Abstract
Adult notocotylid flukes (Digenea: Notocotylidae) were recovered from the ceca of Pitalah ducks (Anas sp.) in Aceh Province, Indonesia. These flukes were morphologically characterized by a median ventral ridge with 2 lateral rows of ventral papillae and the absence of both a ventral sucker and pharynx, consistent with the characteristics of the genus Catatropis. They exhibited a genital pore located just posterior to the oral sucker, 10–11 pairs of ventral papillae, a deep and multi-lobed ovary and testes, a metraterm equal in length to the cirrus sac, and ceca bearing numerous diverticula; accordingly, they were identified as Catatropis indicus Srivastava, 1935. Adult specimens measured 3.01–3.77 mm (average 3.47 mm) in length and 0.98–1.21 mm (average 1.11 mm) in width (n=8). Uterine eggs measured 0.016–0.023 mm (average 0.019 mm) in length and 0.008–0.014 mm (average 0.012 mm) in width (n=20), each bearing 2 long polar filaments. These specimens resembled Catatropis vietnamensis Izrailskaia et al., 2019, and Catatropis pakistanensis Schuster and Wibbelt, 2012, sharing features such as a genital pore immediately posterior to the oral sucker and other morphological traits. However, they differed from C. vietnamensis by having a larger body, ceca with numerous diverticula, and a broader anterior distribution of vitelline follicles. They also differed from C. pakistanensis in possessing a longer esophagus and ceca with multiple diverticula. In 28S rDNA sequence analysis, our specimens showed 99.1% identity with both C. indicus and C. vietnamensis. In contrast, internal transcribed spacer 2 (ITS2) sequence comparisons revealed only 96.0%–96.1% identity with C. vietnamensis (no GenBank data available for C. indicus), suggesting that our specimens are phylogenetically distant from C. vietnamensis. This represents the first report of C. indicus from ducks in Indonesia. A brief review of Catatropis species is provided.
-
Key words: Catatropis indicus, duck (Anas sp.), intestinal fluke, molecular analysis, Indonesia
Introduction
The genera
Catatropis Odhner, 1905, and
Notocotylus Diesing, 1839 (Digenea: Notocotylidae), are trematodes that parasitize the intestines of birds and, less commonly, mammals [
1–
3]. Infected hosts may develop severe conditions such as diarrhea, anemia, malabsorption, weight loss, general debility, or even death [
2]. These genera are morphologically characterized by longitudinal ridges or papillae on the ventral surface, eggs bearing long bipolar filaments, and the absence of both a ventral sucker and pharynx [
1–
3]. The primary distinction lies in body structure:
Catatropis species possess a median ventral ridge flanked by 2 separate rows of ventral papillae, whereas
Notocolytus species exhibit 3 rows of ventral papillae without a median ridge [
1–
3].
Within the genus
Catatropis, at least 30 species have been described (
Table 1) [
2–
31]. Of these, 23 are listed in WoRMS (
https://www.marinespecies.org), while 7 are excluded. Two were omitted due to taxonomic ambiguity (
Catatropis orientalis Harshe, 1932 [
4]) or the lack of deposited type material (
Catatropis vietnamensis Izrailskaia, Besprozvannykh, Tatanova, Nguyen, and Ngo, 2019 [
3]).
Catatropis filamentis Barker, 1911 [
5], and
Catatropis pacifera Noble, 1933 [
6], were excluded by Bayssade-Dufour et al. [
7] and Schuster and Wibbelt [
8] because of structural anomalies in the median ridge.
Catatropis joyeuxi Dvorjadkin, 1989 [
9], was excluded due to the absence of type material [
7]. However, the rationale behind the exclusion of
Catatropis aegyptiacus Ramadan, Abdou, Taha, and Haroun, 2020 [
10], and
Catatropis pakistanensis Shafi, Rehana, and Samad, 1982 (from mammalian host) [
11], remains unclear.
We tentatively included
C. aegyptiacus,
C. orientalis,
C. pakistanensis (from a mammalian host), and
C. vietnamensis in the list of
Catatropis species, while excluding 5 species listed in WoRMS:
Catatropis appendiculata Lutz, 1928 [
12];
Catatropis charadrii Skrjabin, 1915 [
13];
Catatropis gallinulae Johnston, 1928 [
14];
Catatropis johnstoni Martin, 1956 [
15]; and
Catatropis nicolli Cribb, 1991 [
16].
C. appendiculata was not included by McDonald in 1981 [
17] due to the absence of distinguishing features beyond the presence of numerous short diverticula along the intestinal ceca.
C. charadrii was synonymized with
C. verrucosa Odhner, 1905, by Kanev in 1994 based on close morphological similarity [
18].
C. gallinulae was excluded because of an improperly structured median ridge [
7,
8].
C. johnstoni and
C. nicolli were omitted due to the absence of bilateral rows of ventral papillae [
7]. As a result, a total of 22 species are considered tentatively valid in this study (
Tables 1,
2) [
2–
31].
Catatropis verrucosa [synonyms
Fasciola verrucosa Frölich, 1789;
Fasciola anseris Gmelin, 1790;
Monostoma verrucosa (Frölich, 1789) Zedar, 1800;
C. charadrii Skrjabin, 1915] was originally described from wild geese (
Anser anser) in Germany [
19] and later redescribed by Kanev et al. [
18].
Catatropis indicus Srivastava, 1935, was identified based on adult flukes recovered from the rectal ceca of the Indian fowl (
Gallus bankiva murghi) in India [
2].
C. aegyptiacus was recovered from Norway rats (
Rattus norvegicus) in Egypt [
10].
C. chilinae Flores and Brugni, 2003, was found in chickens and ducks experimentally infected with metacercariae encysted in the environment near the freshwater snail (
Chilina dombeiana) in Argentina [
20].
C. chinensis Lai, Sha, Zhang, and Yang, 1984, was collected from chickens and ducks in China [
21].
C. cygni Yamaguti, 1939, was reported from the bird (
Cygnus bewickii jankowskii) in Japan [
22].
C. harwoodi Bullock, 1952, was discovered in the Canadian goose (
Branta canadensis) in the USA [
23].
C. hatcheri Flores and Brugni, 2006, was described from chickens and ducks experimentally infected with metacercariae encysted near the freshwater snail (
Heleobia hatcheri) in Argentina [
24].
Catatropis hisikui Yamaguti, 1939, was reported from the bird (
Anser fabalis serrirostris) in Japan [
22].
Catatropis kashimirensis Khan and Chishti, 1987, was collected from chickens (
Gallus domesticus) in India [
25].
Catatropis lagunae Bayssade-Dufour, Albaret, Fermet-Quinet, and Farhati, 1996, was found in experimental birds (
Anas anser,
Anas platyrhynchos, and
Cairina moschata) infected with metacercariae in France [
7].
C. liara Kossak, 1911 (syn.
Monostomum attenuatum Lühe, 1898), was redescribed based on specimens from the bird (
Phenicopterus roscus) in Tunisia [
26].
C. misrai Gupta and Singh, 1984, was described from Indian birds (
Sarkidiornis melanotos and
Gallinula chloropus) [
27].
C. morosovi Gubanov, Fedorov, Berlovskaya, and Kusnetsova, 1966, was identified from the water vole (
Arvicola terrestris) in Russia [
28].
C. onobae Gonchar and Galaktionov, 2021, was found in birds (
Somateria mollissima) as adult flukes and in snails (
Onoba aculeus) as rediae and cercariae in Russia and Iceland [
29].
C. orientalis Harshe, 1932, was reported from wild ducks (
Dafila acuta acuta) in India [
4].
C. pakistanensis Shafi, Rehana, and Samad, 1982, was recovered from rice rats (
Bandicota bengalensis) in Pakistan [
11].
C. pakistanensis Schuster and Wibbelt, 2012, was identified from Northern shoveler ducks (
Anas clypeata) in Pakistan [
8].
C. poecilorhynchai Gupta and Singh, 1984 (syn.
C. rauschi of Gupta and Jahan, 1977), was found in Indian birds (
Anas poecilorhyncha) [
27].
C. pricei Harwood, 1939, was described based on specimens from the Canadian goose (
Branta canadensis) in the USA [
30].
C. rauschi Sing, 1956, was identified from the pintail duck (
Dafila acuta) in India [
31].
C. vietnamensis Izrailskaia et al., 2019, was collected from experimental ducks (
Anas platyrhynchos) infected with metacercariae encysted near the snail (
Melanoides tuberculata) in Vietnam [
3].
We surveyed intestinal flukes infecting ducks in Aceh Province, Indonesia, and identified adult flukes belonging to the family Notocotylidae. These were characterized by a median ventral ridge flanked by a pair of ventral papillae on each side and by the absence of a ventral sucker and pharynx. Based on these features, we classified our specimens in the genus Catatropis. Detailed morphological examination led to their identification as C. indicus. Molecular analyses of the 28S rDNA and ITS2 genes further supported their placement within the genus Catatropis.
