Abstract
Toxoplasma gondii is an intracellular protozoan parasite capable of causing chronic infection by forming persistent cysts in the brain. Despite its global burden, no approved vaccine exists. Virus-like particle vaccines expressing microneme protein 8 (MIC8) or apical membrane antigen 1 (AMA1) of T. gondii have previously shown efficacy. In this study, we generated recombinant vaccinia viruses (rVVs) expressing MIC8 and AMA1 and evaluated their efficacy against T. gondii ME49 infection. BALB/c mice were intramuscularly immunized with a combination of MIC8 and AMA1 rVVs and challenged orally with T. gondii ME49. Immunization with MIC8+AMA1 rVVs produced a significant increase in T. gondii-specific IgG. Splenocyte analysis revealed robust activation of CD4+ and CD8+ T cells, as well as expansion of memory B cells. The immunized group exhibited an 89.6% reduction in brain cyst count, with significantly improved survival compared to the control group. These findings demonstrate that combining the antigens MIC8 and AMA1 using a vaccinia virus platform can effectively promote both humoral and cellular immunity, supporting its potential as a vaccine strategy against T. gondii ME49.
-
Key words: Toxoplasma gondii, recombinant vaccinia virus, MIC8, AMA1, mouse, protective effects
Introduction
Toxoplasma gondii typically causes asymptomatic infection in healthy individuals, but can establish chronic infections by forming persistent tissue cysts that are highly resistant to clearance in cases of immunosuppression or congenital transmission [
1,
2]. Despite its high global prevalence, no licensed vaccine is currently available, primarily due to challenges in inducing long-term immunity that can control cyst persistence [
3]. This chronic stage is maintained by the parasite’s ability to evade host immunity, presenting a major obstacle for vaccine design.
To date, various vaccine platforms have been explored to develop strategies capable of preventing chronic
T. gondii infection, including DNA vaccines, protein subunits, virus-like particles (VLPs), and live-attenuated
T. gondii [
4]. Among the diverse antigenic targets evaluated, microneme protein 8 (MIC8) [
5] and apical membrane antigen 1 (AMA1) [
6] have emerged as promising candidates due to their critical roles in the active invasion process. Previous studies utilizing VLP platforms have demonstrated that both MIC8 and AMA1 can individually induce strong antigen-specific antibody responses and confer partial protection against
T. gondii infection, including reductions in brain cyst counts and enhanced survival [
7–
10].
In this context, we employed a recombinant vaccinia virus (rVV) platform to enhance the protective efficacy of MIC8 and AMA1. Vaccinia virus vectors are known for their ability to induce both humoral and cellular immune responses, rendering them a complementary alternative to traditional VLP-based vaccines [
11,
12]. Although the rVV platform has previously been explored for other
T. gondii antigens, such as rhoptry proteins 2 and 4, and dense granule antigen 4 [
13–
16], its application to MIC8 or AMA1 has not yet been investigated.
In the present study, we constructed rVVs expressing
T. gondii MIC8 and AMA1 and assessed their immunogenicity and protective efficacy in a murine model of chronic toxoplasmosis. Single-antigen rVV groups were excluded from this study because the immunogenicity of MIC8 and AMA1 has already been well characterized in previous VLP studies [
7,
10]. Instead, this study focused on evaluating whether simultaneous immunization with MIC8 and AMA1 rVVs could broaden the immune response and confer enhanced protection against
T. gondii ME49 infection.
Materials and Methods
Ethics statement
All procedures involving live animals were approved by the Kyung Hee University Institutional Animal Care and Use Committee (approval No. KHSASP-20-648). Experiments using T. gondii were conducted in biosafety level 2 (BSL-2) and animal biosafety level 2 (ABSL-2) facilities.
Animals and T. gondii preparation
Female BALB/c mice (6 weeks old) were obtained from Nara Biotech (Seoul, Korea) and maintained under specific pathogen-free conditions.
T. gondii ME49 was maintained through serial passages in mice, and tissue cysts were isolated from
T. gondii ME49-infected mouse brains [
17,
18].
The sequences of
T. gondii MIC8 (GenBank GQ260152.1, protein ADF36594.1) and AMA1 (GenBank AF010264.1, protein AAB65410.1) were amplified by polymerase chain reaction and cloned into the pRB21 vector. The resulting plasmids were transfected into CV-1 cells that had been pre-infected with wild-type vaccinia virus (western reserve strain) to generate rVVs. The rVVs were propagated in Vero cells, concentrated by 36% sucrose gradient centrifugation, and assessed for antigen expression using ELISA [
14].
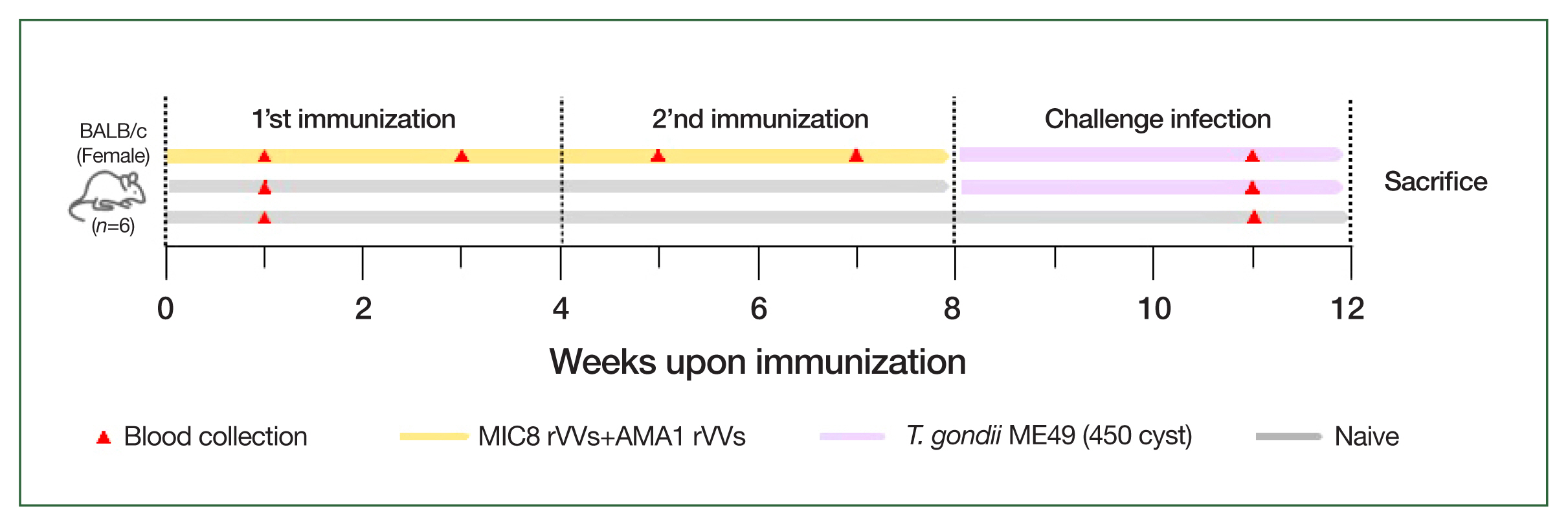
Mice were intramuscularly immunized with a 1:1 mixture of MIC8 and AMA1 rVVs (5× 105 PFU each, total 1×106 PFU) in 50 μl of phosphate-buffered saline under anesthesia. The immunizations were performed twice at 4-week intervals. Four weeks after the boost immunization, the mice were orally challenged with 450 cysts of T. gondii ME49.
