Abstract
Alpacas (Vicugna pacos), native to South America, were recently introduced to Korea, primarily for tourism-related activities. However, information on their parasitic infections in alpacas within the region are limited. This study aimed to investigate the longitudinal prevalence and diversity of gastrointestinal parasites in an alpaca herd on a single farm in Korea and to assess the potential risks for reinfection and interspecies transmission. Between June 2022 and June 2023 (excluding December 2022 and January 2023), fecal samples were collected monthly from a herd of 61 alpacas housed on a farm on Jeju Island, Korea. Coprological examination of 406 samples was performed using a modified McMaster technique, and parasites were identified morphologically. Six gastrointestinal parasite taxa were identified: strongyles, Trichuris sp., capillarids, Moniezia sp., Eimeria lamae, and Eimeria macusaniensis. The overall gastrointestinal parasite prevalence was 11.3%, with strongyles and Trichuris sp. most frequently identified. No seasonal trends were observed, and mixed infections were present in five animals. The prevalence was markedly lower compared with reports from Japan, Poland, and Australia, reflecting the restricted pasture access implemented by Korea’s alpaca management systems, which are primarily tourism-oriented. Neither Nematodirus spp. nor zoonotic parasites were detected. This is the first longitudinal investigation of gastrointestinal parasites in alpacas in Korea. Although the overall prevalence was low, environmental contamination and the risk of reinfection are ongoing concerns. These findings highlight the need for routine surveillance, targeted parasite control, and consistent veterinary oversight, particularly as alpaca populations expand in Korea.
-
Key words: Alpaca, gastrointestinal parasites, prevalence, South Korea
Introduction
Alpacas (
Vicugna pacos), one of the South American camelids (SACs), originate from the high-altitude regions of the Andes in Peru. They have gained popularity in recent years and are now reared in several countries, including the USA [
1], Australia [
2], Europe [
3], and Asia [
4]. Unpublished data obtained upon request from the Animal and Plant Quarantine Agency of Korea (
Supplementary Table S1) indicate that alpacas are regularly imported, and the national population is currently estimated at several hundred.
As with other herbivorous hosts, alpacas harbor diverse gastrointestinal parasites, including nematodes, such as strongyles and
Trichuris spp., and 5 coccidian species shared with llamas (
Eimeria alpacae,
Eimeria lamae,
Eimeria macusaniensis,
Eimeria punoensis, and
Eimeria peruviana). Furthermore, alpacas are susceptible to a range of parasites found in other domestic species, some of which cause severe disease under specific conditions [
5]. Despite the recognized risks of endoparasitism in alpacas, including severe health impacts, financial losses [
5–
8], and zoonotic threats (e.g.,
Cryptosporidium and
Giardia) [
9,
10], data on the prevalence of parasitic infections in alpacas in Korea are lacking. A PubMed literature search using the keywords “alpacas” OR “camelids” AND “Korea” yielded no relevant studies on parasitic infections in this population.
Despite the absence of parasitological surveys on alpacas in Korea, parasite control measures using anthelmintic treatments are routinely implemented. Although fenbendazole is most commonly used, the optimal dosing frequency for effective parasite control and the potential presence of parasites beyond its spectrum of activity remain unelucidated. Therefore, a longitudinal investigation assessing gastrointestinal parasite prevalence following fenbendazole treatment is warranted to inform more sustainable parasite control strategies. Accordingly, the objectives of this study were to determine the longitudinal prevalence and diversity of endoparasites in an alpaca population in Korea and to obtain evidence to inform improved parasite management practices.
Materials and Methods
Ethics statement
This study was approved by the Institutional Animal Care and Use Committee of Cheju Halla University (acceptance No. CHU22040). All procedures complied with the Institutional Animal Care and Use Committee guidelines.
Animals and study design
Monthly inspections were conducted on a farm located on Jeju Island, Korea (elevation 310 m) between June 2022 and June 2023, excluding December 2022 and January 2023 due to weather-related constraints. The alpacas included in the study varied in sex and age (mean 3.93±2.03 years). Because the guidelines of the World Association for the Advancement of Veterinary Parasitology do not specify a recommended sample size for alpaca studies [
11], a farm was selected where the number of alpacas exceeded the minimum sample size recommended for fecal egg count reduction tests in ruminants. Other animals on the farm, including horses, sheep, and goats, were kept separately from the alpacas.