The pathogenicity of
C. indicus is high, as infected birds were found severely ill or dead, displaying pronounced signs such as emaciation, weight loss, anemia, dull plumage, and poor overall condition [
2]. The life cycle of this species was previously studied in Malaysia, where
Bithynia sp. snails served as intermediate hosts. Metacercariae were observed encysted on snail shells, aquatic vegetation, and the surfaces of glass containers, while adult flukes were recovered from experimentally infected chickens [
32]. Additional studies have described the nervous system of the cercariae [
33] and the ultrastructure of the tegumental surface of adult flukes [
34]. The presence of
C. indicus has been confirmed in Australia [
35], Egypt [
36], and Indonesia (this study), but not in other countries.
The present report aims to describe the morphological features of C. indicus collected from the duck intestines in Indonesia. A brief review is also provided on the 22 tentatively valid Catatropis spp. reported in the literature.
Materials and Methods
Ethics statement
All animal experiments were conducted in accordance with the ARRIVE guidelines for animal research (
https://arriveguidelines.org) and were approved by the Institutional Animal Care and Use Committee of the MediCheck Research Institute, Korea Association of Health Promotion, Seoul, Korea (KAHP 201805).
Pitalah ducks (Anas sp.) were collected in Aceh Province, Indonesia, between March and October 2018. A total of 20 ducks were examined, sourced from 2 counties: Aceh Besar and Matang Glumpang. The ducks were euthanized via cervical dislocation, and the entire intestinal tract, including both the small and large intestines, was resected. The intestines were opened longitudinally using scissors and examined under a stereomicroscope for helminth parasites, including trematodes. For morphological analysis, trematode specimens were fixed in 10% formalin on a glass slide and covered with a cover slip. Some specimens were preserved in 70%–90% ethanol for molecular studies.
Morphological study
Formalin-fixed specimens were thoroughly rinsed in water and stained with Semichon’s acetocarmine. They were subsequently dehydrated in a graded ethanol series (70%–100%), cleared in xylene, and mounted on glass slides using Permount (Fisher Scientific, Pittsburgh, PA, USA). The specimens were examined morphologically under a light microscope (CX33, Olympus, Tokyo, Japan). All measurements are expressed in millimeters, unless otherwise specified.
Molecular and phylogenetic analyses
For molecular analyses, sequences of nuclear genes, including 28S ribosomal DNA (28S rDNA) and internal transcribed spacer 2 (ITS2), were obtained. Genomic DNA from each specimen was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. PCR amplification and sequencing were conducted using specific primers: dig12 and LO for 28S rDNA and BR and dig11 for ITS2, under conditions based on previously published protocols [
37,
38]. Multiple sequences were aligned using Clustal W software 2.1 (University College Dublin, Dublin, Ireland) [
39]. A phylogenetic tree, incorporating isolates obtained in this study and reference sequences of trematodes such as
Catatropis sp. and
Notocotylus sp. from the GenBank database, was constructed using the maximum-likelihood method with the Tamura-Nei model [
40] for nucleotide substitution. The tree was visualized using MEGA v6 software [
41], and bootstrap values were calculated based on 1,000 replications.
Results
Helminth species collected
Two species of trematodes and one unidentified species of cestode were recovered from the intestinal tract of the ducks. One trematode species, isolated from the small intestine, was identified as
Echinostoma miyagawai, previously reported by Chai et al. [
42]. The second trematode collected from the large intestine (ceca) was identified as
C. indicus, as confirmed in this study. Specimens of
C. indicus were found in 5 of the 20 ducks (25.0%), with a total of 48 specimens recovered, averaging 9.6 specimens per infected duck.
Family Notocotylidae Lühe, 1909
Genus Catatropis Odhner, 1905
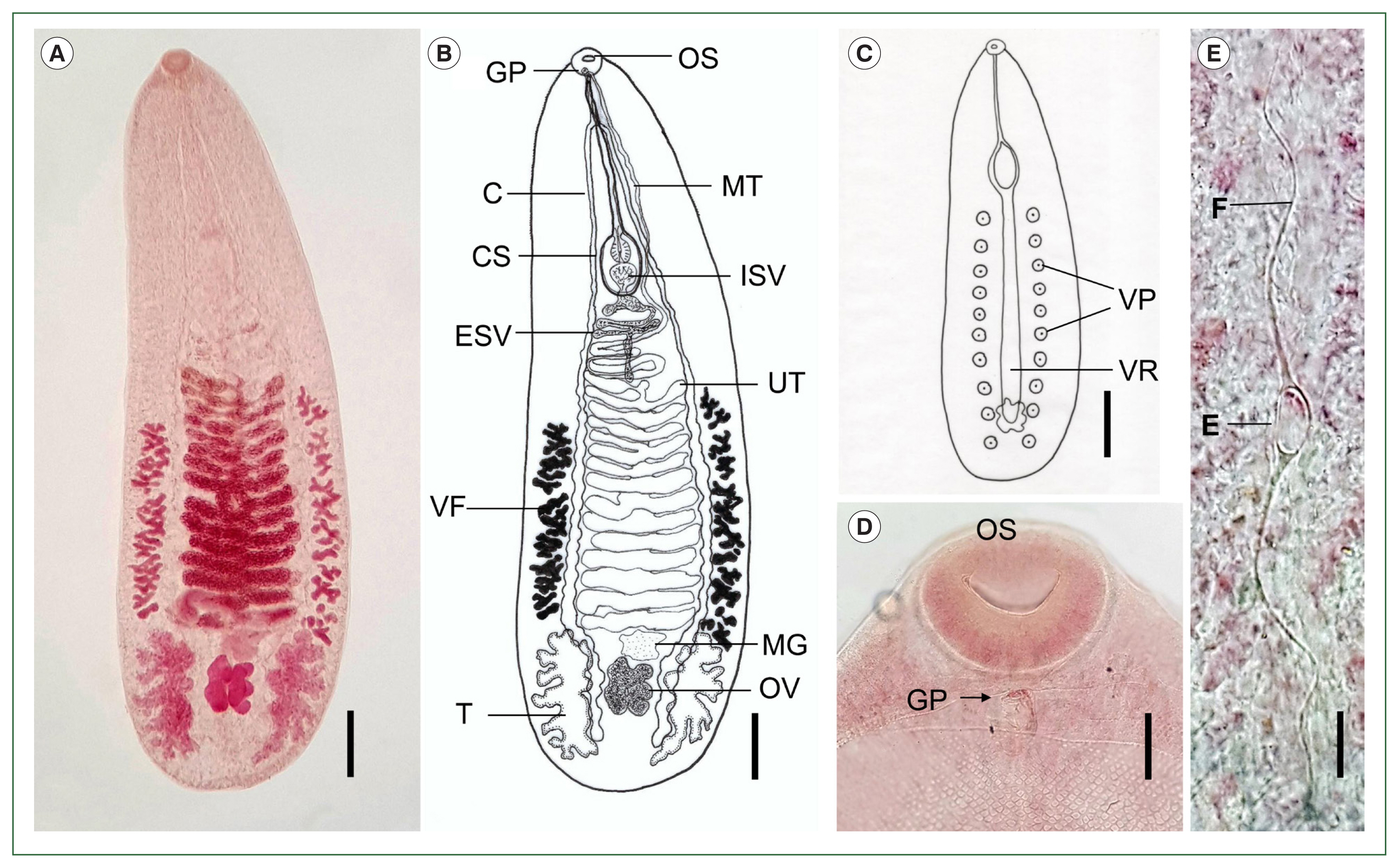
Description (adults, n=8): The body is elongated, flat, and semi-transparent, ventrally concave, tapering anteriorly, and rounded posteriorly, measuring 3.01–3.77 mm in length (average 3.47 mm) and 0.98–1.21 mm in width (average 1.11 mm). The tegument is covered with minute, scale-like spines, which become sparse toward the posterior end. A ventral ridge extends longitudinally from the level of the cirrus sac to the posterior margin of the ovary. Two lateral rows of ventral papillae (10–11 per side) flank the ventral ridge. The first pair of papillae is located near the 15th–16th uterine coils, posterior to the cirrus sac, while the last pair is situated at the level of the posterior two-thirds of the testes. The oral sucker is round and subterminal, with no pharynx or ventral sucker. The short esophagus bifurcates into 2 ceca, which undulate markedly and form numerous fine diverticula projecting both inward and outward. The ceca extend laterally around the uterine coils and terminate near the posterior margin of the testes. The testes are symmetrical, deeply lobed, and located near the posterior extremity of the body. The external seminal vesicle is round or coiled, with an elongated posterior segment that connects to the internal seminal vesicle. The cirrus sac is elongated, tapering anteriorly and broadening posteriorly, and houses the internal seminal vesicle, prostatic gland, and unarmed cirrus. The genital pore is located just posterior to the oral sucker and contains male and female openings; the female pore leads to the metraterm and subsequently to the uterine coils. The metraterm extends approximately two-thirds the length of the cirrus sac. The uterine coil runs from the anterior margin of Mehlis’ gland to the posterior level of the cirrus sac, forming 16–18 coils. Mehlis’ gland varies in shape, most commonly triangular or quadrangular, and is located anterior to the ovary. The ovary is median, positioned between the testes, and extensively lobed into 8–10 lobes. The vitellaria consist of 2 lateral rows of irregularly shaped follicles, located outside the ceca, extending from the anterior edge of Mehlis’ gland to the level of the 12th–13th uterine coils. The eggs are small, oval, and operculate, measuring 0.016–0.023 mm in length (average 0.019 mm) and 0.008–0.014 mm in width (average 0.012 mm), each bearing 2 polar filaments. The excretory vesicle is sac-like and possesses diverticula.