Sample collection and preparation
Mouse sera were collected from the retro-orbital sinus 1 week after the prime and boost immunizations. Spleens were harvested at 4 weeks after challenge infection for analysis of antibody-secreting cells and flow cytometry [
9]. To quantify the brain cysts, the brains of the animals were collected at the same time point and homogenized in phosphate-buffered saline, followed by purification using a sucrose gradient centrifugation method [
19]. Ten microliters of the resulting suspension were placed on a glass slide, and the number of cysts was counted under a light microscope in 3 randomly selected fields. The average number of cysts was calculated for each mouse.
The levels of
T. gondii-specific IgG, IgG2a, IgG2b, and IgA antibodies (SouthernBiotech, Birmingham, AL, USA) were measured via ELISA, as described previously [
19]. Briefly, 96-well plates were coated overnight with
T. gondii lysate antigen (4 μg/ml), blocked with 0.2% gelatin, and then incubated with serially diluted serum. Horse radish peroxidase–conjugated goat anti-mouse antibodies were used as secondary antibodies. The absorbance was measured at 450 nm.
Cell suspensions were prepared from spleens and stimulated with
T. gondii lysate antigen (4 μg/ml) for 5 h. The cells (5×10
6) were stained with fluorochrome-conjugated monoclonal antibodies against CD3, CD4, CD8, CD62L, CD19, CD27, and B220 (BD Biosciences, San Jose, CA, USA). Flow cytometric analysis was conducted using a BD FACS cytometer (BD Biosciences), and data were analyzed as described previously [
9].
All data were analyzed using Prism version 8.4 (GraphPad Software, Boston, MA, USA). Differences between groups were assessed using one-way analysis of variance followed by Tukey’s post hoc test. The significance levels are indicated as follows: *P<0.05, **P<0.01, and ***P<0.001.
Results
Successful generation of rVVs expressing MIC8 and AMA1 antigens
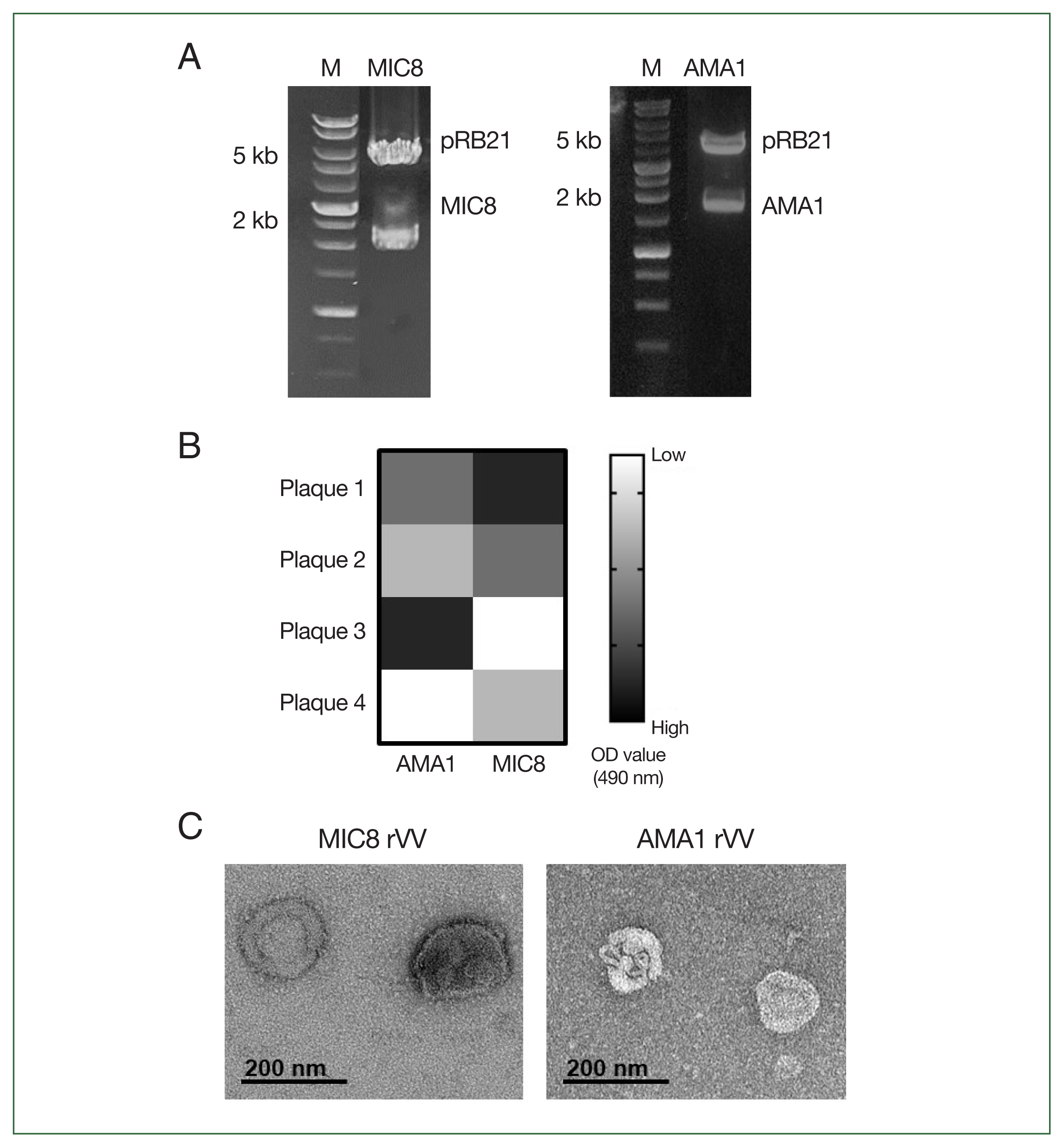
To generate rVVs expressing
T. gondii MIC8 and AMA1, the corresponding genes were cloned into the pRB21 vector and confirmed through restriction enzyme digestion (MIC8 EcoRI/NheI, AMA1 EcoRI/HindIII) (
Fig. 1A). After transfection and infection, individual viral plaques were screened using ELISA, and plaques showing the highest
T. gondii reactivity were selected and expanded (
Fig. 1B). Morphological analysis of purified rVVs by transmission electron microscopy revealed characteristic poxvirus-like virions for both MIC8 and AMA1 rVVs (
Fig. 1C).
