Abstract
Cysteine proteases play key roles in the biology of Plasmodium parasites and are recognized as antimalarial drug targets. Because these enzymes are involved in diverse biological functions, precise regulation is required to prevent unnecessary damage to both parasites and hosts. In this study, we identified an endogenous inhibitor of cysteine protease of Plasmodium vivax (PvICP) and characterized its biochemical properties. PvICP was found to share highly similar structural characteristics with orthologous proteins from other Plasmodium species. Recombinant PvICP (rPvICP) expressed in Escherichia coli showed a broad range of inhibitory activity against falcipain family cysteine proteases, including vivapain-3, vivapain-4, falcipain-3, malapain-2, and malapain-4, with more potent inhibitory activity against vivapain-3 and vivapain-4. rPvICP’s inhibitory activity was not significantly affected by pH, suggesting its broad biological functions. These findings provide new insights into PvICP and lay the groundwork for future studies exploring its biological significance and potential as a therapeutic target in malaria research.
-
Key words: Plasmodium vivax, cysteine protease inhibitors, cysteine proteases
Cysteine proteases play key roles in the biology of
Plasmodium parasites and have been identified as attractive targets for antimalarial drug development [
1,
2]. Falcipains (FPs), which are papain family cysteine proteases in
Plasmodium falciparum, have been extensively studied due to their essential biological functions [
3,
4]. FP-orthologous enzymes have been characterized in other human-infecting species, such as
Plasmodium vivax,
Plasmodium malariae, and
Plasmodium knowlesi, as well as in rodent malaria parasites, such as
Plasmodium berghei and
Plasmodium vinckei [
5–
10]. These enzymes share similar structural, biochemical, and functional properties and play key roles in hemoglobin degradation, erythrocyte rupture, and merozoite invasion [
2,
5,
8].
Due to the crucial roles of FP family enzymes, strict regulation of activity is necessary to control biological functions and avoid inadequate or superfluous damage to parasites and hosts. Various endogenous inhibitors of cysteine protease (ICP) regulating FP family enzymes have been identified and characterized in
Plasmodium species, including
P. falciparum,
P. malariae,
P. berghei, and
Plasmodium yoelii [
11–
14]. Falstatin, the first endogenous ICP identified in
P. falciparum, effectively inhibits FPs and host cysteine proteases, suggesting an essential role in facilitating erythrocyte invasion [
11]. Falstatin orthologs in
P. berghei (PbICP),
P. yoelii (PyICP), and
P. malariae (PmICP) play likely roles in diverse biological processes crucial for parasite survival and development through regulation of both parasite and host cysteine proteases [
12–
14].
Although
P. vivax is the second most prevalent cause of malaria worldwide, there are limited studies on its biology due to the lack of in vitro cultivation methods and lower clinical significance compared to
P. falciparum. Vivapains (VXs), which are FP-orthologous cysteine proteases of
P. vivax, have been previously characterized [
5,
6]. The highly similar biochemical and functional properties between VXs and FPs suggest they play similar biological roles. However, the regulator proteins of VXs have not yet been reported. To address this research gap, the present study investigated the characteristics of an ICP from
P. vivax (PvICP) as a counterpart endogenous inhibitor for VXs.
The gene encoding PvICP (PVX_099035) was identified by data mining the PlasmoDB (
https://plasmodb.org/plasmo/app) and amplified by PCR with the following set of oligonucleotides: 5′-ATGAAACTTTCCAGCCTCTTTTGC-3′ and 5′-TTACGACACGGT CAACTTCAAAAT-3′. Genomic DNA of
P. vivax Salvador I, a reference strain, was used as a template. The thermal conditions for amplification were 94°C for 5 min, 30 cycles at 95°C for 1 min, 50°C for 1 min, and 72°C for 2 min, followed by a final extension at 72°C for 10 min. Subsequently, the PCR product was cloned into the T&A cloning vector (Real Biotech Corporation, Banqiao City, Taiwan) and transformed into
Escherichia coli DH5α competent cells. The nucleotide sequence of the cloned gene was analyzed by automatic DNA sequencing (Genotech, Daejeon, Korea). The nucleotide and deduced amino acid sequences of
PvICP were analyzed using the DNASTAR package (DNASTAR, Madison, WI, USA) and SignalP (
http://www.cbs.dtu.dk/services/SignalP). A phylogenetic tree was constructed based on the amino acid sequences of ICPs from other
Plasmodium species using MEGA7 (
http://www.megasoftware.net) with maximum likelihood estimation via the Jones-Taylor-Thornton model with 1,000 bootstrap replications.
