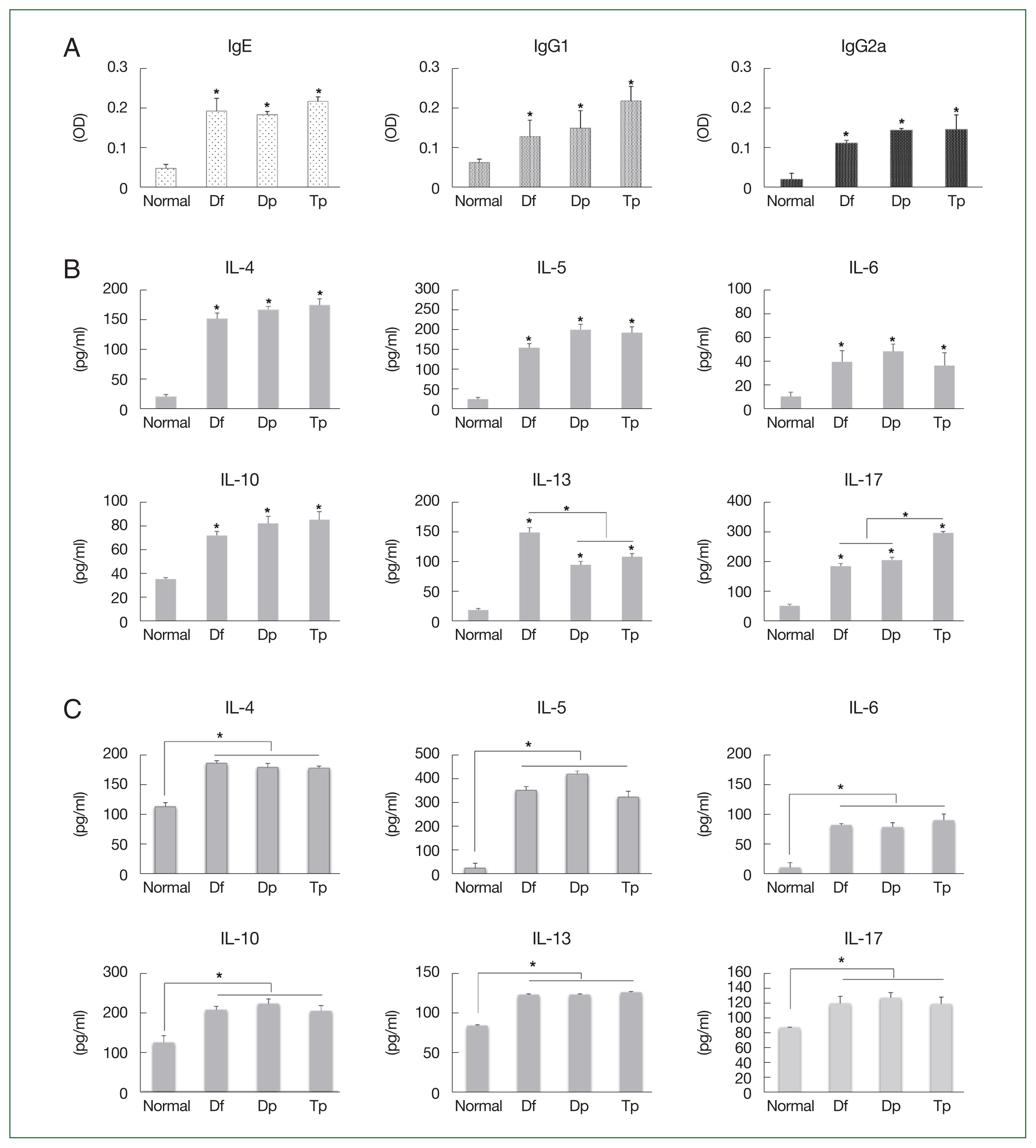
The IgE, IgG1, and IgG2a titers increased in the serum of mite-sensitized mice compared with those in the serum of saline-treated mice (
P<0.05;
Fig. 2A).
The IL-4, IL-5, IL-6, IL-10, IL-13, and IL-17 levels increased in the BALF of HDM- and SM-sensitized mice compared with those in the BALF of saline-treated mice (all
P<0.05;
Fig. 2B). The IL-13 level was higher in the Df-exposed mice, while the IL-17 level was higher in the Tp-exposed mice (
P<0.05;
Fig. 2B).
No significant differences in the Th2 level were observed between the HDM- and SM-sensitized mice. However, the IL-4, IL-5, IL-6, IL-10, IL-13, and IL-17 levels in the splenocytes of mite-exposed mice were higher than those in the saline-treated mice (all
P<0.05;
Fig. 2C).