Taxonomic summary
Host: Pitalah duck (Anas sp.)
Site of infection: large intestine (cecum)
Locality: Aceh Province, Indonesia
Deposition of specimens: Parasite Museum, MediCheck Research Institute, Korea Association of Health Promotion, Seoul 07649, Korea (No. 2024-0001-01~08). Two additional specimens are deposited in the Meguro Parasitological Museum, Tokyo 153-0064, Japan (MPM Coll. No. 25267).
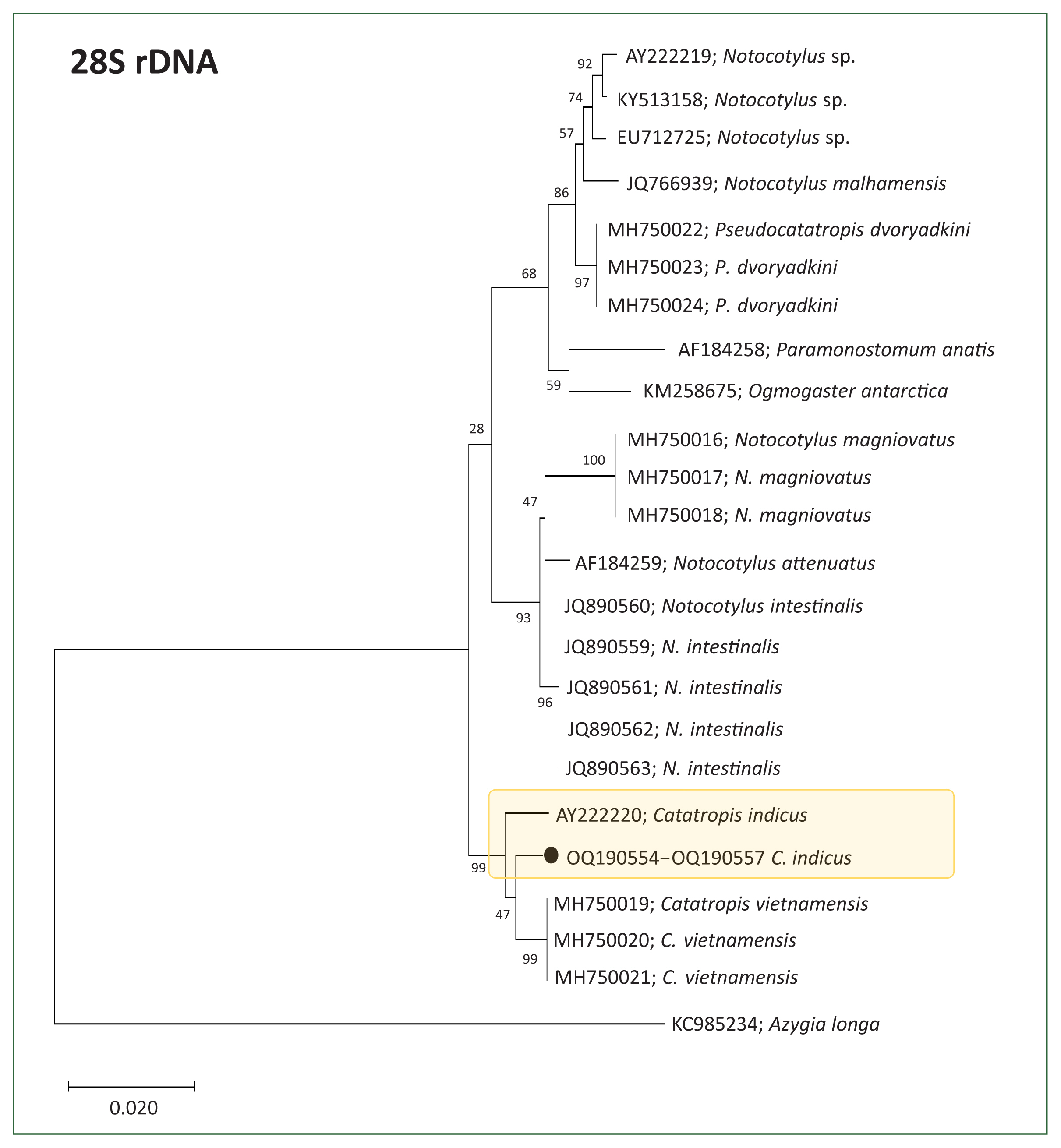
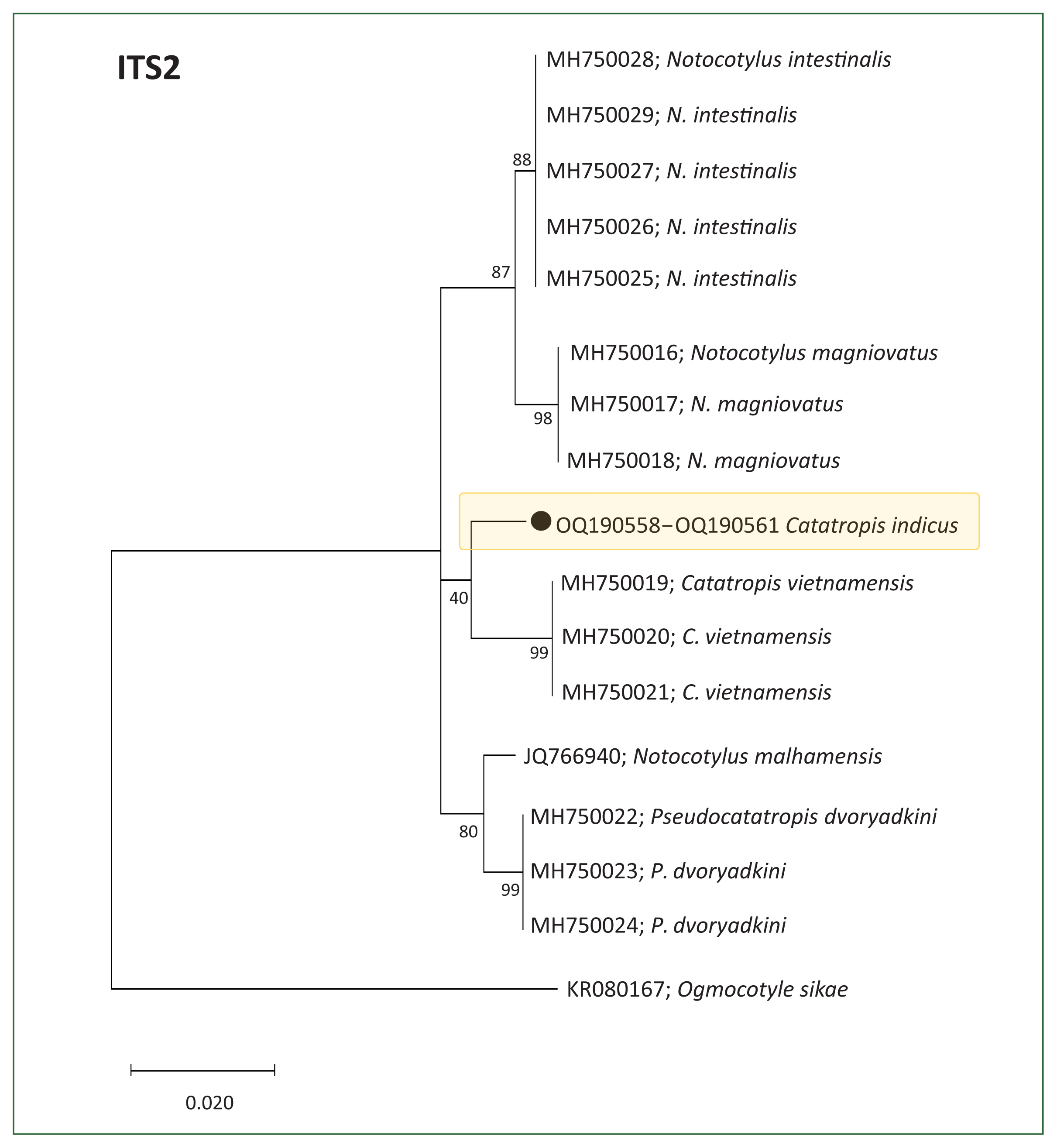
Molecular results and phylogenetic trees
Partial sequences of the 28S rDNA (605 bp) (
n=4, GenBank No. OQ190554–OQ190557) and ITS2 genes (257 bp) (
n=4, GenBank No. OQ190558–OQ190561) from adult flukes were phylogenetically grouped in
Catatropis spp. of the family Notocotylidae (
Figs. 2,
3). The phylogenetic tree based on 28S rDNA showed that our samples clustered closely with a clade containing
C. indicus and
C. vietnamensis, both with 99.1% sequence identity (
Fig. 2). However, the ITS2-based tree indicated that our samples formed a distinct lineage, separate from other members of the Notocotylidae, including
C. vietnamensis (96.0%–96.1%),
Notocotylus magniovatus (95.3%),
N. intestinalis (95.9%–96.1%), and
Pseudocatatropis dvoryadkini (95.7%) (
Fig. 3).