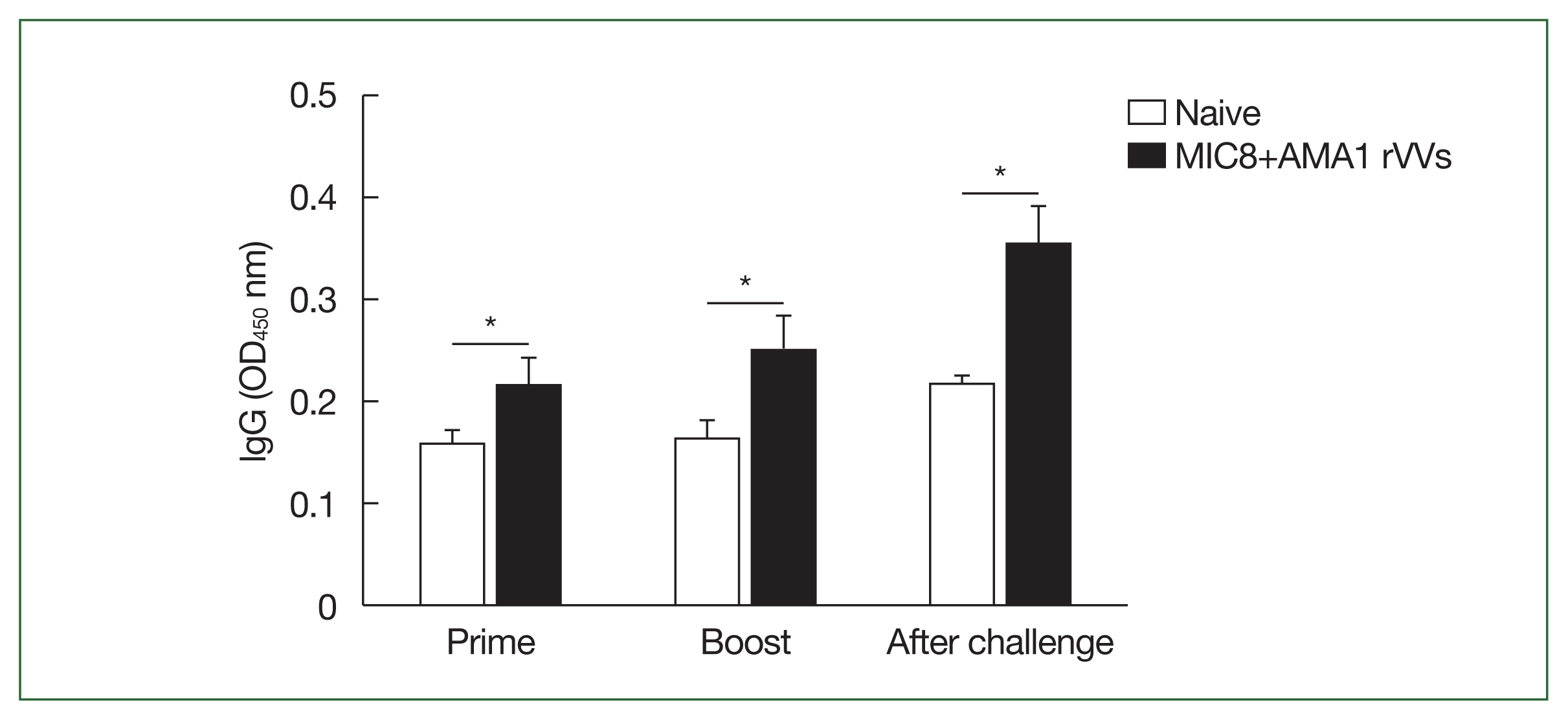
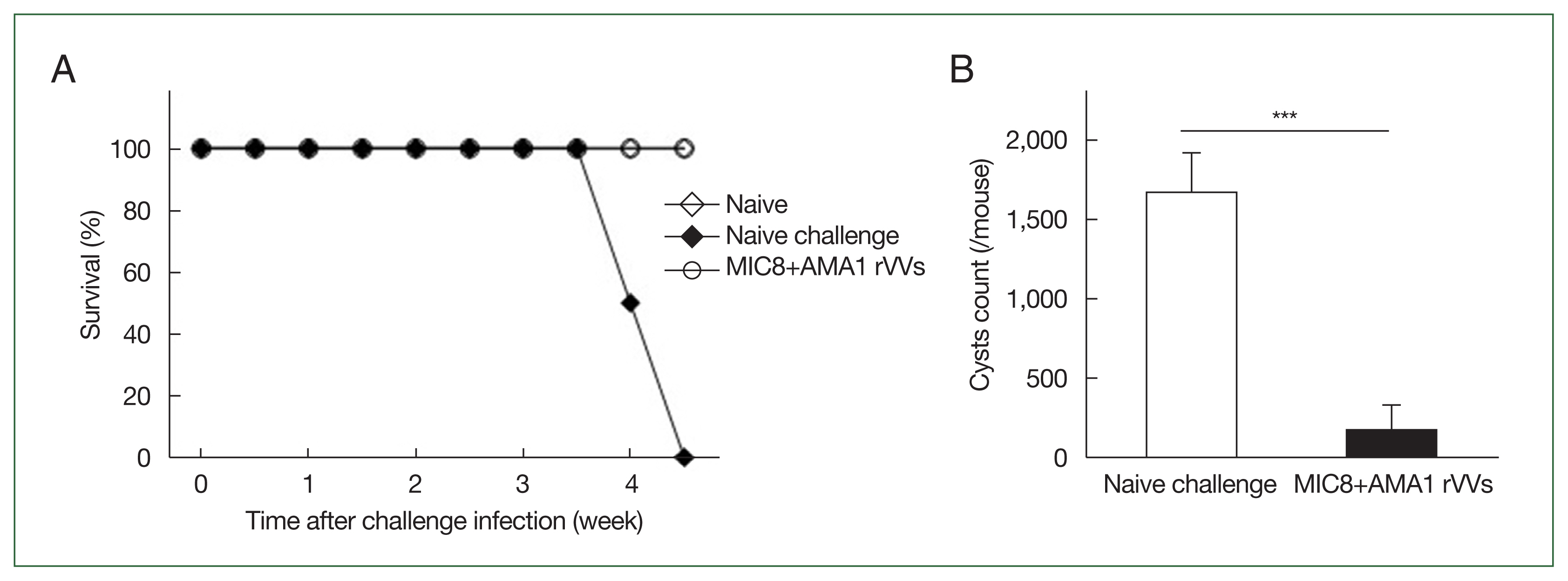
Serum samples were collected and analyzed by ELISA to assess the antibody response elicited by MIC8+AMA1 rVVs immunization (
Fig. 2). Mice immunized with the rVVs showed significantly higher response of
T. gondii–specific IgG compared to unimmunized mice after both prime and boost immunizations; this response was maintained after challenge (
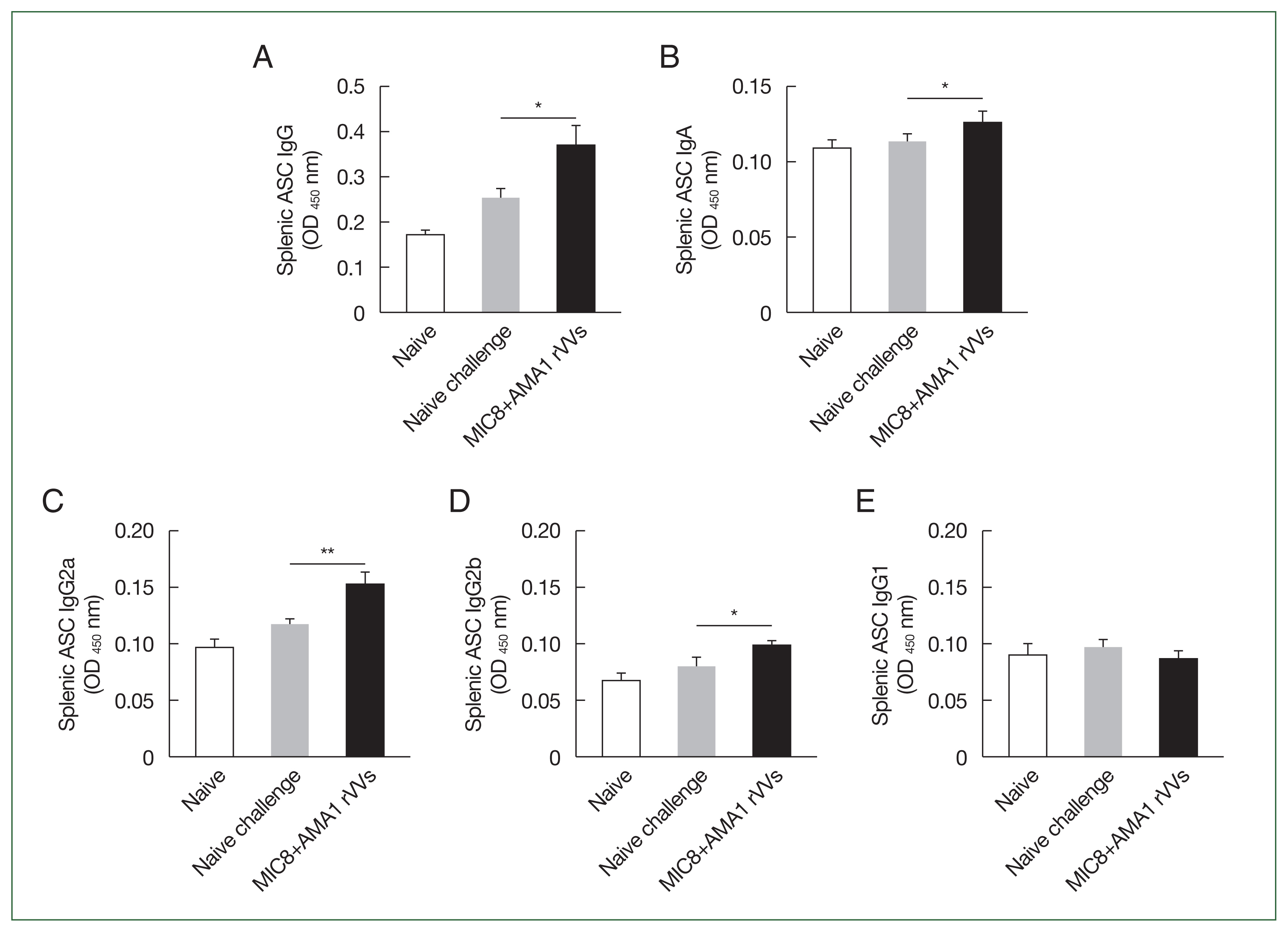
Fig. 3). Splenocytes were cultured on antigen-coated plates for 5 days, and the supernatants were analyzed by ELISA to evaluate the ability of splenic cells to secrete
T. gondii-specific antibodies. Immunized mice showed significantly higher levels of secreted IgG (
Fig. 4A) and IgA (
Fig. 4B) antibodies compared to both naïve mice and those subjected to a naïve challenge. Analysis of antibody subclasses revealed significant increases in IgG2a (
Fig. 4C) and IgG2b (
Fig. 4D), while no significant differences in IgG1 levels were observed (
Fig. 4E).