The study farm’s alpaca population was established in 2019, consistent with a sharp rise in national alpaca imports (
Supplementary Table S1). There were no other alpaca farms in the surrounding area, reducing the likelihood of transmission from external sources. The alpacas in this closed, clinically healthy herd had no history of dietary changes, medical conditions, or medication use, including anthelminthic treatment, during the 6 months preceding the sample collection period. The most recent anthelmintic treatment (fenbendazole) was administered in December 2021. Thus, the likelihood of bias associated with recent antiparasitic interventions was minimal. The alpacas had access to haylage and water ad libitum and received limited quantities of feed concentrate. The pasture area was used exclusively by the alpacas (
Fig. 1A), and they were housed in a barn with wooden partitions (
Fig. 1B). All alpacas remained clinically healthy throughout the study period.
At the end of June 2022 (designated as the baseline), all alpacas were administered an oral commercial anthelminthic agent containing fenbendazole (Fenbenzole; KBNP Inc., Anyang, Korea), which was provided in shared bins. Due to the absence of digital scales, body weight was visually estimated, and doses were based on estimated weights of ~40, 25–30, and 7–10 kg for large, medium, and small alpacas, respectively. The calculated doses (5 mg/kg body weight) were mixed into feed and administered twice on a single day (once in the morning and once in the evening) to account for potential variability in intake from shared feeding. The farm owner administered the treatments according to routine practice.
Coprological examination
Each alpaca was tagged for identification. Fecal samples were collected directly from the rectum of restrained individuals. Samples were not taken if no fecal material was present in the rectum. During the study period, fecal samples were obtained from 31 to 43 alpacas each month. Overall, 61 alpacas were sampled at least once during the study period, and the results were recorded individually for each animal.
The first samples were collected in June 2022, 2 weeks after the anthelmintic treatment. To ensure consistency, collections were performed between 19:00 and 20:00, when the alpacas were housed for the evening. The samples were sealed in labeled plastic bags and shipped overnight to the Department of Parasitology, School of Medicine, Chungbuk National University, Cheongju. They were refrigerated upon receipt and processed within 2 days. The fecal samples underwent quantitative analysis using a modified McMaster technique with a zinc sulfate supersaturated solution (specific gravity 1.18), resulting in a sensitivity of 25 eggs/g. However, due to the low number of positive samples and the use of estimated rather than directly measured body weights, only presence/absence data were used for prevalence. As a result, infection intensity and host factors (age, sex, and estimated weight) were not analyzed.
Results
Morphological identification