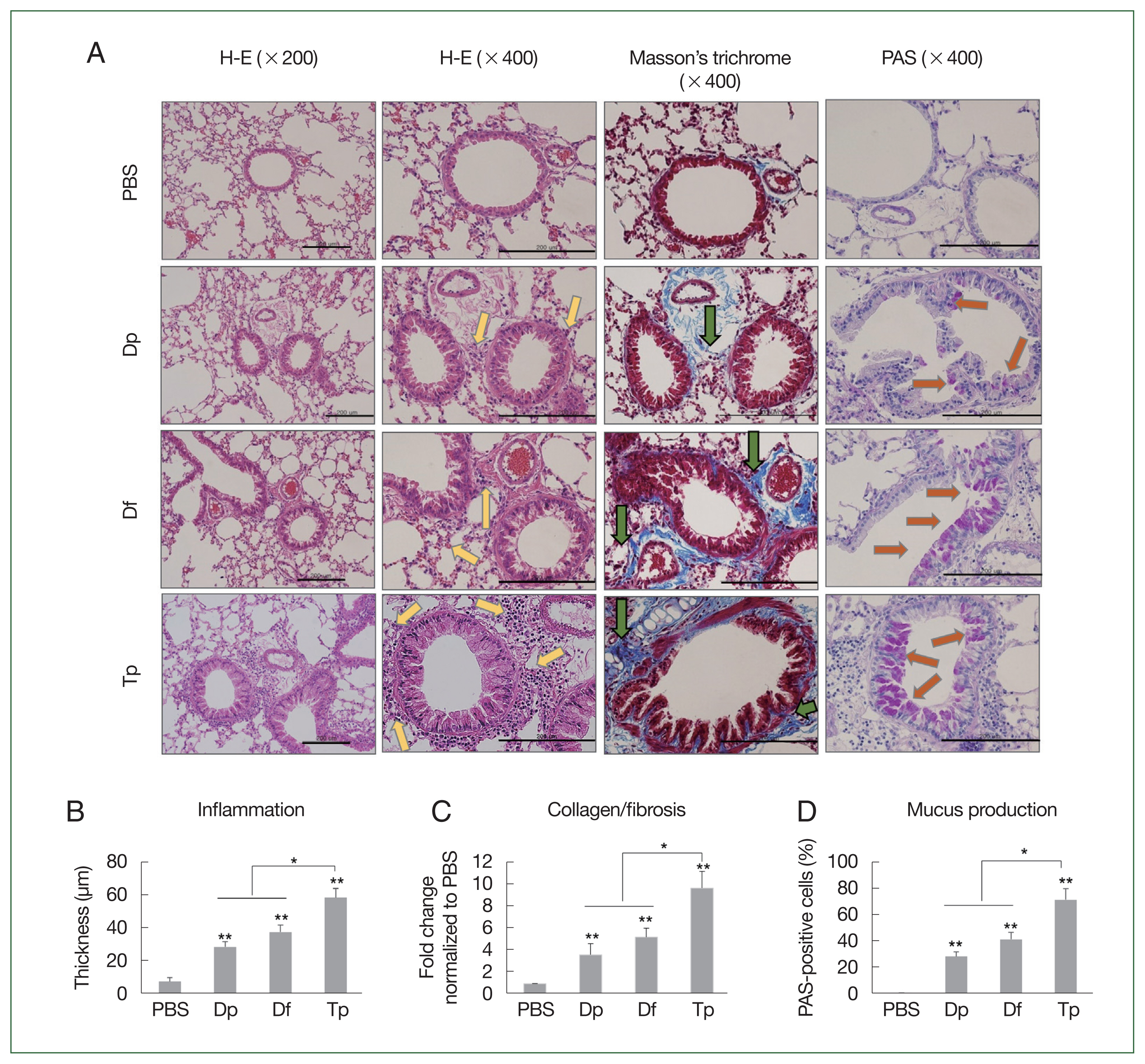
The mice in the mite-sensitized groups showed thicker bronchial mucosa, higher rates of inflammatory cell infiltration in the lung tissues, lower air flux, smaller lung cavities, a higher number of eosinophilic granulocytes and lymphocytes, and more lumen stenosis than those in the saline group did. Moreover, the lesions were more severe in the Tp-sensitized group than in the HDM-sensitized group. Compared with the HDM-sensitized mice, the Tp-sensitized mice showed an increased immune cell infiltration around the bronchial tracts, mucin production, and hyperplasia of lung epithelial cells and goblet cells (
Fig. 3A).
The Masson staining results revealed collagen deposition in the airway epithelium. Mild collagen staining around the small airways and alveolar epithelium was observed in the HDM-sensitized groups. Conversely, the Tp-sensitized group showed a more pronounced Masson staining of collagen than the other mite-sensitized groups did (
Fig. 3A), which indicated that more collagen was deposited around the asthmatic airways in the Tp-exposed mice than in the HDM-exposed mice (
Fig. 3A). Bronchial wall thickening was prominent in Tp-sensitized mice (
Fig. 3B). Collagen expression was significantly increased in the lung tissues of the 3 mite-sensitized groups compared with that in the saline-treated group (
P<0.01). Moreover, collagen expression was significantly higher in the lungs of Tp-sensitized mice than in the HDM-sensitized mice (
P<0.05;
Fig. 3C).
The PAS staining results revealed an increase in the number of goblet cells in the airway mucosa of mice, and this increase was more significant in the mite-sensitized groups than in the saline group. The intensity of PAS staining was higher in the Tp-sensitized group than in the Df- and Dp-sensitized groups, indicating an increased mucus production around the airways. The statistical analysis also revealed the presence of mucus in the PAS-positive cells. Moreover, the number of PAS-positive cells in the lung tissues of mice from the 3 mite-sensitized groups was significantly higher than that in the saline-treated group (
P<0.05). The number of PAS-positive cells was the highest in the lungs of Tp-sensitized mice and was significantly higher than that in the lungs of HDM-sensitized mice (
P<0.01;
Fig. 3D).