Discussion
Flukes of the family Notocotylidae Lühe, 1909, are parasites that primarily infect digestive tracts, particularly the ceca, of birds and mammals [
43]. They are small, monostomatous (lacking a ventral sucker), dorsoventrally flattened, and generally appear elongated or oval. A distinctive feature of this family is the presence of papillae and/or ridges on the ventral surface. The arrangement of these structures serves as a key diagnostic criterion for differentiating genera, including
Hippocrepis Travassos, 1922;
Notocotyloides Dollfus, 1966;
Parapronocephalum Belopol’skaya, 1952;
Ogmocotyle Skrjabin & Schulz, 1933;
Ogmogaster Jägerskiöld, 1891;
Pseudoquinqueserialis Sutton, 1981;
Paramonostomum Lühe, 1909;
Tristriata Belopol’skaya in Skrjabin, 1953;
Catatropis Odhner, 1905;
Notocotylus Diesing, 1839;
Uniserialis Beverley-Burton, 1958;
Tetraserialis Petrov & Chertkova, 1960; and
Quinqueserialis Skvortsov, 1935 [
43]. The proposed functions of the papillae and ridges include facilitating host attachment, secreting digestive enzymes, and nutrient absorption; however, ultrastructural studies indicate that their precise roles remain unclear and warrant further investigation [
43].
Within the genus
Catatropis, 22 species are considered tentatively valid. However, taxonomic ambiguities persist. One such issue involves
C. pakistanensis, which has been reported under the same name on 2 separate occasions (
Tables 1,
2) [
2–
31]. The first report, published in 1982, described specimens collected from the stomach of the rice rat (
Bandicota bengalensis) in Pakistan [
11]. Unaware of this earlier report, Schuster and Wibbelt [
8] later described a different species as
C. pakistanensis based on specimens from the ceca of shoveler ducks (
Anas clypeata), also in Pakistan. Therefore, the
C. pakistanensis specimens from shovelers should be renamed. In addition to this nomenclatural conflict, other taxonomic debates may also arise regarding
Catatropis species. There is an urgent need for expanded molecular data to clarify the taxonomy and evolutionary relationships within this genus.
Our specimens exhibited a median ventral ridge with a pair of ventral papillae on each side, consistent with diagnostic features of the genus
Catatropis. Additional distinguishing characteristics included a genital pore located just posterior to the oral sucker, 10–11 ventral papillae per row, an ovary with 8–10 lobes, and ceca densely filled with numerous fine diverticula. Of the 22 tentatively recognized
Catatropis species, 10 are reported to possess the genital pore anterior to the cecal bifurcation:
C. aegyptiacus [
10],
C. chinensis [
21],
C. harwoodi [
23],
C. indicus [
2],
C. misrai [
27],
C. pakistanensis (from birds) [
8],
C. poecilorhynchai [
27],
C. pricei [
30],
C. rauschi [
31], and
C. vietnamensis [
3] (
Table 2). Our specimens aligned closely with previously published descriptions of
C. indicus. In terms of ventral papillae, our specimens had 10–11 pairs per row. The original description of
C. indicus reported 10–12 ventral papillae [
2], while subsequent redescriptions noted 12–13 papillae [
32,
34,
35]. Among the 22 tentatively valid
Catatropis species, similar counts of ventral papillae were observed in
C. cygni (12–18 pairs),
C. onobae (8–12), and
C. verrucosa (8–12). However, these species differ from our specimens in the position of the genital pore; it is located posterior to the cecal bifurcation in
C. cygni and
C. verrucosa and near the bifurcation in
C. onobae (
Table 2).
Our specimens can also be distinguished from
C. aegyptiacus, which produces notably larger eggs and has been reported in mammalian hosts, particularly rats [
10].
C. chinensis differs in having less branched testes and a more simply lobed ovary [
21].
C. harwoodi is characterized by a genital pore positioned midway between the oral sucker and cecal bifurcation, along with a metraterm that exceeds the length of the cirrus sac [
17,
23].
C. misrai is distinct in having an unspined body and a more restricted distribution of vitelline follicles [
27].
C. pakistanensis (from birds) is characterized by a very short esophagus and ceca that lack prominent diverticula [
8].
C. poecilorhynchai is easily identified by having only 4–6 pairs of ventral papillae [
27,
44].
C. pricei is distinguished by possessing separate genital openings, male (located caudal to the oral sucker) and female (positioned mid-esophagus), along with a less lobulated ovary and testes [
17,
30].
C. rauschi can be differentiated by a metraterm that is longer than the cirrus sac [
31].
C. vietnamensis differs in having a smaller body size, a less lobed ovary, a shorter metraterm, fewer vitelline follicles, and ceca that nearly lack diverticula [
3].
In molecular analyses, the 28S rDNA sequences of our specimens (GenBank No. OQ190554–OQ190557, Indonesia) showed a high identity of 99.1% with both C. indicus (from Australia) and C. vietnamensis (from Vietnam). However, for ITS2 sequences, no reference data for C. indicus were available in GenBank. The highest identity of our specimens based on ITS2 sequences was 96.0%–96.1% with C. vietnamensis (Vietnam), followed by 95.9%–96.1% with N. intestinalis (Vietnam). This result prevents conclusive species-level identification. These molecular findings support the classification of our specimens within the genus Catatropis, but not definitely as C. indicus. Additional molecular data are needed to resolve this taxonomic uncertainty.
The differential diagnosis of species within the genus
Catatropis can be conducted as follows (updated and modified from McDonald [
17]):
1. Parasites of mammals ---------------------------------------------------------------------- 2
Parasites of birds -------------------------------------------------------------------- 4
2. Genital pore anterior to cecal bifurcation, ventral papillae 10–11 pairs -- C. aegyptiacus
Genital pore posterior to cecal bifurcation, ventral papillae ≥12 pairs ----------------- 3
3. Ventral papillae: 12 pairs; vitellaria start far below cirrus sac ----------- C. morosovi
Ventral papillae: 13 pairs; vitellaria start just below cirrus sac ------ C. pakistanensis (rat)
4. Genital pore near cecal bifurcation ------------------------------------------------------- 5
Genital pore anterior to cecal bifurcation ------------------------------------------------ 6
Genital pore posterior to cecal bifurcation ---------------------------------------------- 10
5. Ventral papillae: 8–12 pairs, unequal in size; uterus 14–21 coils -------------- C. onobae
Ventral papillae: 9 pairs, equal in size; uterus 17 coils ------------------- C. kashimirensis
6. Ventral papillae: 4–6 pairs ---------------------------------------------- C. poecilorhynchai
Ventral papillae: 7–9 pairs ----------------------------------------------------------------- 7
Ventral papillae: 9 or 10 pairs -------------------------------------------------------------- 8
Ventral papillae: 10–13 pairs ---------------------------------------------------------- 9
7. Anteriormost ventral papillae far anterior to vitellaria ------------------ C. vietnamensis
Anteriormost ventral papillae immediately posterior to cirrus sac -------- C. harwoodi
8. Body small (1.7–2.1 mm long), cirrus sac long, short esophagus, ceca without diverticula ------------------------------------------------------------------------- C. rauschi
Body small (1.6–2.3 mm long), cirrus sac long, very short esophagus, ceca without diverticula -------------------------------------------------------------------------- C. pricei
Body large (2.9–3.8 mm long), cirrus sac relatively short, short esophagus, ceca with diverticula ----------------------------------------------------------- C. pakistanensis (bird)
9. Ceca with diverticula, uterine loops 16–18, metraterm equal to or longer than cirrus sac, eggs small (16–23 μm) ------------------------------------------------------ C. indicus
Ceca with diverticula, uterine loop 18–20, metraterm equal to or longer than cirrus sac, eggs large (21–25 μm) -------------------------------------------------------- C. misrai
Ceca without diverticula, uterine loop 15–19, metraterm slightly longer than cirrus sac, eggs small (18–21 μm) ----------------------------------------------------- C. chinensis
10. Ventral papillae: 6–9 pairs ------------------------------------------------------------ 11
Ventral papillae: 8–12 pairs ---------------------------------------------------------- 12
Ventral papillae: 12–18 pairs --------------------------------------------------------- 13
11. Cirrus sac long, nearly 1/2 body length --------------------------------------- C. lagunae
Cirrus sac short, less than 1/5 body length ----------------------------------- C. orientalis
12. Body small (1.6–2.4 mm), uterine loops 16–17, eggs 24–29 μm with short or long filament (15–180 μm) ------------------------------------------------------------ C. chilinae
Body large (2.3–2.8 mm), uterine loops 12–17, eggs 19–24 μm with long filament (156-185 μm) -------------------------------------------------------------------- C. hatcheri
Body large (2.3–5.7 mm), uterine loops 12–14, eggs 25–30 μm with short filament (100-150 μm) ------------------------------------------------------------------- C. verrucosa
13. Body small (1.1–1.5 mm), elongated, uterine loops 10, vitellaria begin posterior to mid-body ----------------------------------------------------------------------------- C. liara
Body large (2.4–7.0 mm), tongue-shaped, uterine loops 20, vitellaria begin anterior to mid-body -------------------------------------------------------------------------- C. hisikui
Body large (3.3–6.3 mm), elongated, uterine loops 24–26, vitellaria begin posterior to mid-body --------------------------------------------------------------------------- C. cygni
In conclusion, adult flukes of the genus Catatropis were identified in the large intestine of Pitalah ducks in Indonesia. Morphological analyses allowed us to classify them as C. indicus. Further molecular research on Catatropis species is warranted.