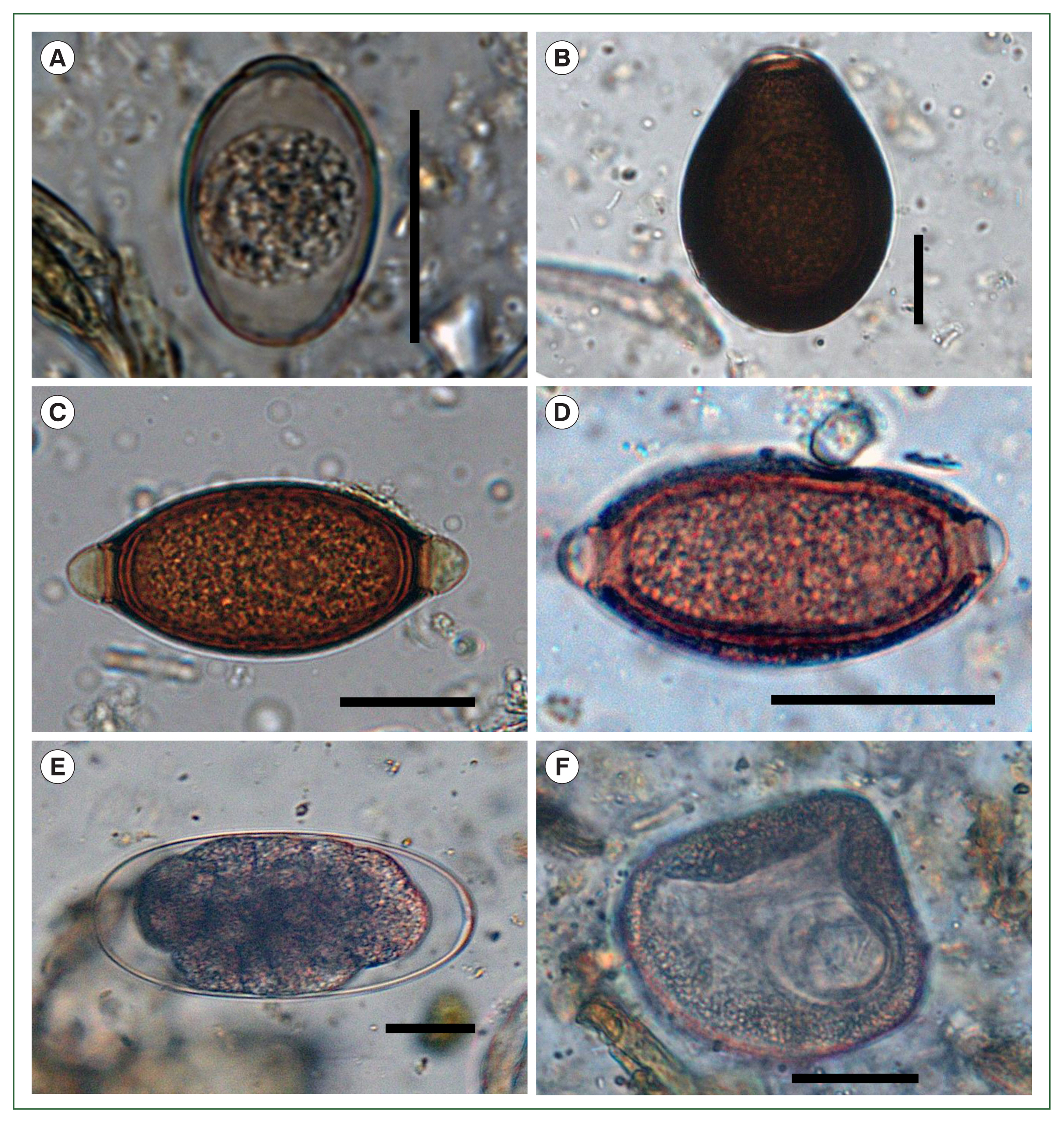
Six parasite taxa were present: 2 coccidian protozoans, 3 nematodes, and 1 cestode species. Based on published descriptions of morphological characteristics, they were identified as strongyles, capillarids,
Trichuris sp.,
Moniezia sp., and 2 types of
Eimeria oocytes [
5]. The smaller
Eimeria oocysts exhibited characteristics consistent with
E. lamae, including an ovoid to ellipsoidal shape, pale coloration, and dimensions of 31–39 (mean 34)×23–26 (mean 25) μm (
Fig. 2A). The larger oocysts were identified as
E. macusaniensis. They were ovoid, with a thicker, darker wall than the smaller
Eimeria oocysts and measured 85–89 (mean 86)×59–63 (mean 61) μm (
Fig. 2B). Both types of
Eimeria oocysts had a micropyle. The
Trichuris eggs measured 67–75 (mean 72)×33–37 (mean 35) μm, with a smooth shell and prominently developed polar plugs (
Fig. 2C). In contrast, the capillarid eggs measured 50–56 (mean 54)×25–28 (mean 27) μm, with a rough shell and slightly prominent polar plugs, distinguishing them from
Trichuris eggs (
Fig. 2D). At least 2 morphological types of strongyle eggs were observed. Although they could not be identified at the species level due to their low count, the eggs were distinguishable by size and shape. The smaller type measured 74–103 (mean 86)×43–48 (mean 46) μm and exhibited a more tapered form than the larger type. The larger type measured 170–190 (mean 182)×93–121 (mean 104) μm and had rounded ends and a parallel-sided central region (
Fig. 2E). The cestode eggs were identified by the presence of a hexacanth larva measuring 65–70 (mean 67)×57–63 (mean 61) μm and were enclosed within a pyriform apparatus. Based on these morphological features, they were identified as
Moniezia sp. (
Fig. 2F).
Among the 406 fecal samples examined, 46 (11.3%) tested positive for at least one type of parasite. Two species of
Eimeria were detected in 10 cases (2.5%), along with four types of helminth eggs: strongyles (15 cases, 3.7%), capillarids (6 cases, 1.5%),
Trichuris sp. (16 cases, 3.9%), and
Moniezia sp. (4 cases, 1.0%) (
Fig. 2). Among the
Eimeria-positive cases, 2 were identified as
E. macusaniensis, while the remainder were
E. lamae. Although some ciliate trophozoites were observed, they were excluded from the results because they were considered components of the normal intestinal flora. Mixed infections were present in 5 individuals: 2 cases of strongyles and
Trichuris sp., and 1 case each of strongyles with
E. lamae, strongyles with capillarids, and
E. macusaniensis with
Moniezia eggs. No triple or higher-order infections were detected.
The universal negativity observed in June (2 weeks after treatment) is consistent with the temporary suppression of nematode egg shedding caused by fenbendazole treatment. The detection of Eimeria spp. and Moniezia spp. in later months may reflect their respective prepatent periods (Eimeria: ~10–12 days and Moniezia: ~35–40 days), suggesting the presence of subclinical infections or parasites in the prepatent period during the initial sampling.