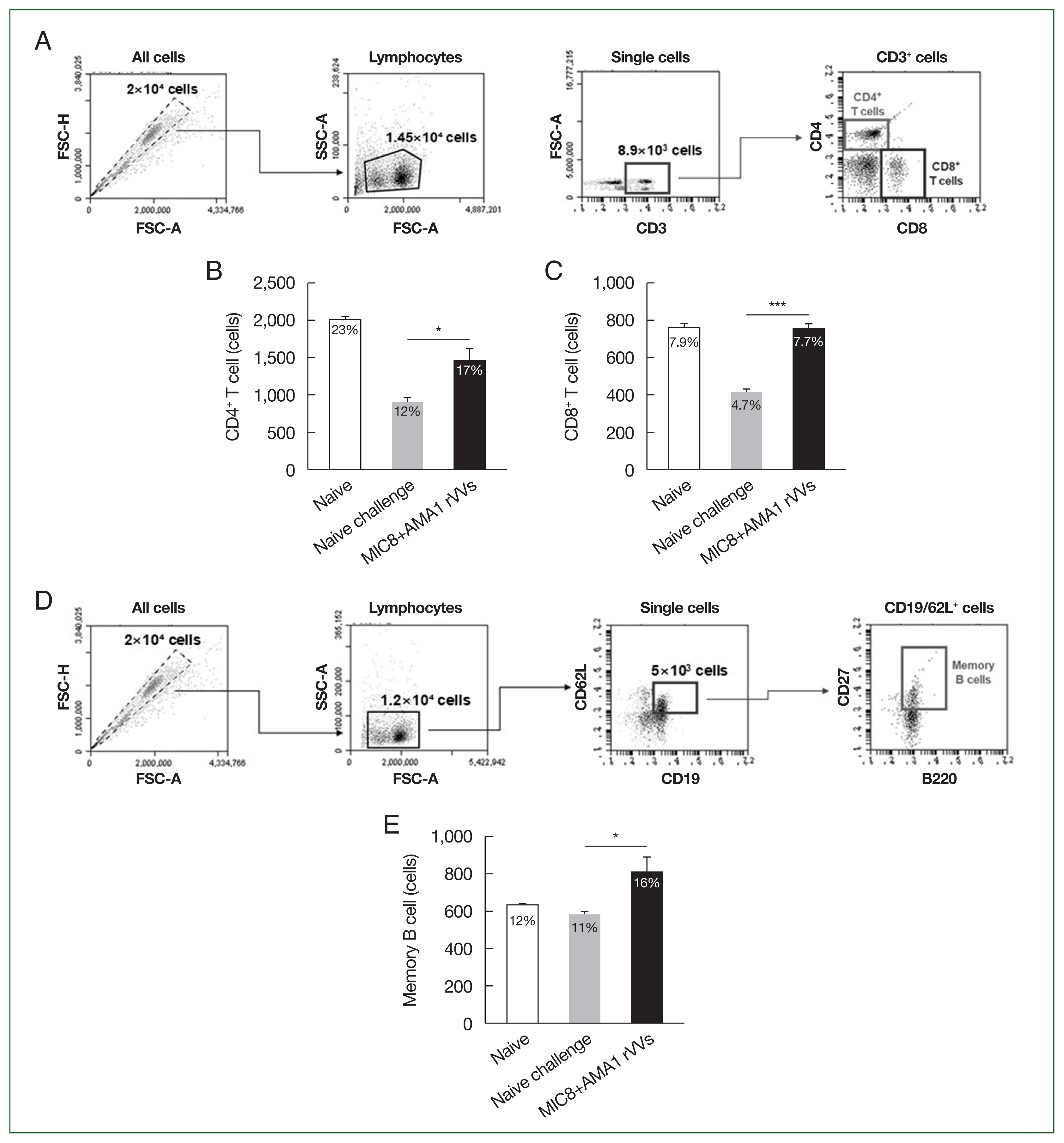
The immune responses were analyzed by flow cytometry using splenocytes collected 4 weeks after challenge. The gating strategy for identifying CD4
+ and CD8
+ T cell subsets is shown in
Fig. 5A. Quantitative analysis revealed a significant increase in the frequencies of both CD4
+ and CD8
+ T cells in the MIC8+AMA1 rVVs compared to both naïve and naïve challenge groups (
Fig. 5B, C). In addition to T cell responses, the frequency of memory B cells (CD19
+, CD62L
+, CD27
+, and B220
+) was assessed (
Fig. 5D). Flow cytometric analysis revealed a significant expansion of memory B cells in the MIC8+AMA1 rVVs compared to the naïve and naïve challenge groups (
Fig. 5E). Together, these data reinforce that the combined MIC8+AMA1 rVVs immunization stimulates responses from both T cells and memory B cells.