Notes
-
Author contributions
Conceptualization: Chai JY
Project administration: Chai JY, Abdullah MBM
Funding acquisition: Chai JY, Jung BK
Methodology: Chang T, Hong S, Shin H
Investigation: Chai JY, Jung BK, Chang T, Hong S, Shin H
Data curation: Chai JY, Jung BK, Chang T, Hong S, Shin H
Formal analysis: Chai JY, Jung BK
Visualization: Chai JY
Validation: Chai JY, Jung BK
Supervision: Chai JY, Abdullah MBM
Writing-original draft: Chai JY
Writing-review and editing: Chai JY, Jung BK
-
Conflict of interest
The authors declare no conflict of interest related to this study.
-
Acknowledgments
We would like to thank the staff of the Korea Association of Health Promotion in Seoul, Korea, for their support with this study. We also appreciate the members of the Permatahati Mazas Foundation in Aceh Besar, Aceh Province, Indonesia, for their cooperation.
Fig. 1
Catatropis indicus (adult) collected from ducks (Anas sp.) in Aceh Province, Indonesia. (A) Adult fluke stained with acetocarmine, showing the oral sucker, uterine coils, vitelline follicles, Mehlis’ gland, ovary, and testes. Ventral view. Scale bar=0.3 mm. (B) Line drawing of the adult fluke shown in (A). Scale bar=0.3 mm. (C) Illustration showing the median ventral ridge (VR) and 10 ventral papillae (VP) on each side. Scale bar=0.5 mm. (D) Location of the genital pore (GP) immediately posterior to the oral sucker (OS). Scale bar=0.05 mm. (E) Egg (E) with a pair of polar filaments (F) in the distal uterine coil. Scale bar=0.018 mm. C, cecum; CS, cirrus sac; ESV, external seminal vesicle; VF, vitelline follicles; T, testis; MT, metraterm; ISV, internal seminal vesicle; UT, uterus; MG, Mehlis’ gland; OV, ovary.
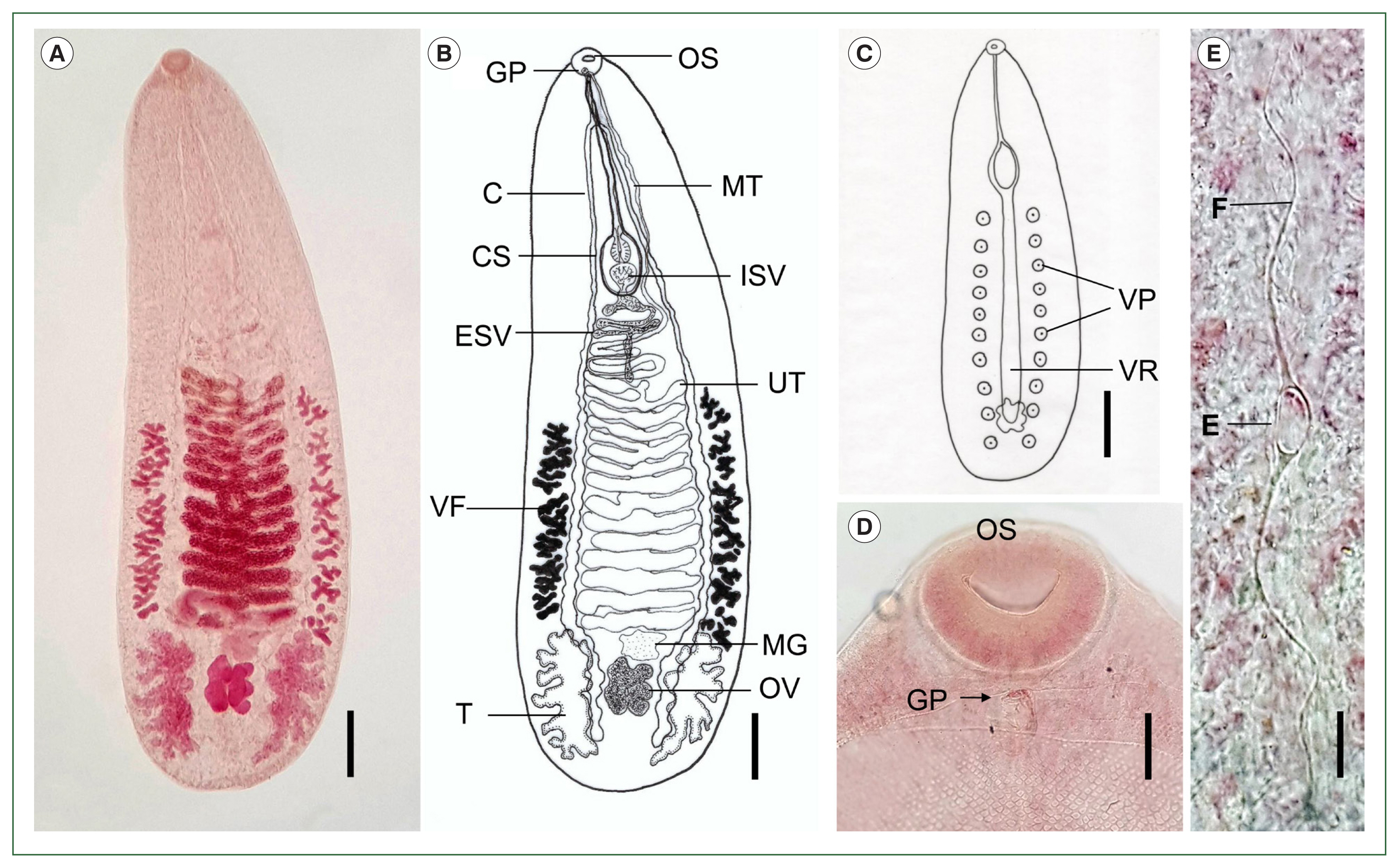
Fig. 2Phylogenetic tree showing Catatropis indicus (n=4, GenBank No. OQ190554-OQ190557) and related species of Catatropis, Notocotylus, and Pseudocatatropis based on 28S rDNA sequences. Azygia longa served as the outgroup. Numeric values on the branches indicate support for the nodes generated by the maximum likelihood method using the Tamura-Nei model for nucleotide substitution. The tree was visualized using MEGA v6 software, and bootstrap values were obtained from 1,000 replicates. (●) indicates the specimens obtained in this study.