Given the low parasite positivity rate (46/406, 11.3%) and the small study population of alpacas (although encompassing a wide age range), statistical comparisons of parasite burden by age, sex, or estimated body weight were unfeasible.
Monthly and individual patterns of parasite infection
Table 1 presents the results recorded for each month from June 2022 to June 2023 (excluding December 2022 and January 2023, when sampling could not be conducted due to weather constraints). In June 2022 (the first month of the study), all 35 sampled alpacas tested negative for parasites. However, at least one positive case was detected in each subsequent month. The highest prevalence occurred in August 2022 (12/43, 27.9%), including 2 cases of mixed infections. Excluding the initial negative results in June 2022, the lowest prevalence rates were recorded in May and June 2023, with only one positive case each month, corresponding to 3.0% and 2.5%, respectively (
Table 1).
Notably, not all alpacas were sampled every month, thereby limiting consistent longitudinal evaluation across individuals. However, 1 alpaca (No. 40;
Supplementary Table S2) tested positive for parasitic infection on four separate occasions, with a different parasite taxa detected each time:
E. lamae, strongyles,
Trichuris sp., and
Moniezia sp. Another alpaca (No. 32) tested positive across multiple months. It was found to carry capillarid eggs in September 2022, tested negative in October 2022, and exhibited a mixed infection of
Trichuris sp. and strongyles in November 2022. In February 2023, it tested positive once again for strongyles and capillarid eggs, but
Trichuris sp. were not detected (
Supplementary Table S2).
Among the 61 alpacas examined at least once, 33 tested negative for all parasites throughout the study period. Thirteen alpacas tested positive in only one month. An additional 13 alpacas tested positive on 2 occasions, which included both consecutive (
n=5) and non-consecutive (
n=8) months. One alpaca tested positive on 3 occasions, and another tested positive on 4 occasions (
Supplementary Table S2).
Discussion
Alpacas are a relatively recent addition to animals raised in Korea. They were first introduced in 2016, with the importation of a male-female pair from Australia. Because alpacas remain a relatively unfamiliar species within the livestock industry in Korea, the prevalence and diversity of parasitic infections have not been investigated. This study presents the first coprological assessment of gastrointestinal parasites in alpacas in Korea.
Due to the inherent limitations of coprological examination, species-level identification was generally unfeasible. However, comparison of morphological characteristics with those previously reported enabled the identification of 2 species of
Eimeria (
E. lamae and
E. macusaniensis) based on distinctive features including size, shape, and the presence or absence of a micropyle [
4,
5,
12].
For other helminths, species-level identification based on egg morphology alone was challenging, particularly for
Trichuris and capillarid eggs, and identification was restricted to the type level, as in previous studies [
4,
5,
12]. For strongyle eggs, classified into 2 morphotypes, identification was only possible at a higher taxonomic level due to the thin, smooth shell characteristic of this group. The smaller strongyle eggs could not be further identified because their size and morphology were similar to those of one or more other species. In contrast, the larger strongyle eggs were presumed to be
Marshallagia marshalli based on their size and morphology being consistent with previous reports in the literature [
5]. Notably, unlike findings from South America [
5], Japan [
4], Poland [
13], and Australia [
2], where
Nematodirus spp. have been identified in alpacas, none were observed in this study. This conclusion was based on the absence of characteristic morphological features, including the distinctive spindle-like oval shape of
Nematodirus spp., with non-parellel margins, which contrast with the parallel-sided, more cylindrical oval shape of
Marshallagia spp. eggs [
5]. The size and morphology of the
Moniezia eggs, particularly the pyriform apparatus, were most consistent with
Moniezia expansa [
14].
The most frequently detected parasites in this study were strongyles and
Trichuris sp. Despite their repeated occurrence, there were no consistent seasonal trends. Two distinct forms of strongyle-type eggs were observed, suggesting the presence of multiple strongylid species, but further identification was limited by morphological overlap and low egg counts. The ongoing detection of unidentified strongyle eggs throughout the study period indicates persistent environmental contamination and reinfection pressure. The alpacas received fenbendazole treatment in June 2022, but no additional anthelmintic treatments were administered thereafter, potentially contributing to infection persistence. The repeated detection of strongyle-type eggs likely reflects sustained transmission within the herd, with contaminated pastures serving as a continual source of reinfection. Similar infection patterns have been reported in other grazing species under conditions of inadequate pasture hygiene and infrequent anthelminthic treatments, allowing parasite life cycles to remain uninterrupted. Under such conditions, integrated control measures, such as routine fecal removal, rotational grazing, and targeted deworming guided by fecal egg count monitoring, are more effective than relying on annual or single-dose deworming treatments [
15].