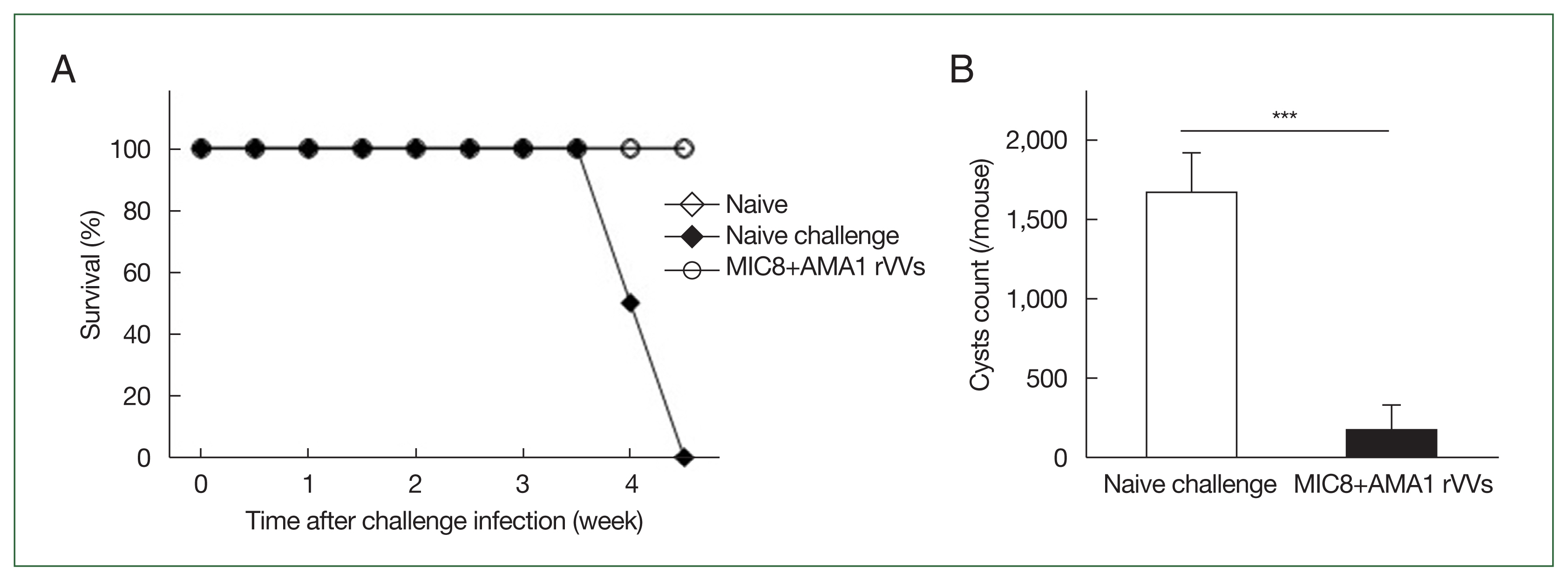
The mice were orally challenged with
T. gondii ME49 cysts and were monitored for survival and the number of brain cysts. All immunized mice survived, whereas those subjected to naïve challenge succumbed by day 31 (
Fig. 6A). Moreover, the number of brain cysts was significantly reduced in the immunized group compared to that in the naïve challenge group (
Fig. 6B), indicating that immunization with MIC8 and AMA1 confers strong protection against
T. gondii ME49.
Discussion
In this study, we evaluated the protective efficacy of rVVs expressing T. gondii MIC8 and AMA1 against T. gondii ME49 in mice. Immunization with MIC8+AMA1 rVVs induced high IgG antibody response, promoted T cell and memory B cell activation, and significantly reduced the cyst count following oral challenge with T. gondii ME49.
Unlike previous studies that assessed the efficacy of MIC8 or AMA1, the present study focused on the combined use of these antigens. While MIC8 has been tested in combination with other antigens in previous VLP-based studies [
7–
9], the current study is the first to assess the immunological synergy between MIC8 and AMA1 using an rVV platform, revealing a robust and multifaceted immune activation.
Importantly, our findings demonstrate that combination rVVs can effectively induce long-term immunity. The strong activation of memory B cells in the combination group supports the idea that simultaneous exposure to multiple antigens can promote durable immune memory [
20]. Targeting both attachment and invasion antigens ensures recall responses upon reinfection or reactivation, leading to improved long-term protection.
Taken together, our results demonstrate that an rVV-based vaccine expressing both MIC8 and AMA1 can induce strong humoral and cellular immunity, including memory B cells, and confer significant protection against T. gondii ME49 infection. These findings highlight the immunological synergy achieved through the co-delivery of functionally complementary antigens via the rVV platform, and further support the broad applicability of this strategy for controlling persistent T. gondii ME49 infections.
Notes
-
Author contributions
Conceptualization: Jin Y
Formal analysis: Kang HJ, Jin Y, Yang ZS
Investigation: Ahmed MA, Quan FS
Methodology: Kang HJ, Yang ZS, Ahmed MA, Quan FS
Supervision: Quan FS
Writing – original draft: Kang HJ
Writing – review & editing: Kang HJ, Quan FS
-
Conflict of interest
Fu-Shi Quan serves as an editor of Parasites, Hosts and Diseases but had no involvement in the decision to publish this article. No other potential conflicts of interest relevant to this study were reported.
-
Acknowledgments
This research was funded by the Core Research Institute Program and the Basic Science Research Program through the National Research Foundation of Korea (NRF), supported by the Ministry of Education (grant No. NRF-2018-R1A6A1A03025124).
Fig. 1Construction and confirmation of recombinant vaccinia viruses (rVVs) expressing Toxoplasma gondii microneme protein 8 (MIC8) and apical membrane antigen 1 (AMA1). (A) MIC8 and AMA1 genes were cloned into the pRB21 vector and verified by restriction digestion. (B) Plaques presenting MIC8 or AMA1 were selected by ELISA. (C) Representative transmission electron microscopy images of MIC8 and AMA1 rVVs. OD, optical density.
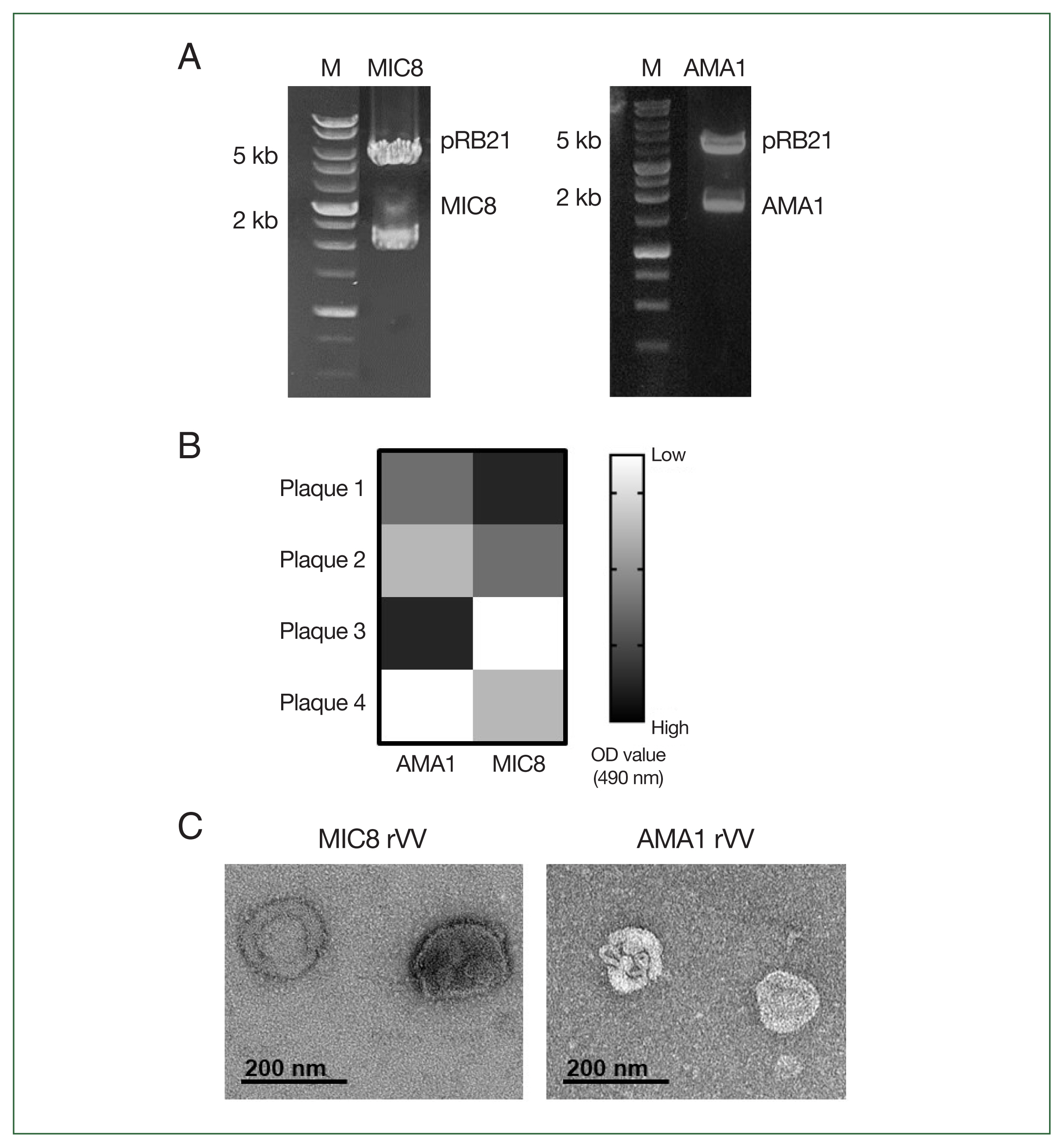
Fig. 2Schematic of the animal experiment design. BALB/c mice were immunized intramuscularly with microneme protein 8 (MIC8)+apical membrane antigen 1 (AMA1) recombinant vaccinia viruses (rVVs) at weeks 0 and 4, followed by oral challenge with Toxoplasma gondii ME49 at week 8. Blood was collected at the indicated time points, and the mice were sacrificed at week 12.