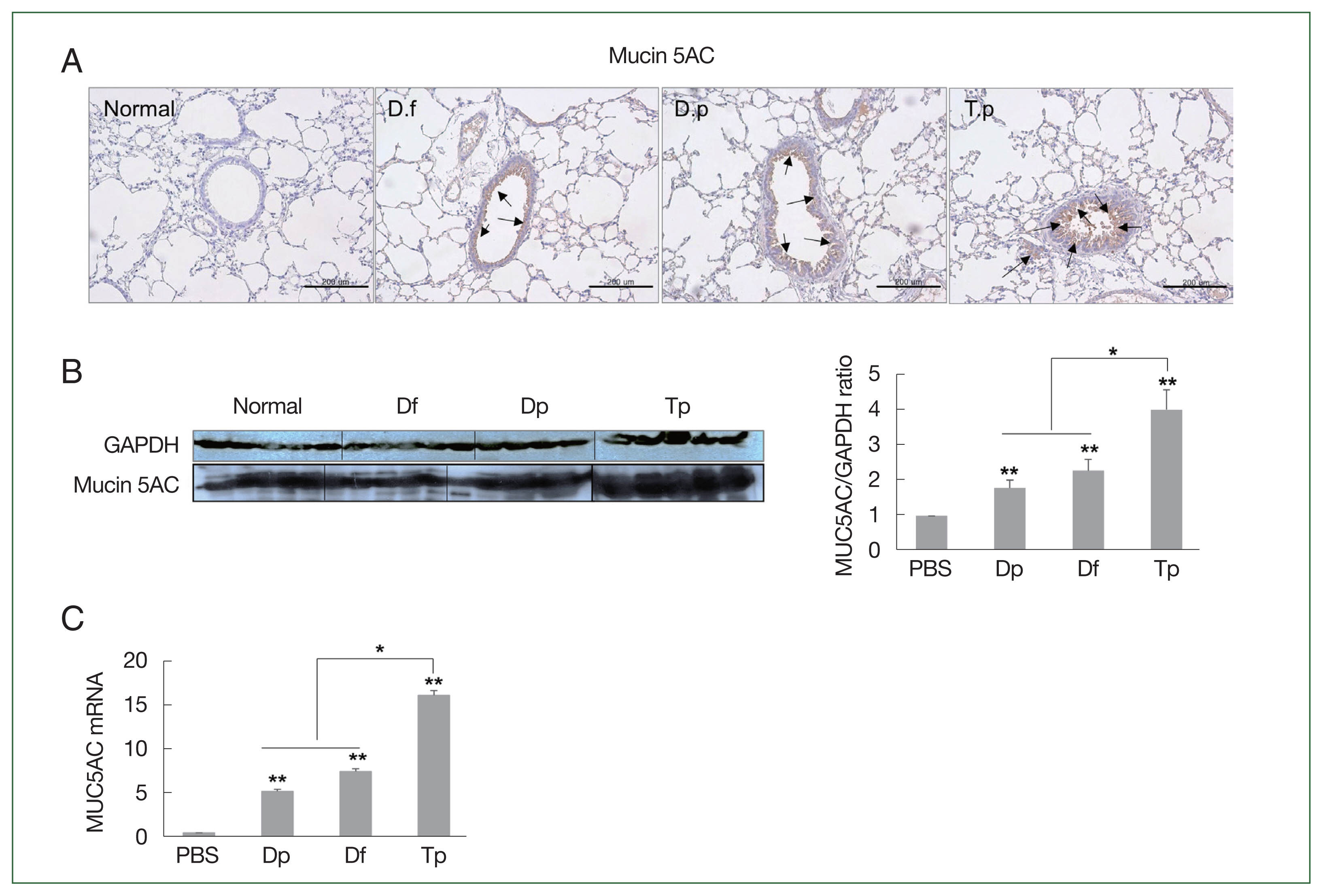
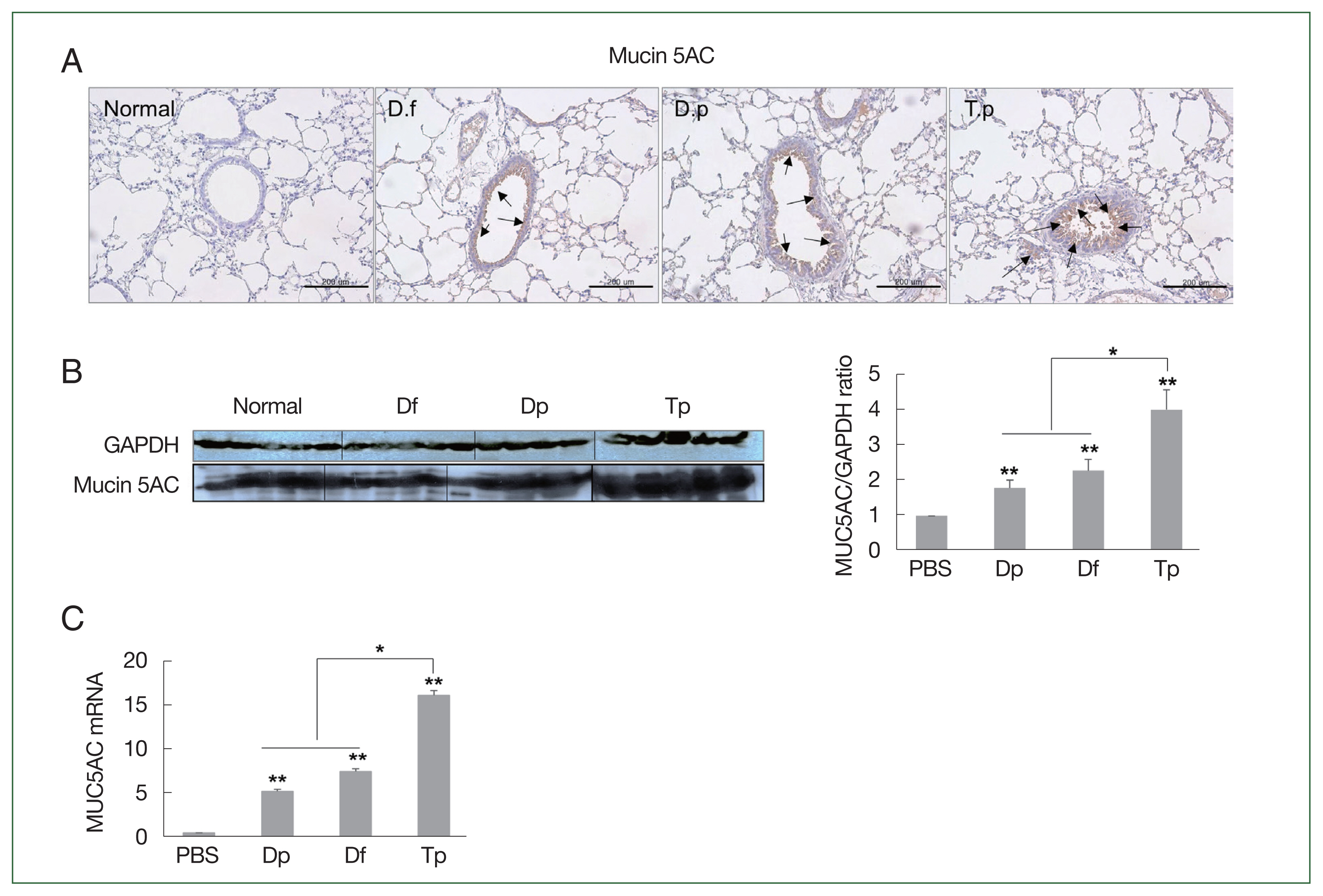
Consistent with the PAS staining results, the MUC5AC protein level was the highest in the lung tissues of the Tp-sensitized group. The expression of MUC5AC was significantly positively correlated with inflammatory cell infiltration and mucus hypersecretion in the airway (
Fig. 4A). Furthermore, the increased expression of MUC5AC was confirmed by the western blotting and RT-qPCR results (
Fig. 4B, C).
MUC5AC expression was significantly higher in the lung tissues of mice from the 3 mite-sensitized groups than that in the lung tissues of mice from the saline-treated group (
P<0.01). Moreover, its expression in the lungs of Tp-sensitized mice was significantly higher than that in the HDM-sensitized mice (
P<0.05;
Fig. 4B, C).
Discussion
Research on allergic asthma induced by HDMs or ovalbumin has mainly focused on the Th2 cell response as a systemic immune response [
6,
20–
23]. Th2 cells secrete IL-5 and IL-13, which play prominent roles in eosinophil activation, resulting in increased IgE and IgG1 production and cell recruitment to allergen-exposed sites [
24–
26]. Although the immune response of the airway mucosa has been investigated in several asthma models generated using ovalbumin and HDM, only a few studies have focused on the airway mucosal immune response to SMs [
4,
20,
21]. Considering the differences in the sources of food and shelter, they may involve distinct mechanisms of disease induction [
5,
11].
Mite feces constitute a major source of HDM allergens, and sensitization to HDMs primarily occurs via fecal pellets [