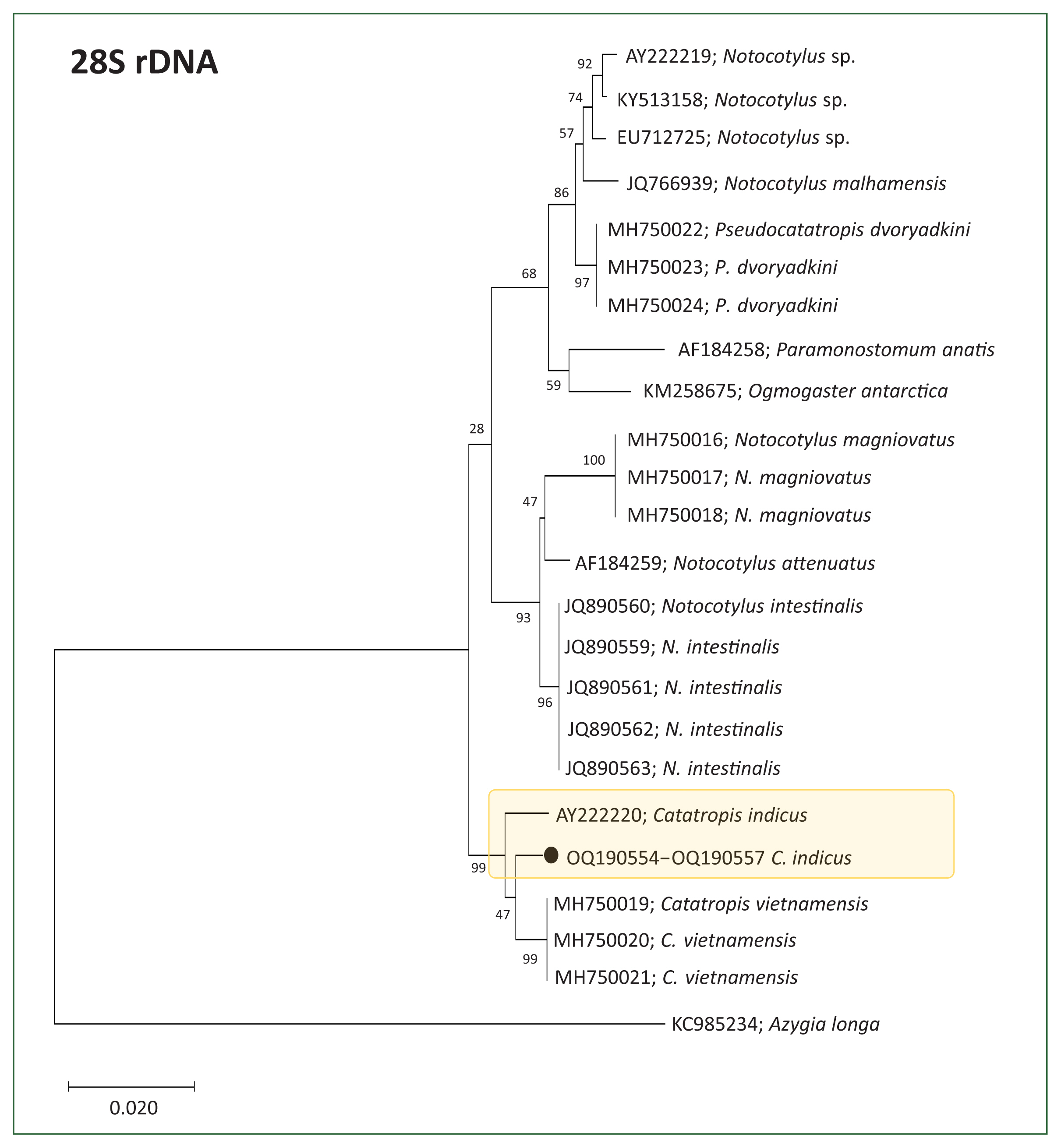
Fig. 3Phylogenetic tree illustrating Catatropis indicus (n=4, GenBank No. OQ190558–OQ190561) and related species of Catatropis, Notocotylus, and Pseudocatatropis based on ITS2 sequences. Ogmocotyle sikae was used as the outgroup. Numeric values at the branches represent node support based on the maximum likelihood method under the Tamura-Nei model. The tree was visualized using MEGA v6 software, and bootstrap values were obtained from 1,000 replicates. (●) indicates the specimens obtained in this study.
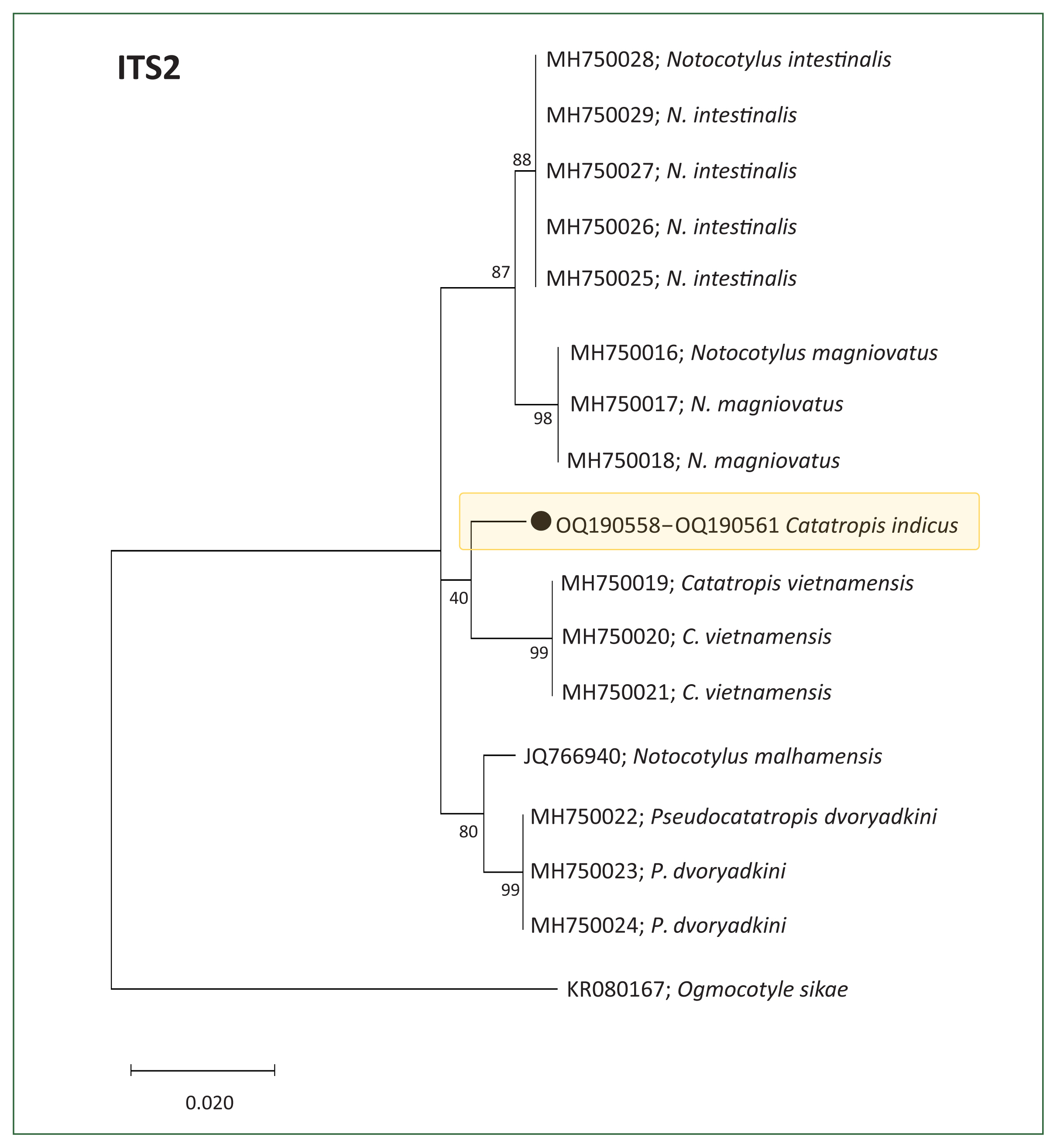
Table 1Thirty Catatropis spp. reported in the literature
Table 1
|
Species name/nominator & year [reference] |
WoRMS (marinespecies.org) |
Schuster and Wibblet [8] |
Tentatively valid species (this study) |
Remarks |
|
C. appendiculata Lutz, 1928 [12] |
Listed |
- |
- |
Not acknowledged as a distinct species (McDonald [17]) |
|
C. aegyptiacus Ramadan et al., 2020 [10] |
- |
- |
Listed |
- |
|
C. charadrii Skrjabin, 1915 [13] |
Listed |
- |
- |
Synonymized with C. verrucosa (Kanev et al. [18]) |
|
C. chilinae Flores and Brugni, 2003 [20] |
Listed |
Listed |
Listed |
- |
|
C. chinensis Lai et al., 1984 [21] |
Listed |
Listed |
Listed |
- |
|
C. cygni Yamaguti, 1939 [22] |
Listed |
Listed |
Listed |
- |
|
C. filamentis Barker, 1911 [5] |
- |
- |
- |
Removed from this genus (Bayssade-Dufour et al. [7]) |
|
C. gallinulae Johnston, 1928 [14] |
Listed |
- |
- |
Removed from this genus (Bayssade-Dufour et al. [7]) |
|
C. harwoodi Bullock, 1952 [23] |
Listed |
Listed |
Listed |
- |
|
C. hatcheri Flores and Brugni, 2006 [24] |
Listed |
Listed |
Listed |
- |
|
C. hisikui Yamaguti, 1939 [22] |
Listed |
Listed |
Listed |
- |
|
C. indicus Srivastava, 1935 [2] |
Listed |
Listed |
Listed |
- |
|
C. johnstoni Martin, 1956 [15] |
Listed |
- |
- |
Removed from this genus (Bayssade-Dufour et al. [7]) |
|
C. joyeuxi Dvorjadkin, 1989 [9] |
- |
- |
- |
No deposition of type material (Schuster and Wibbelt [8]) |
|
C. kashmirensis Khan and Chishti, 1987 [25] |
Listed |
- |
Listed |
- |
|
C. lagunae Bayssade-Dufour et al., 1996 [7] |
Listed |
Listed |
Listed |
- |
|
C. liara Kossak, 1911 [26] |
Listed |
Listed |
Listed |
- |
|
C. misrai Gupta and Singh, 1984 [27] |
Listed |
Listed |
Listed |
- |
|
C. morosovi Gubanow et al., 1966 [28] |
Listed |
Listed |
Listed |
- |
|
C. onobae Gonchar and Galaktionov, 2021 [29] |
Listed |
- |
Listed |
- |
|
C. orientalis Harshe, 1932 [4] |
- |
Listed |
Listed |
Not listed by WoRMS (uncertain taxon) |
|
C. nicolli Cribb, 1991 [16] |
Listed |
- |
- |
Removed from this genus (Bayssade-Dufour et al. [7]) |
|
C. pacifera Noble, 1933 [6] |
- |
- |
- |
Removed from this genus (Bayssade-Dufour et al. [7]) |
|
C. pakistanensis Shafi et al., 1982 [11] |
- |
Listed |
Listed |
- |
|
C. pakistanensisa Schuster and Wibbelt, 2012 [8] |
Listed |
- |
Listed |
- |
|
C. poecilorhynchai Gupta and Singh, 1984 [27] |
Listed |
Listed |
Listed |
- |
|
C. pricei Harwood, 1939 [30] |
Listed |
Listed |
Listed |
- |
|
C. rauschi Sing, 1956 [31] |
Listed |
Listed |
Listed |
- |
|
C. verrucosa Odhner, 1905 [19] |
Listed |
Listed |
Listed |
- |
|
C. vietnamensis Izrailskaia et al., 2019 [3] |
- |
- |
Listed |
Not listed by WoRMS (no deposition of type material) |
Table 2Twenty-two tentatively valid Catatropis spp. included in this study
Table 2
|
Species |
Host |
Position of genital pore |
No. of ventral papillae |
Country of original report |
|
C. aegyptiacus
|
Mammal |
Anterior to ceca bifurcation |
10–11 pairs |
Egypt |
|
C. chilinae
|
Bird |
Posterior to ceca bifurcation |
9–11 pairs |
Argentina |
|
C. chinensis
|
Bird |
Anterior to ceca bifurcation |
12 pairs |
China |
|
C. cygni
|
Bird |
Posterior to ceca bifurcation |
12–18 pairs |
Japan |
|
C. harwoodi
|
Bird |
Anterior to ceca bifurcation |
7–9 pairs |
USA |
|
C. hatcheri
|
Bird |
Posterior to ceca bifurcation |
10–12 pairs |
Argentina |