To produce recombinant PvICP (rPvICP), a fragment of PvICP lacking the N-terminal signal peptide region was amplified using the specific primers 5′-GTCGACCAAAACACG TACTCCTTTGACATC-3′ containing a 5′ SalI site and 5′-CTGCAGTTACGACACGGT CAACTTCAAAAT-3′ harboring a 5′ PstI site. The PCR product was then cloned into a T&A cloning vector (Real Biotech Corporation) and transformed into E. coli DH5α. The resulting plasmid was digested with SalI and PstI, ligated into the pQE-9 vector (Qiagen, Hilden, Germany), and transformed into E. coli M15. Expression of rPvICP was induced using 1 mM of isopropyl-1-thio-β-D-galactopyranoside (Duchefa Biochemie, Haarlem, The Netherlands). rPvICP was purified with nickel-nitrilotriacetic acid chromatography (Qiagen) according to the manufacturer’s instructions. The purification and purity of rPvICP were analyzed by 12% SDS-PAGE and immunoblotting with anti-His antibody (Qiagen). The concentration of rPvICP was determined using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
The inhibitory activity of rPvICP against cysteine proteases was determined by measuring the residual enzyme activity after incubating each enzyme with rPvICP as previously described [
8,
14]. The cysteine proteases used in this assay were papain (Sigma, St. Louis, MO, USA), VX-3, VX-4, FP-3, malapain (MP)-2, and MP-4. Recombinant VX-3, VX-4, FP-3, MP-2, and MP-4 were produced using previously reported methods [
14]. The effects of pH and temperature on the rPvICP’s inhibitory activity against the enzymes was investigated using methods similar to those previously described [