27]. Recent studies on the microbiomes of
D. farinae,
D. pteronyssinus, and
T. putrescentiae have reported different microbiota compositions. The major allergens in
D. farinae and
D. pteronyssinus belong to Groups 1, 2, and 23, whereas those in
T. putrescentiae belong to Groups 2 and 3. Moreover, Group 1 allergens comprise cysteine proteases that destroy the tight junctions of epithelial cells and are present in the gut of mites. Group 2 allergens are MD-2–like lipid-binding proteins that are also found in the gut. Group 3 allergens, which are the major allergens in
T. putrescentiae, exhibit serine protease activity [
28,
29]. Although SMs are a source of allergic asthma, their mechanisms of allergy induction and pathogenesis have not yet been determined.
In this study, we described the distinct immune responses and histological lung lesions caused by HDMs and the SM Tp using a murine model. The Tp-sensitized mice showed the highest degree of airway resistance and histological changes, despite being challenged with the same concentration of mite protein extract (100 μg/ml) as the mice in the Df- and Dp-sensitized groups. The number of total cells, eosinophils, and neutrophils increased the most in the Tp-challenged mice. Allergic asthma was characterized by AHR, chronic eosinophil inflammation, and elevated serum IgE, IgG1, and IgG2a levels in the HDM- and SM-exposed mouse asthma model. However, no significant differences between the IL-4, IL-5, and IL-6 levels in the BALF of mice challenged with the HDM or SM extracts were observed. However, the IL-17 level was the highest in the BALF of Tp-exposed mice.