The limited efficacy of fenbendazole against cestodes and protozoa, as well as the timing of Eimeria and Moniezia oocyst/egg detection in this study, suggests delayed patency or residual infections rather than treatment failure. Although pasture access was minimal, reinfection through contaminated housing, feed, or shared pens cannot be excluded.
Although
Moniezia sp. was the least frequently detected helminth in this study, its presence is significant because it is typically associated with domestic ruminants, such as sheep and goats. Thus, the possibility of parasite spillover must not be overlooked, particularly in management systems where SACs cohabit with or share pastures with other livestock species. While this study does not directly confirm interspecies transmission, the detection of
Moniezia in alpacas is concerning because SACs are known hosts of parasites commonly found in domestic ruminants [
8].
Moniezia spp. have been detected in small ruminants in Korea. However, most data originate from studies conducted 2–5 decades ago [
16,
17]. Given the limited and outdated nature of these reports, reassessment of
Moniezia prevalence in sympatric domestic ruminants is warranted. Routine parasitological monitoring in comanaged livestock systems may help elucidate potential transmission pathways, thereby informing more effective, integrated parasite control strategies.
The protozoan infections in this study were limited to
Eimeria spp., represented by 2 distinct oocyst morphotypes corresponding to
E. lamae and
E. macusaniensis, both of which have been previously reported in SACs from South America [
18]. This suggests that
E. lamae and
E. macusaniensis were introduced through the importation of animals. Although oocyst counts were low and clinical signs were absent, regular monitoring is advised, particularly in young or immunocompromised individuals. In contrast, ciliate trophozoites were considered incidental findings, likely representing normal intestinal flora.
Intriguingly, some alpacas tested positive for parasites on multiple occasions throughout the study, while others consistently tested negative. Such variations in individual infection patterns suggest that a single fecal examination is insufficient to accurately assess parasitic infection in alpacas. This may be attributed to several factors, including intermittent shedding, low parasite burdens, and sampling variability due to small fecal volumes. In cases of light infection, the uneven distribution of parasite eggs within the fecal sample may increase the likelihood of false-negative results. Therefore, repeated sampling and standardized collection protocols are recommended to improve diagnostic accuracy.
Although zinc sulfate flotation with high specific gravity may have limited sensitivity for detecting small protozoan parasites, such as Cryptosporidium and Giardia, the absence of these organisms in this study likely reflects true negativity, as none of the alpacas displayed clinical signs suggesting protozoan infection. Furthermore, based on previous diagnostic experience, infections with these parasites typically result in easily detectable oocyst shedding. Nevertheless, this methodological limitation should be taken into account when interpreting the findings.
Although self-cure and intermittent egg shedding have been proposed in herbivores such as sheep and goats, these mechanisms are not well-characterized in alpacas. Given the generally low egg/oocyst counts observed in this study, transition from a positive to negative status may reflect random variation in sampling rather than true parasite clearance. Fecal samples were collected per rectum, and eggs or oocysts may not have been uniformly distributed within the fecal bolus in cases of light infections. Thus, some negative results may reflect the absence of diagnostic stages in the specific fecal aliquot analyzed, rather than the actual absence of infection.
Compared with studies performed in other countries, the overall prevalence of gastrointestinal parasites observed in this study is relatively low. A Japanese survey of 53 alpacas identified
Nematodirus sp. (13.2%),
Trichuris sp. (11.3%), and capillarids (5.7%), as well as strongyle-type eggs in >50% of animals, despite quarterly ivermectin treatment [
4]. A large-scale survey in Australia of 1,688 alpacas across 13 farms reported a gastrointestinal nematode prevalence of 61%, with
Trichostrongylus spp.,
Haemonchus spp., and
Camelostrongylus mentulatus as the predominant species [
2]. Similarly, a Polish study on 248 alpacas from 43 farms reported high prevalence rates of trichostrongyles (46%) and
Nematodirus spp. (33.9%), while that of
Moniezia sp. was low (2%) [
13].
Direct comparisons across studies are limited due to differences in sample sizes, management practices, climate, and diagnostic methodology. However, the relatively low parasite prevalence in this study may be attributed to the unique husbandry conditions in Korea. Unlike countries where alpacas are raised for fiber, meat, or transport [
18], alpaca farming in Korea is primarily tourism-oriented. As such, animals are typically housed in confined settings with limited pasture access, thereby reducing exposure to contaminated environments and the risk of reinfection.