14]. All assays were performed in triplicate. The SD of the values obtained from each individual experiment were calculated.
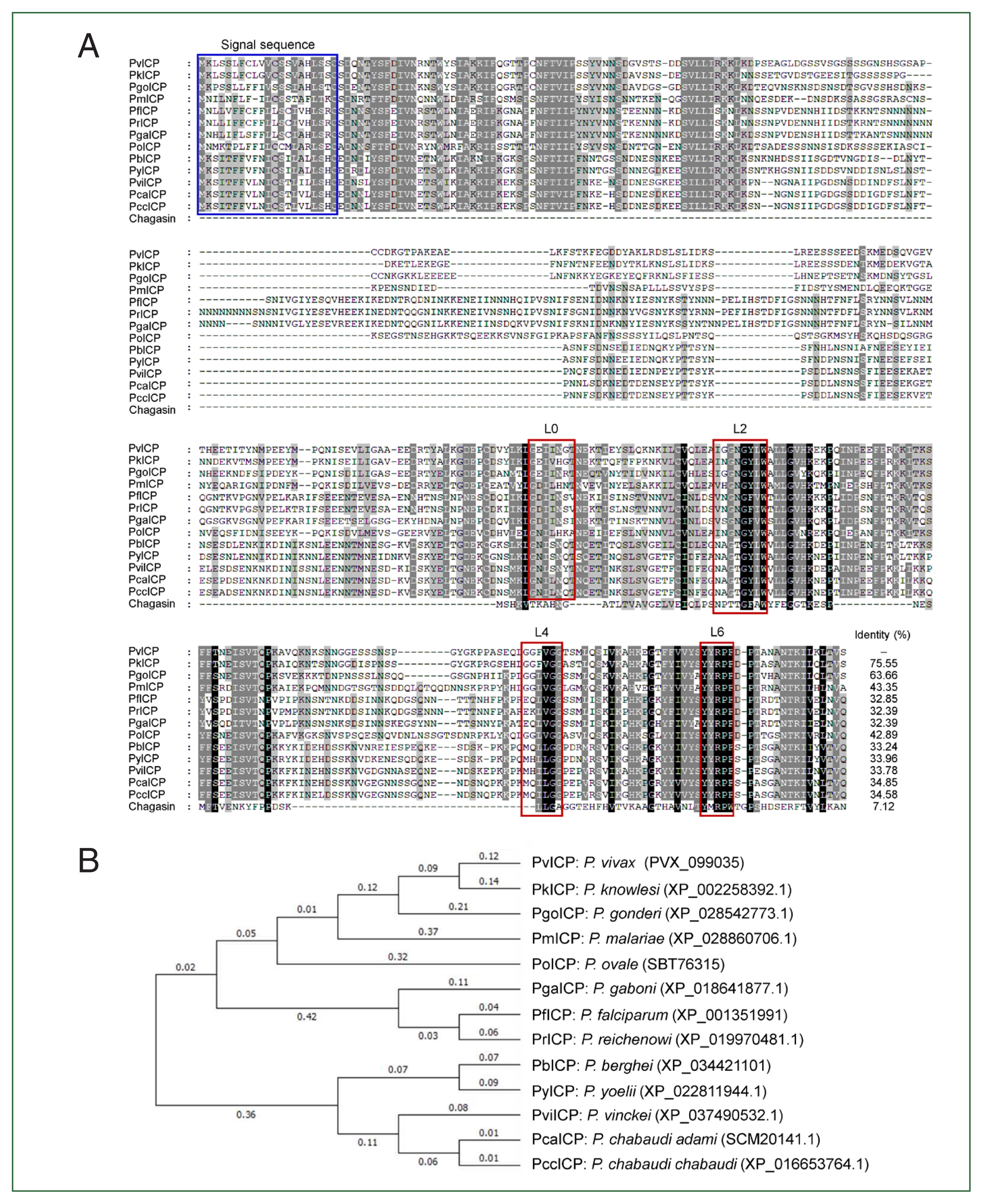
PCR amplification of
PvICP exhibited a single product with an approximate size of 1.8 kbp. Sequence analysis of cloned
PvICP revealed that the gene comprised two exons separated by an intron of 645 bp in the N-terminal region. The open reading frame of the gene consisted of 1,095 nucleotides encoding 364 amino acids. Multiple sequence alignment and phylogenetic analyses of plasmodial ICPs at the amino acid level revealed that PvICP showed high amino acid sequence identities with ICPs of two
Plasmodium species, namely
P. knowlesi (75.55%) and
Plasmodium gonderi (63.66%) (
Fig. 1A, B). Sequence identities with other plasmodial ICPs ranged from 34.58 to 43.35%. Like other plasmodial ICPs, PvICP had a C-terminal chagasin-like domain with a longer N-terminal region, resulting in a large size of approximately 40 kDa. The potential signal peptide sequence with a length of 21 amino acids was well-conserved in all plasmodial ICPs. Four typical loops—L0, L2, L4, and L6—that bind directly to the active site of counterpart cysteine proteases [
15,
16] were well-conserved in all plasmodial ICPs, although minor amino acid variations were observed among the proteins. The high conservation of these structural loops in plasmodial ICPs across different
Plasmodium parasite species suggests their functional relevance.