In lung epithelial cells, IL-17A induces mucus production and goblet cell metaplasia, a hallmark of asthma and cystic fibrosis [
30,
31]. Moreover, IL-17-driven inflammation likely promotes the progression of chronic lung disease [
32,
33]. The results of the lung pathology examination revealed that the inflammatory response in SM-sensitized mice was more severe than that in the HDM-sensitized mice. Furthermore, the degree of collagen deposition, presence of goblet cells that secrete mucin, and MUC5AC level at the RNA/transcript and protein levels were significantly increased in the SM-sensitized groups, indicating that SMs elicit a greater mucosal immune response compared with HDMs. Importantly, IL-17A has been shown to promote MUC5AC expression and goblet cell hyperplasia in nasal polyps via the nuclear factor-kappa-B activator 1-mediated pathway [
34].
MUC5AC transcripts are localized to goblet cells within the tracheal and bronchial epithelium [
35–
37]. MUC5AC is one of the major mucins that form the mucous gel layer coating the apical surfaces of the airways and is primarily produced by goblet cells within the epithelial lining of the trachea and bronchi [
34–
36]. Moreover, MUC5AC expression and protein production are upregulated in patients with asthma, and the excessive production of mucins is critical in the development of mucus metaplasia in the asthmatic airway epithelium [
35,
37,
38]. MUC5AC overproduction is also a key feature of the allergic responses required for airway AHR [
35–
38].
In summary, our findings revealed that airway inflammation was more severe and mucin hypersecretion was more prevalent in the SM-sensitized mice than in the HDM-sensitized mice. Furthermore, Tp sensitization was strongly correlated with asthma mucosal immunity. Understanding the differential pathological parameters, including lung function, immune responses, and histopathological changes associated with HDM and SM exposure, has important implications for asthma pathophysiology. This study suggests that even though both allergens elicit similar symptoms in patients with asthma, the corresponding treatment strategies used should depend on the mite species, as lung damage and mucus overexpression caused by SM exposure are more severe than those caused by HDMs.
Notes
-
Author contributions
Conceptualization: Kim EM
Data curation: Kim EM, Kim JY
Formal analysis: Kim EM
Funding acquisition:Yong TS
Investigation: Kim EM, Kim JY, Kwak YS Yi MH
Methodology: Kim EM, Yi MH
Project administration: Kim EM
Resources: Kim JY, Kwak YS, Yi MH, Yong TS
Software: Kim EM
Supervision: Yong TS
Validation: Kim EM
Visualization: Kim EM
Writing – original draft: Kim EM
Writing – review & editing: Kim EM Yong TS
-
The authors declare no financial conflicts of interest.
Acknowledgments
We would like to thank Mr. Sung-Hyun Nam and Mr. In-Yong Lee for providing the HDM samples.
Fig. 1(A) Schematic representation of the protocols for mite-induced airway inflammation. BALB/c mice (n=10 per group) were challenged with mite protein extracts (100 μg/ml) of D. farinae (Df) and D. pteronyssinus (Dp) (HDMs) as well as T. putrescentiae (Tp; SMs) in 10 ml of PBS for 10 min through an air-compressing nebulizer in a chamber. PBS was used as a negative control. The extracts were applied on Days 7, 14, 21, 28, 35, 42, 60, and 61; the mice were euthanized on Day 63. (B) Airway hyperresponsiveness (AHR) was determined as airway resistance to the inhaled methacholine concentration. (C) The total number of cells in the bronchoalveolar lavage fluid and the relative proportions of cells, eosinophils, neutrophils, lymphocytes, and macrophages. *P<0.01 compared with the PBS/PBS group for the mice in the protocol groups (n=10 per group).