Although zoonotic parasites were not detected in this study, previous reports of
Cryptosporidium parvum transmission from alpacas to humans underscore the importance of continued surveillance, particularly because asymptomatic animals may shed infectious oocysts [
10]. With the growing popularity of alpaca farming in Korea, often characterized by close human-animal contact in tourism settings, such risks highlight the need for structured herd health programs. However, veterinary involvement remains limited, and anecdotal reports of sudden death in the absence of preceding clinical signs are concerning. Collectively, these issues indicate the need for improved veterinary oversight, including routine health monitoring, diagnostic investigations, and necropsies, to safeguard animal health and mitigate potential public health risks.
This study has several limitations. First, it was conducted on a single farm on Jeju Island, with a relatively small and variable number of animals sampled each month. Thus, the findings may not be generalizable to the broader alpaca population in Korea. Second, the lack of comprehensive demographic data prevented the assessment of age-specific prevalence and risk factors. Nevertheless, this study is the first longitudinal investigation of gastrointestinal parasites in alpacas in Korea, demonstrating the feasibility and value of year-round parasitological monitoring. The detection of multiple gastrointestinal parasite taxa (although at a low prevalence) indicates the presence of persistent infections that may remain undetected in the absence of routine surveillance. Given the reliance on single-dose or routine deworming on many farms, periodic diagnostic screening may offer more accurate assessments, thereby facilitating more targeted and effective parasite control.
Such an approach may reduce unnecessary anthelmintic use and help slow the development of drug resistance, a growing global concern in livestock management. Nationwide studies involving larger sample sizes across multiple farms are crucial for developing comprehensive, evidence-based strategies for the sustainable control of parasites in alpaca herds under Korean farming conditions. This study provides foundational data for informing evidence-based parasite control in alpacas, highlighting the importance of coordinated, national surveillance efforts tailored to Korea’s emerging alpaca industry.
Notes
-
Author contributions
Conceptualization: Ryu SH, Choe S
Data curation: Ryu SH, Choe S
Formal analysis: Lee HW, Opara E
Investigation: Ryu SH, Lee HW, Opara E, Choe S
Methodology: Ryu SH, Choe S
Resources: Ryu SH, Choe S
Software: Ryu SH, Choe S
Validation: Ryu SH, Forbes E, Choe S
Visualization: Ryu SH
Writing – original draft: Ryu SH, Forbes E
Writing – review & editing: Ryu SH, Forbes E, Lee HW, Opara E, Choe S
-
Conflict of interest
The authors declare no conflict of interest related to this study.
-
Acknowledgments
This work was supported by the research grant of the Chungbuk National University in 2020. In addition, the authors gratefully acknowledge Youngchan Kwon, owner of the alpaca farm, for his valuable cooperation during the year-long sample collection process.
Supplementary Information
Fig. 1Outdoor and indoor environments for the alpacas during the study period. (A) Daytime outdoor grazing area designated solely for the alpacas. (B) Nighttime indoor housing facility with wooden partitions used to accommodate the alpacas.

Fig. 2Microscopic images of parasitic eggs and oocysts identified in fecal samples from the alpacas (Vicugna pacos) in this study. (A) Eimeria lamae, (B) Eimeria macusaniensis, (C) Trichuris sp., (D) a capillarid egg, (E) a strongyle egg, and (F) Moniezia sp. Scale bars=25 μm.