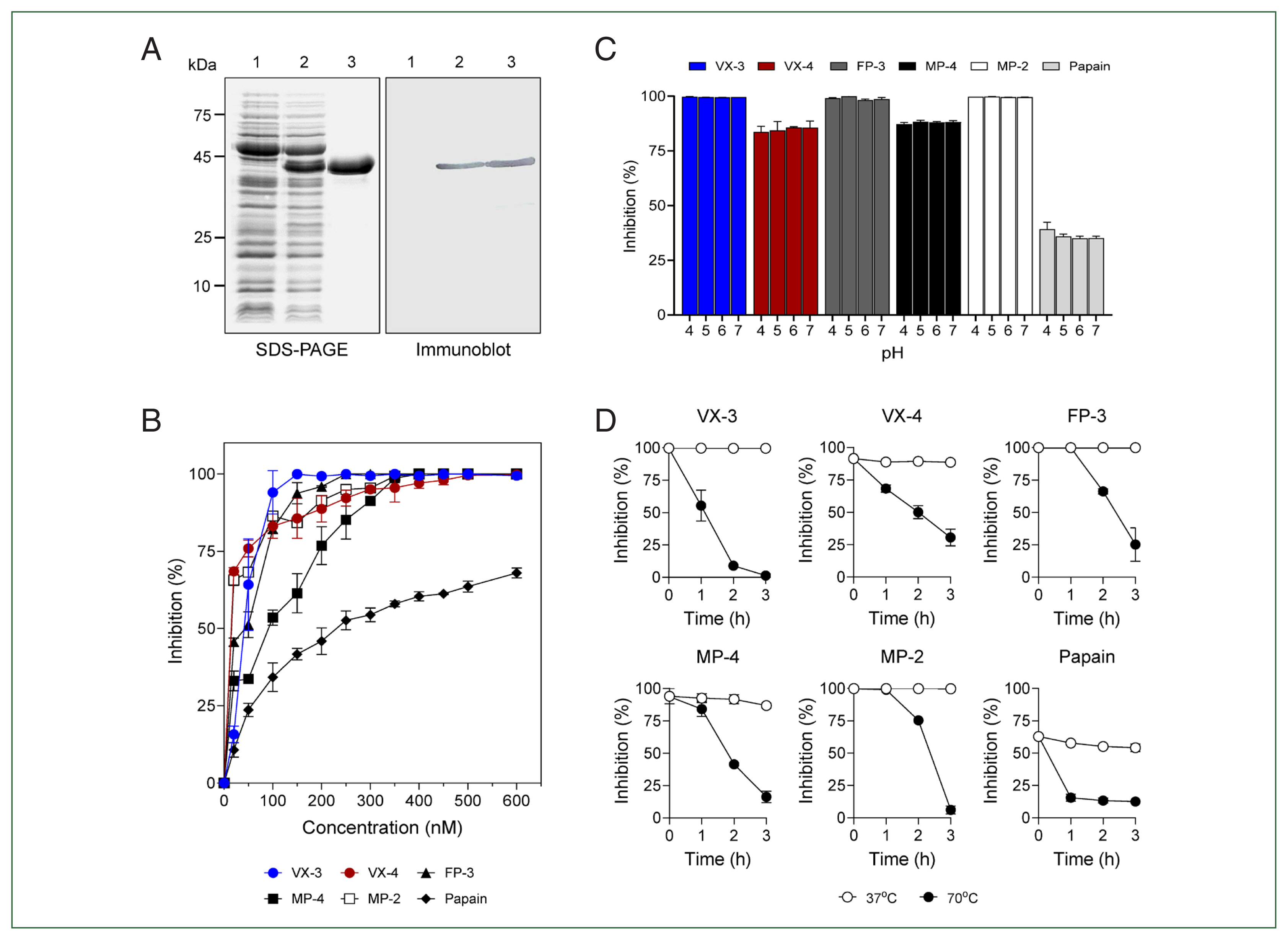
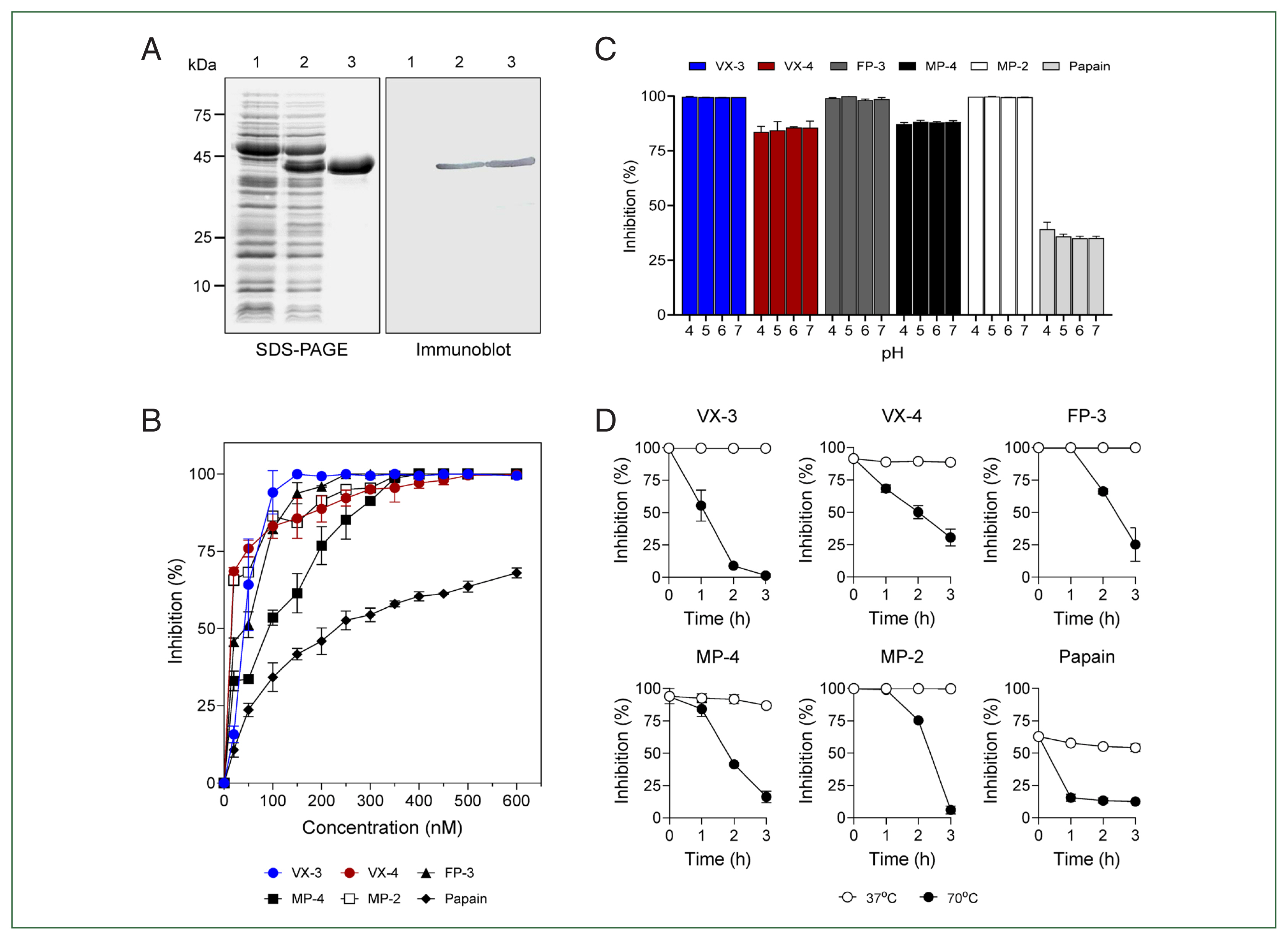
A partial fragment of PvICP that excluded the N-terminal signal peptide sequence was expressed in
E. coli, resulting in a soluble recombinant protein with an expected molecular size of 38.5 kDa (
Fig. 2A). The inhibitory activity of purified rPvICP was analyzed against several cysteine proteases, including VX-3, VX-4, FP-3, MP-2, MP-4, and papain. rPvICP exhibited promising inhibition activity for the plasmodial enzymes in a dose-dependent manner (
Fig. 2B). The broad-range inhibitory activity of rPvICP for FP family enzymes from different
Plasmodium species suggest structural conservation of plasmodial ICPs and potent target enzymes in
Plasmodium parasites. Notably, rPvICP inhibited FP-3 family enzymes (VX-3, FP-3, and MP-2) more effectively than VX-4 and MP-4 (
Fig. 2B). Moreover, rPvICP showed lower inhibitory activity for papain. These findings suggest that PvICP has more suitable structural properties for inhibiting FP-3 family enzymes. However, further studies are necessary to understand the underlying molecular interactions between PvICP and VXs. The inhibitory activity of rPvICP was not significantly affected by pH, resulting in a broad range of inhibitory activity against the tested enzymes under different pH conditions (
Fig. 2C). Similar pH-independent broad inhibitory activity has been reported in other plasmodial ICPs [
11,
14]. rPvICP was thermo-labile, rapidly losing its inhibitory activity at 70°C. However, it was stable at 37°C with no significant loss of inhibitory activity (
Fig. 2D). Similar thermo-labile properties have been reported for PmICP, an ICP of
P. malariae [
14].