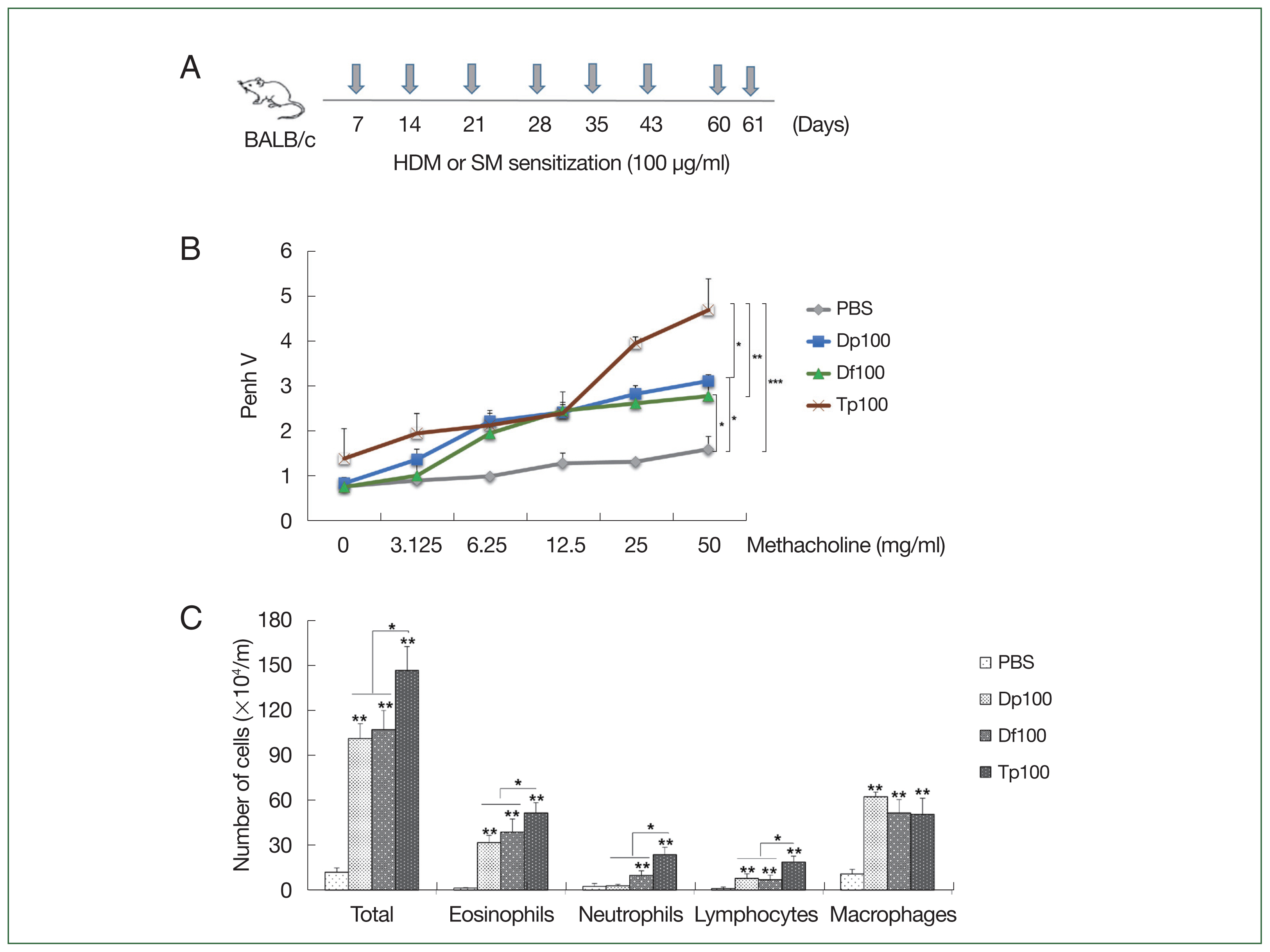
Fig. 2Profiles of the immunoglobulin and cytokines induced by the protein extracts of D. farinae, D. pteronyssinus, and T. putrescentiae. (A) Serum levels of IgE, IgG1, and IgG2a measured using an ELISA as optical density (OD) values. (B) Levels of cytokines from the bronchoalveolar lavage fluid (BALF). (C) Levels of cytokines from cultured splenocytes. Data were expressed as the mean±SD. *P<0.01 (n=10 mice/group).
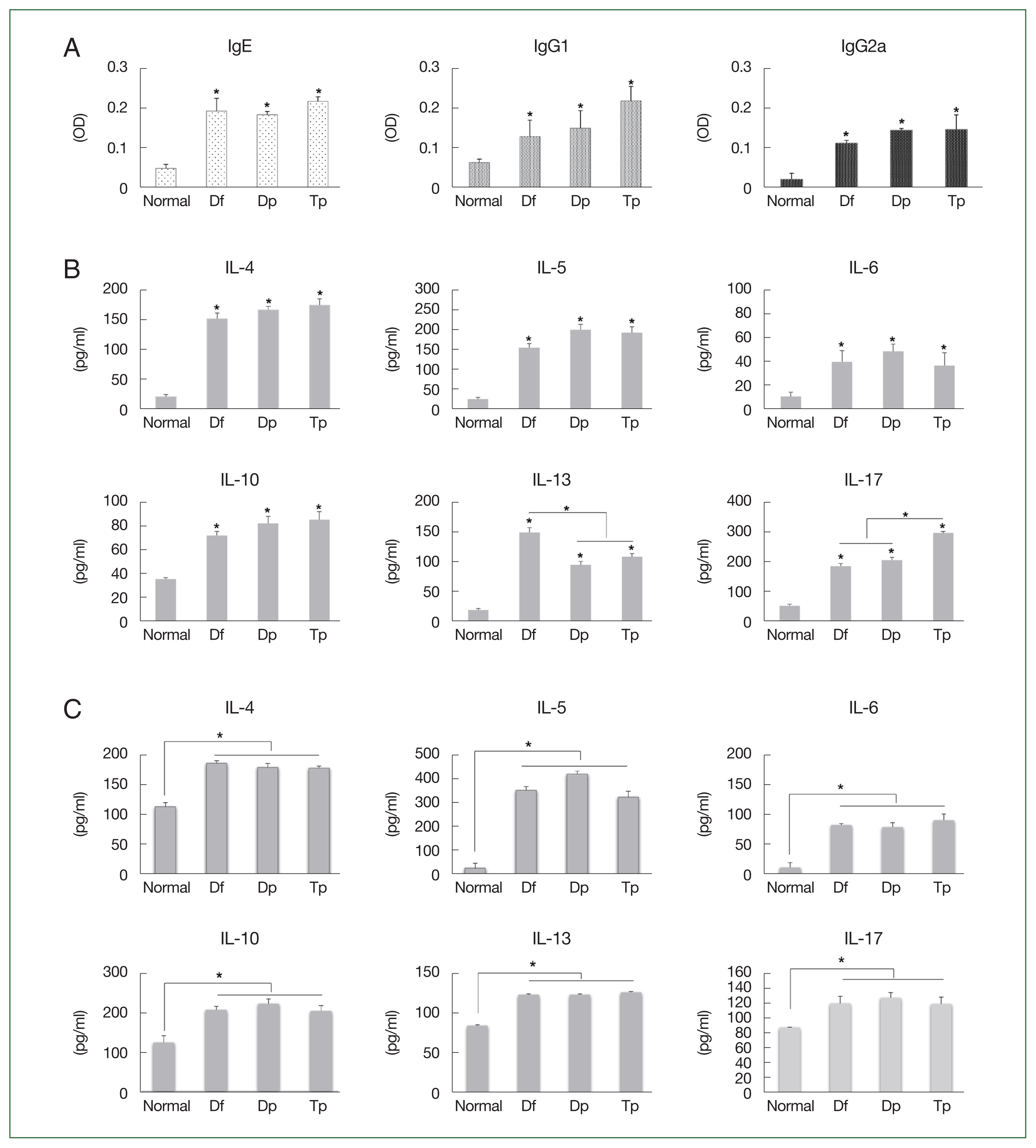
Fig. 3Histopathology induced by the protein extracts of D. farinae, D. pteronyssinus, and T. putrescentiae. (A) Representative hematoxylin and eosin, Masson’s trichrome, PAS, and toluidine blue-stained lung section photomicrographs are shown for each group. The HDM-exposed mice showed apparent inflammatory infiltrates (yellow arrows) including peribronchial and perivessel inflammation, thickness in the bronchial epithelial cells, collagen deposition (green arrows, blue staining for Masson’s trichrome), and numerous mucus-containing cells (PAS, orange arrows) compared with the PBS-treated control mice. Scale bar=200 μm. (B–D) Analyses of the epithelial layer thickness and airway collagen/fibrosis (blue), presented as the fold change (blue/total area in each group normalized to the PBS group), and the number of PAS-positive cells. Data were expressed as the mean±SD. *P<0.01 (n=10 mice/group).

Fig. 4Protein expression of MUC5AC in the lung tissues of mice challenged with the protein extracts of D. farinae, D. pteronyssinus, and T. putrescentiae (100 μg/ml). The black arrow indicates the MUC5AC-positive goblet cells (scale bar=200 μm). (A) Expression of MUC5AC in the lung tissues, as detected using western blotting. (B) The representative MUC5AC-immunostained lung sections 24 h after the final challenge with the mite extracts. *P<0.05 and **P<0.01. (C) RNA expression of MUC5AC in the lung tissues as determined using RT-qPCR.
References
- 1. Braman SS. The global burden of asthma. Chest 2006;130(Suppl):4-12.