|
C. hisikui
|
Bird |
Posterior to ceca bifurcation |
15–17 pairs |
Japan |
|
C. indicus
|
Bird |
Anterior to ceca bifurcation |
10–13 pairs |
India |
|
C. kashmirensis
|
Bird |
Near ceca bifurcation |
9 pairs |
India |
|
C. lagunae
|
Bird |
Posterior to ceca bifurcation |
6–9 pairs |
France |
|
C. liara
|
Bird |
Posterior to ceca bifurcation |
>12 pairs |
Tunisia |
|
C. misrai
|
Bird |
Anterior to ceca bifurcation |
12 pairs |
India |
|
C. morosovi
|
Mammal |
Posterior to ceca bifurcation |
13 pairs |
Russia |
|
C. onobae
|
Bird |
Near ceca bifurcation (3 areas) |
8–12 pairs |
Russia and Iceland |
|
C. orientalis
|
Bird |
Posterior to ceca bifurcation |
7–8 pairs |
India |
|
C. pakistanensis (mammal) |
Mammal |
Posterior to ceca bifurcation |
12 pairs |
Pakistan |
|
C. pakistanensis (bird) |
Bird |
Anterior to ceca bifurcation |
9–10 pairs |
Pakistan |
|
C. poecilorhynchai
|
Bird |
Anterior to ceca bifurcation |
4–6 pairs |
India |
|
C. pricei
|
Bird |
Anterior to ceca bifurcation |
9–11 pairs |
USA |
|
C. rauschi
|
Bird |
Anterior to ceca bifurcation |
10 pairs |
India |
|
C. verrucosa
|
Bird |
Posterior to ceca bifurcation |
8–12 pairs+1 (center) |
Germany |
|
C. vietnamensis
|
Bird |
Anterior to ceca bifurcation |
9 pairs |
Vietnam |
Table 3Morphology and measurements (mm) of Catatropis indicus adults compared with previous reports and a closely related species
Table 3
|
Characters |
C. indicus This study (n=8) |
C. indicus Srivastava (1935) [2] (n=?) |
C. indicus Rohde and Lee (1967) [32] (n=20) |
C. vietnamensis Izrailskaia et al. (2019) [3] (n=10) |
|
Body (L×W) |
3.01–3.77×0.98–1.21 |
4.20–4.60×1.20 |
2.22–4.34×0.83–1.17 |
1.46–1.60×0.52–0.55 |
|
Oral sucker (L×W) |
0.14–0.17×0.11–0.16 |
0.14–0.20 (diam.) |
0.13–0.17×0.14–0.21 |
0.09×0.11–0.12 |
|
Esophagus (L) |
0.14–0.25 |
0.20–0.26 |
- |
0.10–0.12 |
|
Cirrus sac (L×W) |
0.98–1.20×0.15–0.22 |
0.87–1.20×0.17–0.20 |
0.91–1.13×0.14–0.21 |
0.51–0.60×0.05–0.08 |
|
Testis, left (L×W) |
0.54–0.75×0.26–0.33 |
0.75–0.99×0.20–0.30 |
0.50–0.86×0.20–0.35 |
0.21–0.28×0.17–0.18 |
|
Testis, right (L×W) |
0.59–0.78×0.27–0.35 |
0.75–0.99×0.20–0.30 |
0.50–0.86×0.20–0.35 |
0.27–0.29×0.15–0.17 |
|
Ovary (L×W) |
0.24–0.35×0.20–0.26 |
0.26–0.35×0.26–0.35 |
0.18–0.40×0.15–0.29 |
0.12–0.16×0.12 |
|
No. of ovarian lobes |
8–10 lobes |
Irregularly lobed |
Slightly lobed |
4 lobes |
|
Mehlis’ gland (L×W) |
0.20–0.25×0.15–0.23 |
0.23–0.26×0.17–0.26 |
- |
0.06–0.08×0.09–0.10 |
|
No. of uterine loops |
16–18 |
18 |
17–18 |
15–18 |
|
Genital pore (position) |
Immediately behind oral sucker |
Immediately behind oral sucker |
Immediately behind oral sucker |
Immediately behind oral sucker |
|
Metraterm (L) |
0.90–1.20 |
0.87–1.20 |
0.91–1.13 |
0.18–0.19 |
|
Vitelline fields (L) |
0.87–1.70 |
1.20–1.50 |
0.61–1.33 |
0.27–0.39 |
|
No. of ventral papillae (glands) on each side |
10–11 |
10–12 |
12–13 |
9 |
|
Eggs (L×W) |
0.016–0.023×0.008–0.014 |
0.0170–0.020×0.008–0.010 |
0.022–0.027×0.009–0.011 |
0.019–0.023×0.008–0.012 |
|
Length of filaments |
0.140–0.180 |
- |
0.150–0.230 |
- |
|
Ceca diverticula |
Numerous |
Numerous |
Numerous |
Almost no |
References
- 1. Schell SC. How to Know the Trematodes. W.C. Brown Co; Dubuque, USA. 1970, p 335.
- 2. Srivastava HD. On a new species of Catatropis Odhner, 1905, from an Indian fowl-Gallus bankiva murghi. Proc Acad Sci (UP India) 1935;4(3):283-287.
- 3. Izrailskaia AV, Besprozvannykh VV, Tatanova YV, Nguyen HM, Ngo HD. Developmental stages of Notocotylus magniovatus Yamaguti, 1934, Catatropis vietnamensis n. sp., Pseudocatatropis dvoryadkini n. sp., and phylogenetic relationships of Notocotylidae Lühe, 1909. Parasitol Res 2019;118(2):469-481. https://doi.org/10.1007/s00436-018-6182-2
- 4. Harsche KR. On two new species of trematodes from Allahabad. Allahabad Univ Stud 1932;8:32-46.
- 5. Barker FD. Parasites of the American muskrat (Fiber zibethicus). J Parasitol 1915;1(4):184-197.