Overall, this study has increased our understanding on rPvICP. Functional expression of rPvICP offers crucial materials for future studies on the nature of PvICP. The similar structural and biochemical properties observed in plasmodial ICPs—particularly those from human-infecting Plasmodium species—provide crucial insights for developing specific inhibitors as effective antimalarial drugs targeting FP family cysteine proteases in malaria parasites. Although the biological functions of PvICP in P. vivax are not fully understood, the high structural and biochemical similarities with other plasmodial ICPs suggest that PvICP may share functional relevance with other plasmodial ICPs. Further studies on the expression patterns and localization of PvICP across different developmental stages of P. vivax and the molecular interactions of PvICP with VXs and host cysteine proteases are needed to better understand the biological significance of PvICP.
Notes
-
Author contributions
Conceptualization: Võ TC, Na BK
Data curation: Võ TC, Lê HG
Formal analysis: Võ TC, Kang JM, Lê HG
Investigation: Võ TC, Kang JM, Lê HG
Methodology: Võ TC, Kang JM, Lê HG
Project administration: Na BK
Supervision: Na BK
Visualization: Võ TC, Lê HG
Writing—original draft: Võ TC, Lê HG
Writing—review & editing: Kang JM, Na BK
-
Conflict of interest
The authors declare no conflict of interest related to this study.
-
Acknowledgments
This work was partially supported by the National Research Foundation of Korea (NRF) grant funded by the government of Korea (MSIT) (RS-2025-02413635).
Fig. 1Sequence and phylogenetic analyses of inhibitors of cysteine protease (ICP) from
Plasmodium vivax (PvICP) and other plasmodial ICPs. (A) Multiple sequence alignment. Deduced amino acid sequences of ICPs from
Plasmodium species and chagasin (Q966X9) were aligned. Dashes represent gaps introduced to maximize alignment. The predicted N-terminal signal sequence is marked with a blue box. Amino acid residues corresponding to L0, L2, L4, and L6 loops, which are conserved in plasmodial ICPs [
15], are presented with red boxes, respectively. The percentage of identity among sequences is represented by different shading: black (>88%), dark grey (75%–88%), light grey (37%–75%), and no shading (<37%). (B) Phylogenetic analysis.

Fig. 2Expression and biochemical properties of recombinant inhibitors of cysteine protease from Plasmodium vivax (rPvICP). (A) Expression of rPvICP. Lane 1, non-induced Escherichia coli lysate; lane 2, isopropyl-1-thio-β-D-galactopyranoside–induced E. coli lysate; lane 3, purified rPvICP. (B) Inhibitory activity of rPvICP against falcipain (FP) family plasmodial cysteine proteases and papain. Each enzyme (200 nM) was incubated with different concentrations of rPvICP (0–600 nM) for 20 min, and residual activity was assayed. (C) Effect of pH. rPvICP (200 nM) was incubated with each enzyme (200 nM) in different pHs (pH 4–7) for 20 min, and the residual enzyme activity was assayed. (D) Thermal stability. rPvICP was pre-incubated in 50 mM sodium acetate (pH 6.0) at 37°C or 70°C for the indicated time. The residual inhibitory activity of rPvICP for each enzyme was assayed. All experiments were performed in triplicate, and mean and SD values were calculated. VX, vivapain; MP, malapain.