https://doi.org/10.1378/chest.130.1_suppl.4S
- 2. Wong QYA, Lim JJ, Ng JY, Malipeddi P, Lim YYE, et al. An updated prevalence of asthma, its phenotypes, and the identification of the potential asthma risk factors among young Chinese adults recruited in Singapore. World Allergy Organ J 2023;16(3):100757.
https://doi.org/10.1016/j.waojou.2023.100757
- 3. Tauro S, Su YC, Thomas S, Schwarze J, Matthaei KI, et al. Molecular and cellular mechanisms in the viral exacerbation of asthma. Microbes Infect 2008;10(9):1014-1023.
https://doi.org/10.1016/j.micinf.2008.07.037
- 4. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004;169(3):378-385.
https://doi.org/10.1164/rccm.200308-1094OC
- 5. Kim CR, Jeong KY, Yi MH, Kim HP, Shin HJ, et al. Cross-reactivity between group-5 and -21 mite allergens from Dermatophagoides farinae, Tyrophagus putrescentiae and Blomia tropicalis
. Mol Med Rep 2015;12(4):5467-5474.
https://doi.org/10.3892/mmr.2015.4093
- 6. Wypych TP, Marzi R, Wu GF, Lanzavecchia A, Sallusto F. Role of B cells in TH cell responses in a mouse model of asthma. J Allergy Clin Immunol 2018;141(4):1395-1410.
https://doi.org/10.1016/j.jaci.2017.09.001
- 7. Reboux G, Valot B, Rocchi S, Scherer E, Roussel S, et al. Storage mite concentrations are underestimated compared to house dust mite concentrations. Exp Appl Acarol 2019;77(4):511-525.
https://doi.org/10.1007/s10493-019-00376-2
- 8. Arlian LG, Geis DP, Vyszenski-Moher DL, Bernstein IL, Gallagher JS. Antigenic and allergenic properties of the storage mite Tyrophagus putrescentiae
. J Allergy Clin Immunol 1984;74(2):166-171.
https://doi.org/10.1016/0091-6749(84)90281-1
- 9. Morales M, Iraola V, Leonor JR, Bartra J, Rodríguez F, et al. Different sensitization to storage mites depending on the co-exposure to house dust mites. Ann Allergy Asthma Immunol 2015;114(1):36-42.
https://doi.org/10.1016/j.anai.2014.10.005
- 10. Yi MH, Kim M, Yong TS, Kim JY. Investigating the microbiome of house dust mites in South Korea. Front Allergy 2023;4:1240727.