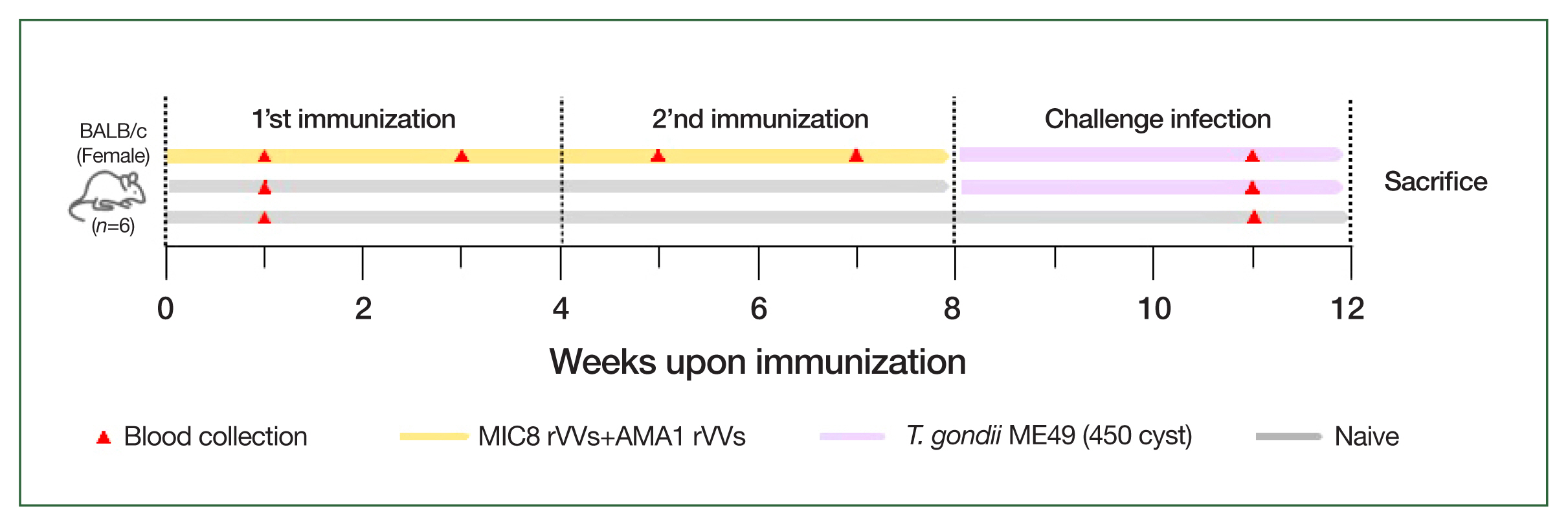
Fig. 3Induction of Toxoplasma gondii-specific antibody responses in serum. The IgG levels in serum were measured by ELISA at prime, boost, and after challenge. Mice immunized with microneme protein 8 (MIC8)+apical membrane antigen 1 (AMA1) recombinant vaccinia viruses (rVVs) exhibited significantly increased antibody responses compared to naïve mice. OD, optical density. *P<0.05.
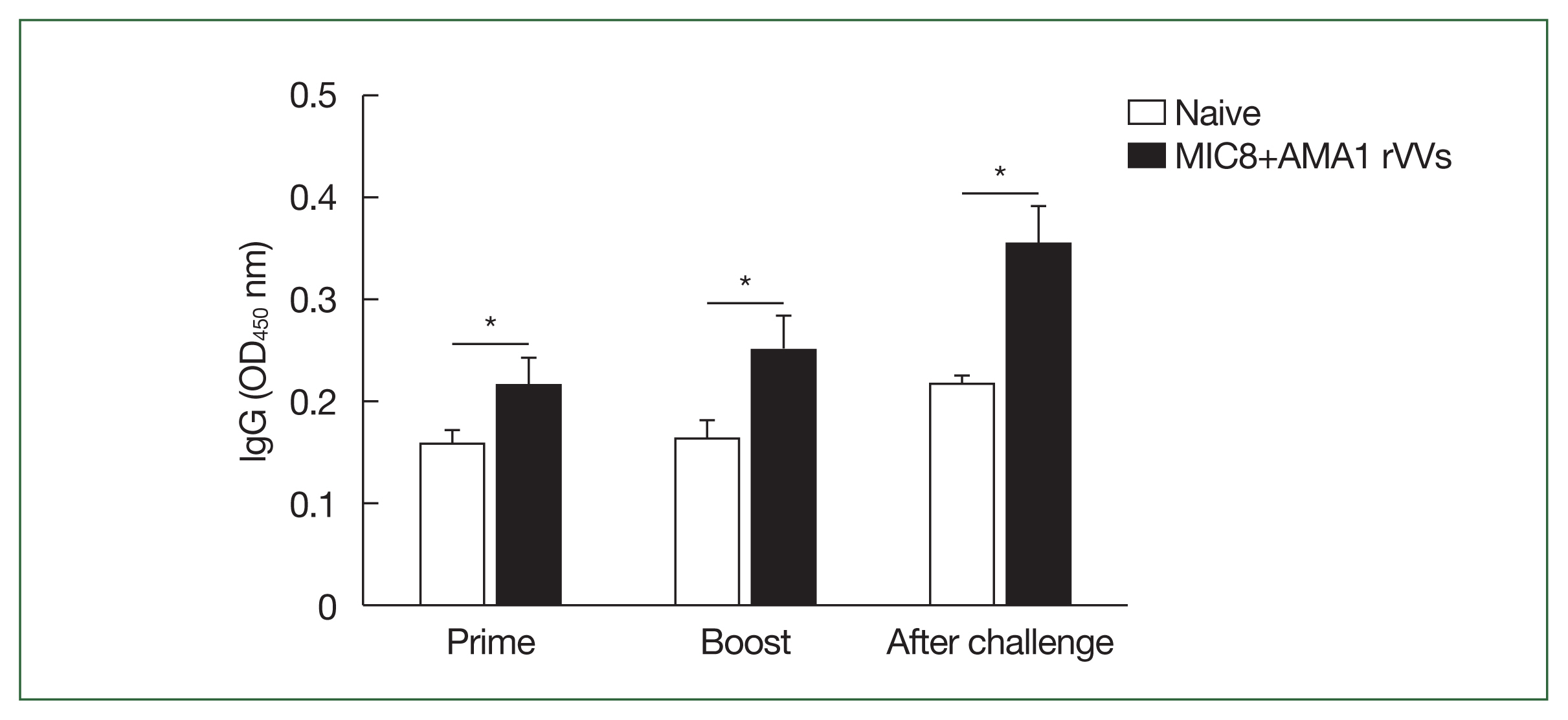
Fig. 4Evaluation of Toxoplasma gondii-specific antibody production in cultured splenocytes. Splenocytes were cultured on T. gondii-coated plates for 5 days, and the secreted antibodies were measured by ELISA. (A) The IgG, (B) IgA, (C) IgG2a, (D) IgG2b, and (E) IgG1 responses are shown. Significant increases were observed in IgG, IgA, IgG2a, and IgG2b in the microneme protein 8 (MIC8)+apical membrane antigen 1 (AMA1) recombinant vaccinia viruses (rVVs) group. ASC, antibody secreting cell; OD, optical density. *P<0.05, **P<0.01.