- 6. Noble AE. Two new trematodes from the American coot. Trans Am Microsc Soc 1933;52(4):353-360.
- 7. Bayssade-Dufour C, Albaret JL, Fermet-Quinet H, Farhati K. Catatropis lagunae n. sp., Trematoda, Notocotylidae, parasite d’oiseaux de mer. Can Field Nat 1996;110(3):392-402.
- 8. Schuster RK, Wibbelt G. Catatropis pakistanensis n. sp. (Trematoda: Notocotylidae) from Northern shovelers, Anas clypeta (Anatidae: Aves) from Pakistan with some remarks on the history of Catatropis species. Helminthologia 2012;49(1):43-48. https://doi.org/10.2478/s11687-012-0007-0
- 9. Dvoryadkin VA. Species compositions and specificities of development of trematodes of Notocotylidae family from the South Far East of USSR. In Lebedev BI ed, Investigation in Parasitology Academic of Sciences of the USSR. Vladivostok. USSR; 1989, pp 97-104.
- 10. Ramadan MM, Abdou NE, Taha RG, Haroun SH. Scanning electron microscopy of Catatropis aegyptiacus n. sp. (Trematoda: Notocotylidae) from Norway brown rat, Rattus norvegicus (Muridae: Mammalia) from Egypt. J Egypt Soc Parasitol 2020;50(1):209-214.
- 11. Shafi MM, Rehana R, Khurshid S. Catatropis pakistanensis (Trematoda: Notocotylidae): a new species from new host, rice rat (Bandicota bengalensis) in Pakistan. Pak J Agric Res 1982;3(1):34-39.
- 12. Lutz A. Estudios sobre Trematodes observados en Venezuela. In Lutz A ed, Estudios de Zoologica y Parasitologia Venezolanas. Diptera. Rio de Janeiro, Brazil; 1928, pp 101-125.
- 13. Skrjabin KI. Trematodes dans les oiseaux d’Oural. Annaire du Musee Zoologique de l’Academie Imperiale des Sciences 1915;9(4):270.
- 14. Johnston TH. New trematodes from the Australian water-hen, Gallinula tenebrosa. Rec South Aust Mus 1929;4:135-142.
- 15. Martin WE. The life cycle of Catatropis johnstoni n. sp. (Trematoda: Notocotylidae). Trans Am Microsc Soc 1956;75(1):117-128.
- 16. Cribb TH. Notocotylidae (Digenea) from the Australian water rat Hydromys chrysogaster Geoffroy, 1804 (Muridae). Syst Parasitol 1991;18:227-237. https://doi.org/10.1007/BF00009362
- 17. McDonald ME. Key to Trematodes Reported in Waterfowl. US Department of the Interior. Fish and Wildlife Service; Washington DC, USA. 1981, pp 1-157.
- 18. Kanev I, Vassilev I, Dimitrov V, Radev V. Life-cycle, delimitation and redescription of Catatropis verrucosa (Frölich, 1789) Odhner, 1905 (Trematoda: Notocotylidae). Syst Parasitol 1994;29:133-148. https://doi.org/10.1007/BF00009809
- 19. Odhner T. Die Trematoden des arktischen Gbietes. Fauna Arctica 1905;4(2):291-372.
- 20. Flores V, Brugni N. Catatropis chilinae n. sp. (Digenea: Notocotylidae) from Chilina dombeiana (Gastropoda: Pulmonata) and notes on its life-cycle in Patagonia, Argentina. Sys Parasitol 2003;54(2):89-96. https://doi.org/10.1023/a:1022593810479
- 21. Lai G, Sha G, Zhang T, Yang M. A new species of Catatrophis-Catatropis chinensis sp. nov. Chin J Anim Vet Sci 1984;15(2):121-124.
- 22. Yamaguti S. Studies on the helminth fauna of Japan. Part 25. Trematodes of birds, IV. Jpn J Zool 1939;8(2):129-210.
- 23. Bullock WL. Two new species of monostomes from the Canada goose with a review of Paramonostomum alveatum (Mehlis in Creplin, 1846). J Parasitol 1952;38(5):371-378. https://doi.org/10.2307/3273915
- 24. Flores V, Brugni N. Catatropis hacheri n. sp. (Digenea: Notocotylidae) from Heleobia hatcheri (Prosobranchia: Hydrobiidae) and notes on its life-cycle in Patagonia, Argentina. Syst Parasitol 2006;63(2):111-118. https://doi.org/10.1007/s11230-005-9004-8
- 25. Khan AR, Chishti MZ. The incidence of trematode parasites in a common Kashmiri fowl population and study of Catatropis kashmirensis sp. nov. (Digenea: Notocotylidae Lühe, 1909). Revista Parasitol 1987;4(3):355-360.
- 26. Kossak W. Über monostomiden. Zool Jahrb 1911;31:491-590.
- 27. Gupta PC, Singh RB. On two species of the genus Catatropis Odhner, 1905 (Digenea: Notocotylidae) from avian hosts in India. Indian J Helminthol 1984;35:122-128.
- 28. Gubanov NM, Fedorov KP, Berlovskaya LI, Kuznetsova LG. Catatropis morosovi n. sp. from the water vole of the central Yakutsk region. Mater Nauch Konf Vses Obscestva Gel’mint 1966 Part 3:97-100.
- 29. Gonchar A, Galaktionov KV. It is marine: distinguishing a new species of Catatropis (Digenea: Notocotylidae) from its freshwater twin. Parasitology 2021;148(1):74-83. https://doi.org/10.1017/S0031182020001808
- 30. Harwood PD. Notes on Tennessee helminths. IV. North American trematodes of the subfamily Notocotylinae. J Tennessee Acad Sci 1939;14:332-340.
- 31. Singh KS. Catatropis rauschi n. sp. (Family: Notocotylidae Lühe, Trematoda) from the pintail, Dafia acuta from India. J Zool Soc India 1956;8:43-46.
- 32. Rohde K, Lee FO. Life cycle of Catatropis indica Srivastava, 1935 (Trematoda: Notocotylidae). Z Parasitenkd 1967;29(2):137-148. https://doi.org/10.1007/BF00260201
- 33. Rohde K. Das nervensystem der cercarie von Catatropis indica srivastava, 1935 (Digenea: Notocotylidae) und der geschlechsreifen form von Diaschitorchis multitesticularis Rohde, 1962 (Digenea: Pronocephalidae). Z Morphol Tiere 1968;62(1):77-102.
- 34. Tandon V, Roy B. Stereoscan observations on the tegumental surface of Catatropis indicus Srivastava, 1935. Acta Parasitol 1996;41(3):115-119.
- 35. Koch M. First record and description of Catatropis indicus Srivastava 1935 (Digenea: Notocotylidae), in Australia. Queensland Museum, Memoirs of Queensland Museum; South Brisbane, Australia. 2002, pp 147-153.
- 36. Yousif F, Bardicy SE, Tadros M, Ayoub M. First record of Catatropis indicus Srivastava (Notocotylidae) from Gabbiella senaariensis Kuster (Bithyniidae) in Egypt. Austr J Basic Appl Sci 2011;5(9):724-728.
- 37. Tkach V, Pawlowski J, Mariaux J. Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int J Parasitol 2000;30(1):83-93. https://doi.org/10.1016/s0020-7519(99)00163-0
- 38. Tkach VV, Pawlowski J, Sharpilo VP. Molecular and morphological differentiation between species of the Plagiorchis vespertilionis group (Digenea, Plagiorchiidae) occurring in European bats, with a re-description of P. vespertilionis (Müller, 1780). Syst Parasitol 2000;47(1):9-22. https://doi.org/10.1023/a:1006358524045
- 39. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007;23(21):2947-2948. https://doi.org/10.1093/bioinformatics/btm404
- 40. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993;10(3):512-526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
- 41. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 2018;35(6):1547-1549. https://doi.org/10.1093/molbev/msy096
- 42. Chai JY, Jung BK, Chang T, Shin H, Cho J, et al. Echinostoma miyagawai Ishii, 1932 (Echinostomatidae) from ducks in Aceh Province, Indonesia with special reference to its synonymy with Echinostoma robustum Yamaguti, 1935. Korean J Parasitol 2021;59(1):35-45. https://doi.org/10.3347/kjp.2021.59.1.35
- 43. Barton DP, Blair D. Family Notocotylidae Lühe 1909. In Jones A, Bray RA, Gibson DI eds, Key to the Trematoda. 2:CABI Publishing; London, UK. 2005, p 745.
- 44. Gupta V, Jehan A. Some trematodes from avian hosts of India. An Inst Biol Ser Zool 1977;48(1):13-26.
 , Bong-Kwang Jung2,3,*
, Bong-Kwang Jung2,3,* , Taehee Chang4
, Taehee Chang4 , Sooji Hong2
, Sooji Hong2 , Hyejoo Shin2
, Hyejoo Shin2 , Marzuki Bin Muhammad Abdullah5
, Marzuki Bin Muhammad Abdullah5