https://doi.org/10.3389/falgy.2023.1240727
- 11. Jeong KY, Choi SY, Lee JH, Lee IY, Yong TS, et al. Standardization of house dust mite extracts in Korea. Allergy Asthma Immunol Res 2012;4(6):346-350.
https://doi.org/10.4168/aair.2012.4.6.346
- 12. Park JW, Taube C, Joetham A, Takeda K, Kodama T, et al. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med 2004;169(6):726-732.
https://doi.org/10.1164/rccm.200307-1042OC
- 13. Jeong KY, Lee IY, Lee J, Ree HI, Hong CS, et al. Effectiveness of education for control of house dust mites and cockroaches in Seoul, Korea. Korean J Parasitol 2006;44(1):73-79.
https://doi.org/10.3347/kjp.2006.44.1.73
- 14. Munhbayarlah S, Park JW, Ko SH, Ree HI, Hong CS. Identification of Tyrophagus putrescentiae allergens and evaluation of cross-reactivity with Dermatophagoides pteronyssinus
. Yonsei Med J 1998;39(2):109-115.
https://doi.org/10.3349/ymj.1998.39.2.109
- 15. Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: utility and limitations. J Asthma Allergy 2017;10:293-301.
https://doi.org/10.2147/JAA.S121092
- 16. Pomés A, Chapman MD, Wünschmann S. Indoor allergens and allergic respiratory disease. Curr Allergy Asthma Rep 2016;16(6):43.
https://doi.org/10.1007/s11882-016-0622-9
- 17. Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report 2011;2011(32):1-14.
- 18. Ishmael FT. The inflammatory response in the pathogenesis of asthma. J Am Osteopath Assoc 2011;111(Suppl):11-17.
- 19. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120(Suppl):94-138.
https://doi.org/10.1016/j.jaci.2007.09.043
- 20. Ye L, Mou Y, Wang J, Jin ML. Effects of microRNA-19b on airway remodeling, airway inflammation and degree of oxidative stress by targeting TSLP through the Stat3 signaling pathway in a mouse model of asthma. Oncotarget 2017;8(29):47533-47546.
https://doi.org/10.18632/oncotarget.17258
- 21. Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, et al. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol 2003;162(6):2069-2078.
https://doi.org/10.1016/S0002-9440(10)64338-6
- 22. Iuvone T, Den Bossche RV, D’Acquisto F, Carnuccio R, Herman AG. Evidence that mast cell degranulation, histamine and tumour necrosis factor alpha release occur in LPS-induced plasma leakage in rat skin. Br J Pharmacol 1999;128(3):700-704.
https://doi.org/10.1038/sj.bjp.0702828
- 23. MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol 1999;20(3):379-387.
https://doi.org/10.1165/ajrcmb.20.3.3291
- 24. Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol 1999;21(4):480-489.
https://doi.org/10.1165/ajrcmb.21.4.3659
- 25. Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, et al. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res 2001;50(12):616-624.
https://doi.org/10.1007/PL00000243
- 26. Paul DL. New functions for gap junctions. Curr Opin Cell Biol 1995;7(5):665-672.
https://doi.org/10.1016/0955-0674(95)80108-1
- 27. Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature 1981;289(5798):592-593.
https://doi.org/10.1038/289592a0
- 28. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009;457(7229):585-588.
https://doi.org/10.1038/nature07548
- 29. Kim JY, Yi MH, Lee S, Lee IY, Yong D, et al. Microbiome and mycobiome interaction in house dust mites and impact on airway cells. Clin Exp Allergy 2021;51(12):1592-1602.
https://doi.org/10.1111/cea.13962
- 30. Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, et al. IL-17 receptor signaling in the lung epithelium is required for mucosal chemokine gradients and pulmonary host defense against K. pneumoniae
. Cell Host Microbe 2016;20(5):596-605.
https://doi.org/10.1016/j.chom.2016.10.003
- 31. Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep 2015;10(2):239-252.
https://doi.org/10.1016/j.celrep.2014.12.017
- 32. Ritzmann F, Lunding LP, Bals R, Wegmann M, Beisswenger C. IL-17 cytokines and chronic lung diseases. Cells 2022;11(14):2132.
https://doi.org/10.3390/cells11142132
- 33. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006;7(1):135.
https://doi.org/10.1186/1465-9921-7-135
- 34. Xia W, Bai J, Wu X, Wei Y, Feng S, et al. Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway. PLoS One 2014;9(6):e98915.
https://doi.org/10.1371/journal.pone.0098915
- 35. Kanoh S, Tanabe T, Rubin BK. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy 2011;41(12):1747-1756.
https://doi.org/10.1111/j.1365-2222.2011.03852.x
- 36. Wang X, Li Y, Luo D, Wang X, Zhang Y, et al. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. Sci Rep 2017;7:42675.
https://doi.org/10.1038/srep42675
- 37. Yan F, Li W, Zhou H, Wu Y, Ying S, et al. Interleukin-13-induced MUC5AC expression is regulated by a PI3K-NFAT3 pathway in mouse tracheal epithelial cells. Biochem Biophys Res Commun 2014;446(1):49-53.
https://doi.org/10.1016/j.bbrc.2014.02.051
- 38. Nakagome K, Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx 2011;38(5):555-563.
https://doi.org/10.1016/j.anl.2011.01.011