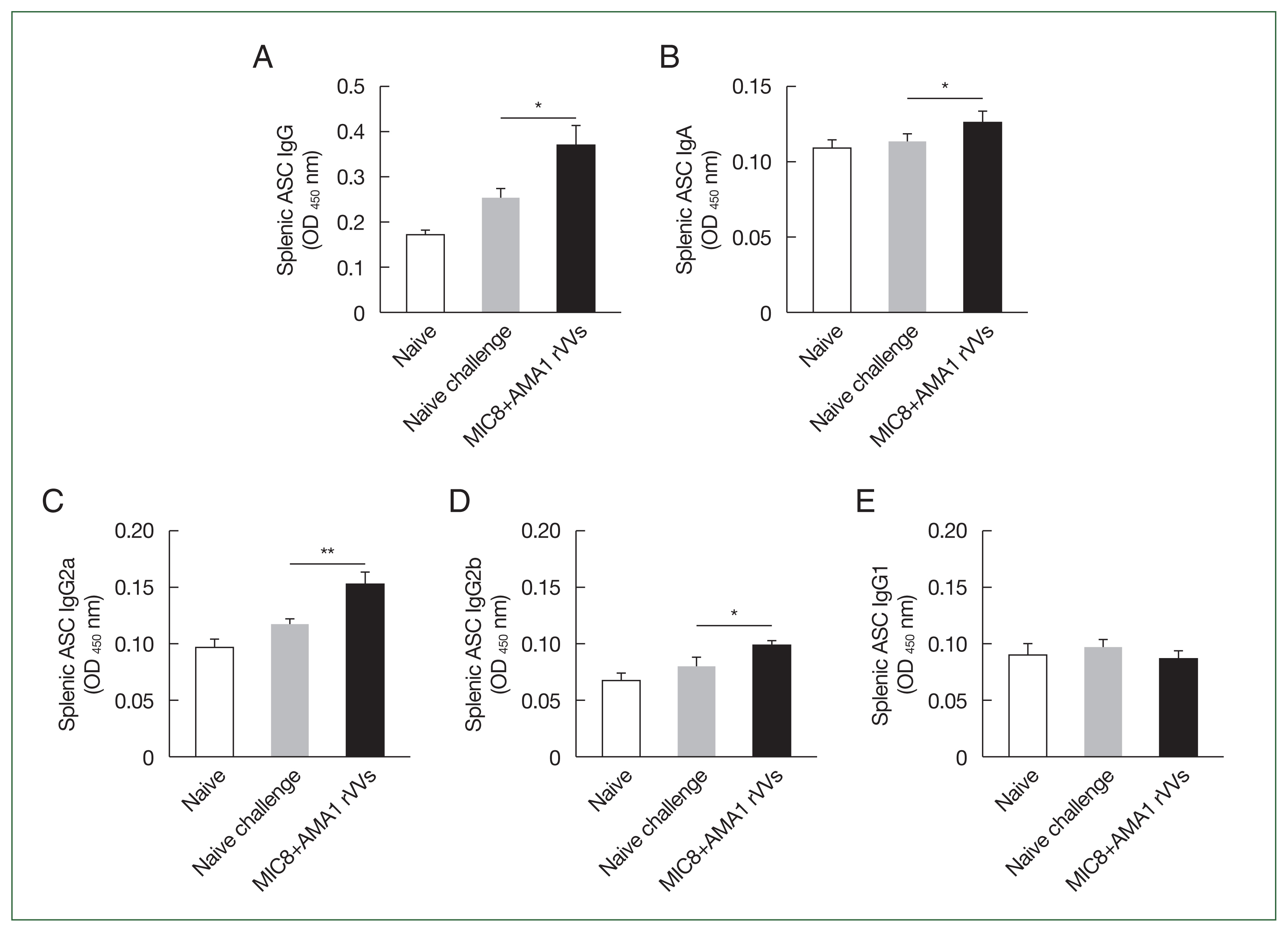
Fig. 5Activation of splenic T cells and memory B cells after MIC8+AMA1 rVVs immunization. Splenocytes were analyzed by flow cytometry to evaluate lymphocyte activation. (A) Gating strategy for CD4+ and CD8+ T cells. (B) CD4+ and (C) CD8+ T cell frequencies. (D) Gating strategy for memory B cells. (E) Frequency of memory B cells. Significant increases were observed in all subsets in the microneme protein 8 (MIC8)+ apical membrane antigen 1 (AMA1) recombinant vaccinia viruses (rVVs) group. FSC-H, forward scatter–height; FSC-A, forward scatter–area; SSC-A, side scatter–area. *P<0.05, ***P<0.001.