References
- 1. Rosenthal PJ. Cysteine proteases of malaria parasites. Int J Parasitol 2004;34(13–14):1489-1499. https://doi.org/10.1016/j.ijpara.2004.10.003
- 2. Rosenthal PJ. Falcipains and other cysteine proteases of malaria parasites. Adv Exp Med Biol 2011;712:30-48. https://doi.org/10.1007/978-1-4419-8414-2_3
- 3. Rosenthal PJ. Falcipain cysteine proteases of malaria parasites: an update. Biochim Biophys Acta Proteins Proteom 2020;1868(3):140362. https://doi.org/10.1016/j.bbapap.2020.140362
- 4. Patra J, Rana D, Arora S, Pal M, Mahindroo N. Falcipains: biochemistry, target validation and structure-activity relationship studies of inhibitors as antimalarials. Eur J Med Chem 2023;252:115299. https://doi.org/10.1016/j.ejmech.2023.115299
- 5. Na BK, Shenai BR, Sijwali PS, Choe Y, Pandey KC, et al. Identification and biochemical characterization of vivapains, cysteine proteases of the malaria parasite Plasmodium vivax. Biochem J 2004;378(Pt 2):529-538. https://doi.org/10.1042/BJ20031487
- 6. Na BK, Bae YA, Zo YG, Choe Y, Kim SH, et al. Biochemical properties of a novel cysteine protease of Plasmodium vivax, vivapain-4. PLoS Negl Trop Dis 2010;4(10):e849. https://doi.org/10.1371/journal.pntd.0000849
- 7. Prasad R, Atul , Soni A, Puri SK, Sijwali PS. Expression, characterization, and cellular localization of knowpains, papain-like cysteine proteases of the Plasmodium knowlesi malaria parasite. PLoS One 2012;7(12):e51619. https://doi.org/10.1371/journal.pone.0051619
- 8. Lê HG, Kang JM, Võ TC, Yoo WG, Lee KH, et al. Biochemical properties of two Plasmodium malariae cysteine proteases, malapain-2 and malapain-4. Microorganisms 2022;10(1):193. https://doi.org/10.3390/microorganisms10010193
- 9. Ramjee MK, Flinn NS, Pemberton TP, Quibell M, Wang Y, et al. Substrate mapping and inhibitor profiling of falcipain-2, falcipain-3 and berghepain-2: implications for peptidase anti-malarial drug discovery. Biochem J 2006;399(1):47-57. https://doi.org/10.1042/BJ20060422
- 10. Singh A, Shenai BR, Choe Y, Gut J, Sijwali PS, et al. Critical role of amino acid 23 in mediating activity and specificity of vinckepain-2, a papain-family cysteine protease of rodent malaria parasites. Biochem J 2002;368(Pt 1):273-281. https://doi.org/10.1042/BJ20020753
- 11. Pandey KC, Singh N, Arastu-Kapur S, Bogyo M, Rosenthal PJ. Falstatin, a cysteine protease inhibitor of Plasmodium falciparum, facilitates erythrocyte invasion. PLoS Pathog 2006;2(11):e117. https://doi.org/10.1371/journal.ppat.0020117
- 12. Rennenberg A, Lehmann C, Heitmann A, Witt T, Hansen G, et al. Exoerythrocytic Plasmodium parasites secrete a cysteine protease inhibitor involved in sporozoite invasion and capable of blocking cell death of host hepatocytes. PLoS Pathog 2010;6(3):e1000825. https://doi.org/10.1371/journal.ppat.1000825
- 13. Pei Y, Miller JL, Lindner SE, Vaughan AM, Torii M, et al. Plasmodium yoelii inhibitor of cysteine proteases is exported to exomembrane structures and interacts with yoelipain-2 during asexual blood-stage development. Cell Microbiol 2013;15(9):1508-1526. https://doi.org/10.1111/cmi.12124
- 14. Lê HG, Kang JM, Võ TC, Nguyễn TD, Jung M, et al. Inhibitor of cysteine protease of Plasmodium malariae regulates malapains, endogenous cysteine proteases of the parasite. Pathogens 2022;11(5):605. https://doi.org/10.3390/pathogens11050605
- 15. Hansen G, Heitmann A, Witt T, Li H, Jiang H, et al. Structural basis for the regulation of cysteine-protease activity by a new class of protease inhibitors in Plasmodium. Structure 2011;19(7):919-929. https://doi.org/10.1016/j.str.2011.03.025
- 16. Wang SX, Pandey KC, Scharfstein J, Whisstock J, Huang RK, et al. The structure of chagasin in complex with a cysteine protease clarifies the binding mode and evolution of an inhibitor family. Structure 2007;15(5):535-543. https://doi.org/10.1016/j.str.2007.03.012
Citations
Citations to this article as recorded by