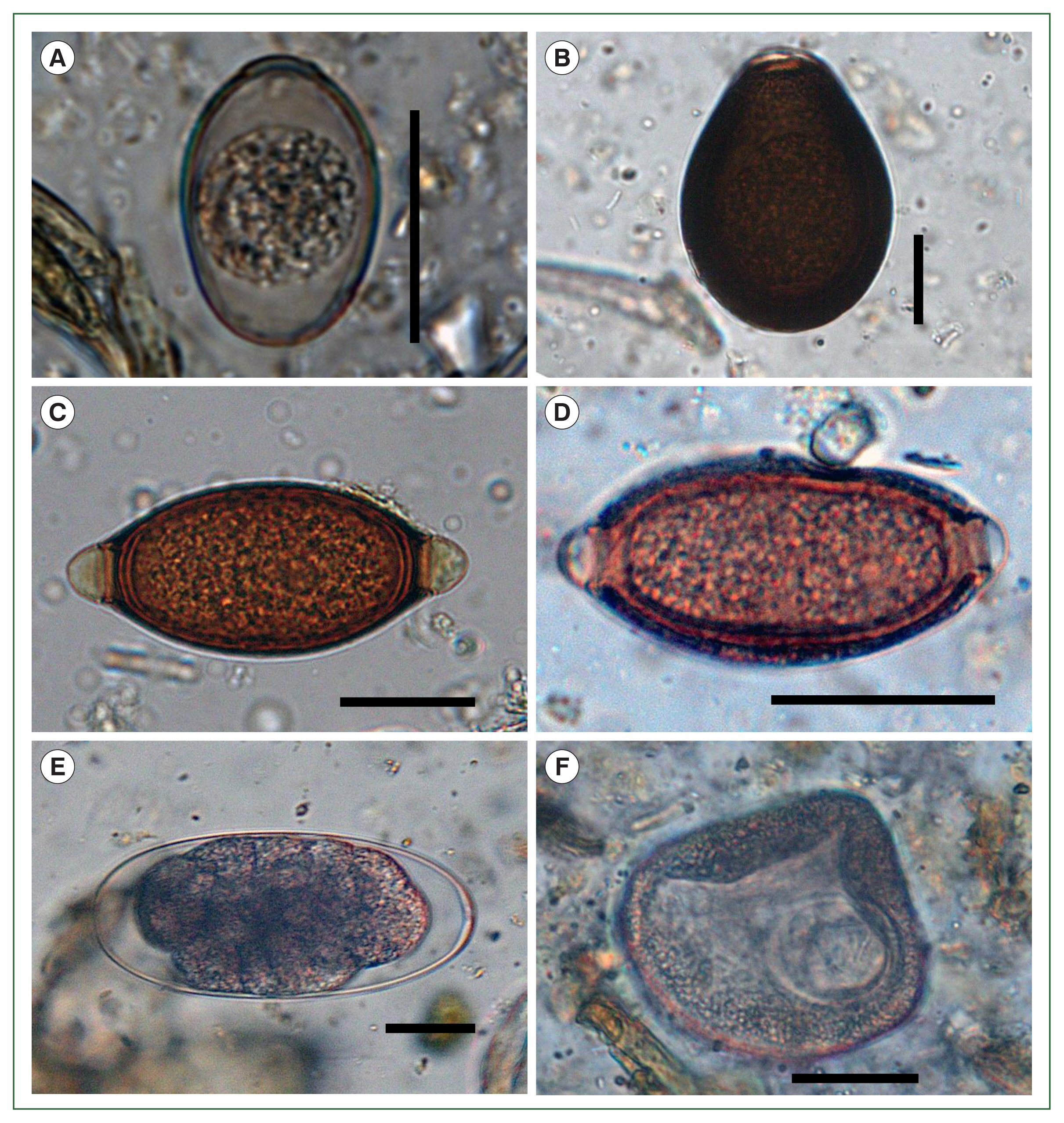
Table 1Monthly prevalence of gastrointestinal parasites among alpacas (Vicugna pacos) in Korea
Table 1
|
Species |
|
Jun 2022 (n=35) |
Jul (n=30) |
Aug (n=43) |
Sep (n=40) |
Oct (n=35) |
Nov (n=39) |
Feb 2023 (n=40) |
Mar (n=40) |
Apr (n=31) |
May (n=33) |
Jun (n=40) |
|
Protozoa |
Eimeria lamae
|
0 (0.0) |
0 (0.0) |
5 (11.6) |
2 (5.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (2.5) |
0 (0.0) |
1 (3.0) |
0 (0.0) |
|
Eimeria macusaniensis
|
0 (0.0) |
0 (0.0) |
0 (0.0) |
1 (2.5) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Nematoda |
Trichuris sp. |
0 (0.0) |
5 (16.7) |
1 (2.3) |
2 (5.0) |
1 (2.9) |
3 (7.7) |
1 (2.5) |
2 (5.0) |
1 (3.2) |
0 (0.0) |
0 (0.0) |
|
Capillarids |
0 (0.0) |
0 (0.0) |
2 (4.7) |
1 (2.5) |
2 (5.7) |
0 (0.0) |
1 (2.5) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Strongyles |
0 (0.0) |
1 (3.3) |
4 (9.3) |
1 (2.5) |
1 (2.9) |
2 (5.1) |
4 (10.0) |
0 (0.0) |
1 (3.2) |
0 (0.0) |
1 (2.5) |
|
|
Cestoda |
Moniezia sp. |
0 (0.0) |
0 (0.0) |
2 (4.7) |
2 (5.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
|
Total |
|
0 (0.0) |
6 (20.0) |
12 (27.9) |
8 (20.0) |
4 (11.4) |
4 (10.3) |
5 (12.5) |
3 (7.5) |
2 (6.5) |
1 (3.0) |
1 (2.5) |
References
- 1. Cebra CK, Stang BV. Comparison of methods to detect gastrointestinal parasites in llamas and alpacas. J Am Vet Med Assoc 2008;232(5):733-741. https://doi.org/10.2460/javma.232.5.733
- 2. Rashid MH, Stevenson MA, Vaughan JL, Saeed MA, Campbell AJD, et al. Epidemiology of gastrointestinal nematodes of alpacas in Australia: II. A longitudinal study. Parasitol Res 2019;118(3):901-911. https://doi.org/10.1007/s00436-019-06236-7
- 3. Halsby K, Twomey DF, Featherstone C, Foster A, Walsh A, et al. Zoonotic diseases in South American camelids in England and Wales. Epidemiol Infect 2017;145(5):1037-1043. https://doi.org/10.1017/S0950268816003101
- 4. Hyuga A, Matsumoto J. A survey of gastrointestinal parasites of alpacas (Vicugna pacos) raised in Japan. J Vet Med Sci 2016;78(4):719-721. https://doi.org/10.1292/jvms.15-0546
- 5. Fowler ME. Parasites. In Fowler ME ed, Medicine and Surgery of Camelids. 3rd ed. Wiley-Blackwell Ames; USA. 2010, pp 231-271.