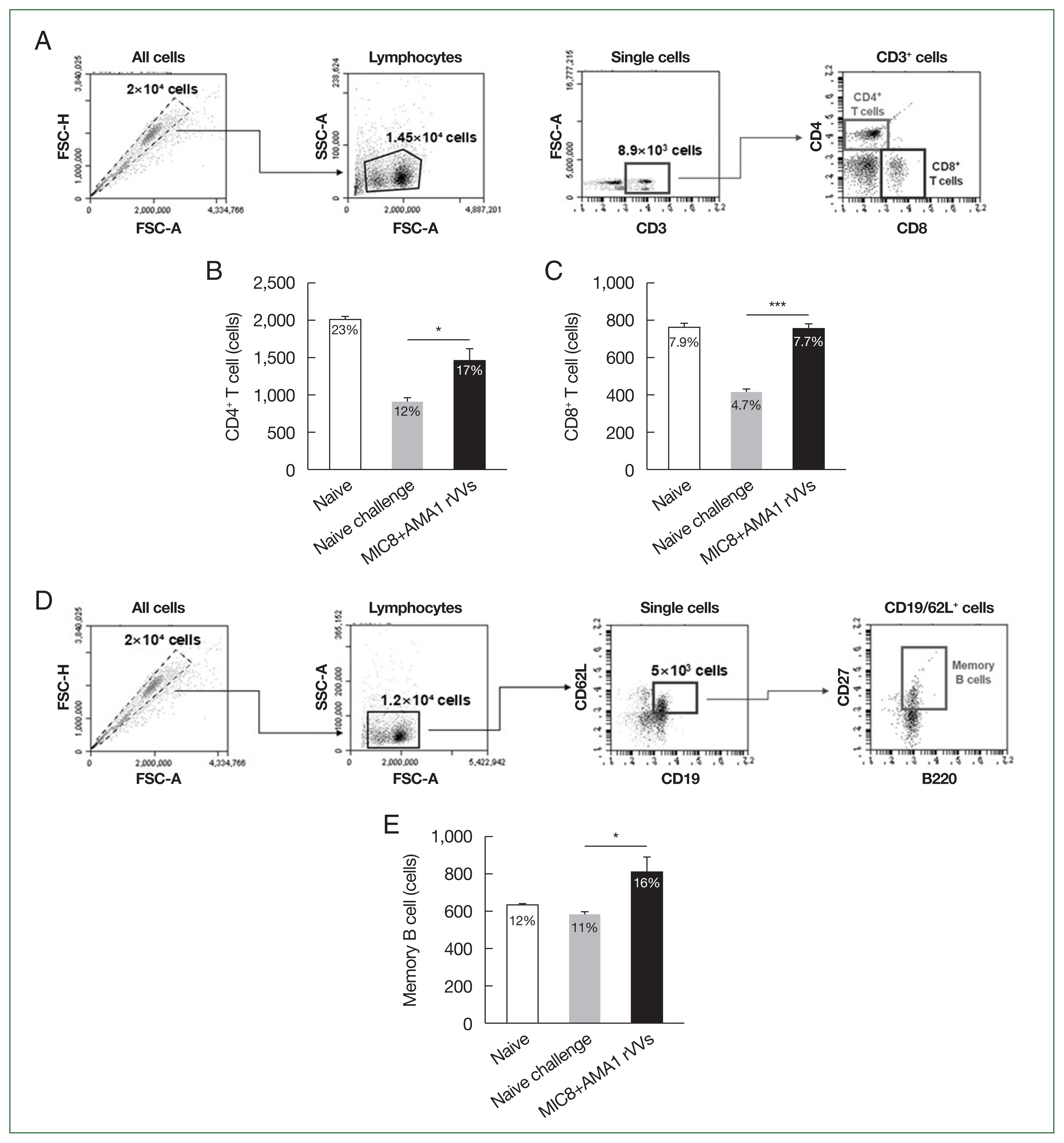
Fig. 6Immunization with microneme protein 8 (MIC8)+apical membrane antigen 1 (AMA1) recombinant vaccinia viruses (rVVs) protects against Toxoplasma gondii ME49. Mice were orally challenged with T. gondii ME49 cysts after immunization. (A) Survival was monitored for 5 weeks after the challenge infection. (B) Brain cysts were counted at 30 days after the challenge infection. Immunized mice exhibited complete survival and significantly reduced brain cyst count compared to those subjected to naïve challenge. ***P<0.001.
References
- 1. Zhao XY, Ewald SE. The molecular biology and immune control of chronic Toxoplasma gondii infection. J Clin Invest 2020;130(7):3370-3380. https://doi.org/10.1172/JCI136226
- 2. Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 1998;11(2):267-299. https://doi.org/10.1128/CMR.11.2.267
- 3. Zhang NZ, Chen J, Wang M, Petersen E, Zhu XQ. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev Vaccines 2013;12(11):1287-1299. https://doi.org/10.1586/14760584.2013.844652
- 4. Chu KB, Quan FS. Recent progress in vaccine development targeting pre-clinical human toxoplasmosis. Parasites Hosts Dis 2023;61(3):231-239. https://doi.org/10.3347/PHD.22097
- 5. Liu MM, Yuan ZG, Peng GH, Zhou DH, He XH, et al. Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol Res 2010;106(5):1079-1084. https://doi.org/10.1007/s00436-010-1742-0
- 6. Najm R, Ruivo MTG, Penarete-Vargas DM, Hamie M, Mouveaux T, et al. Invasion of Toxoplasma gondii bradyzoites: molecular dissection of the moving junction proteins and effective vaccination targets. Proc Natl Acad Sci U S A 2023;120(5):e2219533120. https://doi.org/10.1073/pnas.2219533120
- 7. Mao J, Eom GD, Yoon KW, Heo SI, Kang HJ, et al. Protective humoral immunity induced by virus-like particles expressing Toxoplasma gondii CST1 or MIC8. Acta Trop 2025;261:107501. https://doi.org/10.1016/j.actatropica.2024.107501
- 8. Lee SH, Kang HJ, Lee DH, Quan FS. Protective immunity induced by incorporating multiple antigenic proteins of Toxoplasma gondii into influenza virus-like particles. Front Immunol 2019;9:3073. https://doi.org/10.3389/fimmu.2018.03073
- 9. Lee SH, Chu KB, Kang HJ, Quan FS. Virus-like particles containing multiple antigenic proteins of Toxoplasma gondii induce memory T cell and B cell responses. PLoS One 2019;14(8):e0220865. https://doi.org/10.1371/journal.pone.0220865
- 10. Kim MJ, Lee SH, Kang HJ, Chu KB, Park H, et al. Virus-like particle vaccine displaying Toxoplasma gondii apical membrane antigen 1 induces protection against T. gondii ME49 infection in mice. Microb Pathog 2020;142:104090. https://doi.org/10.1016/j.micpath.2020.104090
- 11. Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol 2004;172(10):6265-6271. https://doi.org/10.4049/jimmunol.172.10.6265
- 12. Harbour JC, Lyski ZL, Schell JB, Thomas A, Messer WB, et al. Cellular and humoral immune responses in mice immunized with vaccinia virus expressing the SARS-CoV-2 spike protein. J Immunol 2021;206(11):2596-2604. https://doi.org/10.4049/jimmunol.2100054
- 13. Roque-Reséndiz JL, Rosales R, Herion P. MVA ROP2 vaccinia virus recombinant as a vaccine candidate for toxoplasmosis. Parasitology 2004;128(Pt 4):397-405. https://doi.org/10.1017/s0031182003004761
- 14. Yoon KW, Chu KB, Kang HJ, Kim MJ, Eom GD, et al. Orally administrated recombinant vaccinia virus displaying ROP4 induces protection against Toxoplasma gondii challenge infection. Vaccines (Basel) 2022;10(2):152. https://doi.org/10.3390/vaccines10020152
- 15. Yoon KW, Eom GD, Mao J, Kim MJ, Heo SI, et al. Protection induced by recombinant vaccinia virus targeting the ROP4 of Toxoplasma gondii in mice. Exp Parasitol 2025;269:108900. https://doi.org/10.1016/j.exppara.2025.108900
- 16. Zhang G, Huong VT, Battur B, Zhou J, Zhang H, et al. A heterologous prime-boost vaccination regime using DNA and a vaccinia virus, both expressing GRA4, induced protective immunity against Toxoplasma gondii infection in mice. Parasitology 2007;134(Pt 10):1339-1346. https://doi.org/10.1017/S0031182007002892
- 17. Kang HJ, Mao J, Kim MJ, Yoon KW, Eom GD, et al. The detection of Toxoplasma gondii ME49 infections in BALB/c mice using various techniques. Parasites Hosts Dis 2023;61(4):418-427. https://doi.org/10.3347/PHD.23048
- 18. Eom GD, Chu KB, Kang HJ, Kim MJ, Yoon KW, et al. Protective mucosal and systemic immunity induced by virus-like particles expressing Toxoplasma gondii cyst wall protein. PLoS One 2023;18(4):e0283928. https://doi.org/10.1371/journal.pone.0283928
- 19. Kang HJ, Lee SH, Kim MJ, Chu KB, Lee DH, et al. Influenza virus-like particles presenting both Toxoplasma gondii ROP4 and ROP13 enhance protection against T. gondii infection. Pharmaceutics 2019;11(7):342. https://doi.org/10.3390/pharmaceutics11070342
- 20. Bugya Z, Prechl J, Szénási T, Nemes É, Bácsi A, et al. Multiple levels of immunological memory and their association with vaccination. Vaccines (Basel) 2021;9(2):174. https://doi.org/10.3390/vaccines9020174