- 6. Cheney JM, Allen GT. Parasitism in llamas. Vet Clin North Am Food Anim Pract 1989;5(1):217-225. https://doi.org/10.1016/s0749-0720(15)31011-2
- 7. Windsor RH, Teran M, Windsor RS. Effects of parasitic infestation on the productivity of alpacas (Lama pacos). Trop Anim Health Prod 1992;24(1):57-62. https://doi.org/10.1007/BF02357238
- 8. Ballweber LR. Ecto- and endoparasites of New World camelids. Vet Clin North Am Food Anim Pract 2009;25(2):295-310. https://doi.org/10.1016/j.cvfa.2009.02.003
- 9. Gomez-Puerta LA, Lopez-Urbina MT, Alarcon V, Cama V, Gonzalez AE, et al. Occurrence of Giardia duodenalis assemblages in alpacas in the Andean region. Parasitol Int 2014;63(1):31-34. https://doi.org/10.1016/j.parint.2013.10.003
- 10. Starkey SR, Johnson AL, Ziegler PE, Mohammed HO. An outbreak of cryptosporidiosis among alpaca crias and their human caregivers. J Am Vet Med Assoc 2007;231(10):1562-1567. https://doi.org/10.2460/javma.231.10.1562
- 11. Kaplan RM, Denwood MJ, Nielsen MK, Thamsborg SM, Torgerson PR, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet Parasitol 2023;318:109936. https://doi.org/10.1016/j.vetpar.2023.109936
- 12. Franz S, Wittek T, Joachim A, Hinney B, Dadak AM. Llamas and alpacas in Europe: endoparasites of the digestive tract and their pharmacotherapeutic control. Vet J 2015;204(3):255-262. https://doi.org/10.1016/j.tvjl.2015.04.019
- 13. Maria Pyziel-Serafin A, Raboszuk A, Klich D, Orłowska B, Sierociuk D, et al. Two centrifugal flotation techniques for counting gastrointestinal parasite eggs and oocysts in alpaca faeces. J Vet Res 2022;66(3):389-393. https://doi.org/10.2478/jvetres-2022-0039
- 14. Soulsby EJ. Helminths, Arthropods and Protozoans of Domesticated Animals. 7th ed. Lea and Febiger; Philadelphia, USA. 1982, pp 93-94.
- 15. Horner A, Bamford NJ, Stear MJ, Piedrafita D, Jabbar A, et al. Strongyle egg shedding and egg reappearance periods in horses with pituitary pars intermedia dysfunction. Vet Parasitol 2024;328:110176. https://doi.org/10.1016/j.vetpar.2024.110176
- 16. Lee WC, Lee KW. Epizoological survey on infestation rate of helminths in Korean native cattle. Kisaengchunghak Chapchi 1971;9(2):54-57. https://doi.org/10.3347/kjp.1971.9.2.54
- 17. Jeong JM, Jo SW, Kwak KH, Seo SY. A case of Moniezia expansa infection in goat. Korean J Vet Serv 2006;29(2):123-128. https://doi.org/10.7853/.1970.0.0
- 18. Díaz P, Panadero R, López R, Cordero A, Pérez-Creo A, et al. Prevalence and risk factors associated to Eimeria spp. infection in unweaned alpacas (Vicugna pacos) from Southern Peru. Acta Parasitol 2016;61(1):74-78. https://doi.org/10.1515/ap-2016-0008